Abstract
In Eastern Canada, an increasing number of pests, predators and pathogens in downriver areas (close to the ocean) have led the aquaculture industry to consider growing oysters in upriver areas (close to the river source). In this study, oyster growth performance was compared between downriver and upriver environments by means of stock transfer experiments within the Richibucto estuary. In May 2009, seed oysters (~26 mm shell height) originating from two downriver sites (salinity ~20–30 ‰) were transferred upriver (salinity ~5–20 ‰). Follow-up measurements in October 2009 revealed that the seed transferred upriver grew and survived as well as seed that remained downriver, while the mortality rates of adult oysters (shell height ~66 mm) were lower at the upriver site. Meat content was unaffected in adult oysters transferred upriver. However, there were indications that the upriver environment promoted shell growth (mm) in adult oysters. Oysters transferred upriver had a gain in shell height (LSM ± SE) over the oysters that remained downriver (2.7 ± 0.5 vs. 1.8 ± 0.5 mm) and in shell width [Median (95 % CI); 2.8 mm (1.9, 3.6) vs. 1.0 mm (0.3, 1.2)]. Therefore, the holding of adult oysters upriver during the spring–summer period confers productivity advantages on top of protection from diseases and predation. By contrast, productivity losses were recorded when relocating adult oysters originating from the upriver environment. More specifically, final organic meat content were approximately 35 % less in adult oysters transferred downriver compared to those that remained upriver (0.48 ± 0.04 vs. 0.74 ± 0.04 g). Results suggest that transfers along the river impact physiological processes such as gametogenesis and shell formation in adult oysters.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Oyster culture in Atlantic Canada is generally carried out in the downriver (close to the ocean) area, in the shallow bays and estuaries where salinity varies between ~18 and 30 ‰. It is well documented that maximum growth and survival for eastern oysters (Crassostrea virginica) are in the vicinity of 14–28 ‰ (Medcof and Needler 1941; Loosanoff 1953; Chanley 1958; Shumway 1996). However, the downriver area also provides a suitable environment for disease vectors causing high mortality in wild and cultured stocks. For example, a 58 % mortality rate in oyster spat reared downriver (12–18 ‰) compared to 0 % upriver (9–14 ‰) was attributed to Perkinsus marinus infections, causative agent of Dermo disease (Albright et al. 2007; Bushek et al. 2012). Similarly, juvenile oyster disease (JOD) that primarily affects young oysters (15–25 mm in shell height) was also observed at culture sites where salinities were above 18 ‰ (Boettcher et al. 2006).
As oysters experience a higher incidence of disease downriver, incorporating an upriver (close to the river source) site in the production cycle of an oyster operation could provide benefits to the industry (Paynter and Burreson 1991; Ewart and Ford 1993; McGladdery and Zurbrigg 2006; Ford and Smolowitz 2007). Given that parasite infection is positively correlated with salinity, an area that experiences low-salinity events for part of the seasonal cycle may protect oyster stocks and hence increase overall productivity of the farms (Ford and Haskin 1988; Ragone and Burreson 1993; Elston and Ford 2011). For example, in the 1950s, the oyster industry in Delaware Bay benefited from using the low-salinity environment of the upper Bay to protect seed stocks from an endoparasitic pathogen, Haplosporidium nelsoni, responsible for MSX disease (Elston and Ford 2011). In recent years, severe mortality (~90 %) within the Bras d’Or Lakes in Cape Breton, NS (15–20 ‰) was also caused by an outbreak of MSX disease (McGladdery and Zurbrigg 2006). Within the Gulf of St. Lawrence (Fig. 1), oysters are considered resistant to Malpeque disease (Mary Stephenson, Fisheries and Oceans, pers. comm. November 12, 2014). Previous studies indicated a clear spatial gradient of reduced threats with reduced salinity (Paynter and Burreson 1991; Chu et al. 1993; Bushek et al. 2012). Also, population differentiation in sessile organisms may exist along a salinity gradient, thus providing a fitness advantage for marine invertebrates exposed to heterogeneity environments often present upriver (Sanford and Kelly 2011). As a result, oyster growers in Atlantic Canada are increasingly considering the use of the upriver area. Moreover, predaceous and competing species, such as oyster drills, starfish, boring sponges and mussels, are less abundant in the upriver area (Wallace 1966).
In Atlantic Canada, incorporating an upriver site in the early stage of the production cycle would entail unidirectional transfers of seed from the downriver areas. Growers depend on natural spat collected from the downriver areas to replenish their stocks (Méthé and Léger 1994). Since oyster larvae (2 week planktonic stages) travel predominantly downstream (North et al. 2010), spat presumably originate from parents located all along the riverbed of the estuary. As a result, spat collected downriver should be well adapted to upriver and downriver conditions. Eierman and Hare (2013) showed that larval pools from downriver areas (salinity 30 ‰) had similar survival rates across different salinities (10 and 30 ‰) in a laboratory setting. Conceivably, unidirectional transfers of seed stock upriver may prove to be an acceptable practice for the industry to reduce exposure to disease and predaceous activities in young stocks.
Information regarding the tolerance of adult oysters to transfers along the salinity gradient of an estuary is more limited than for seed oysters. As sessile organisms, adult oysters spend a lifetime adjusting to local conditions. For example, Loosanoff (1953) showed that C. virginica oysters from a low-salinity environment have a greater capacity to close and open their shells and resume pumping activities at lower salinities than those living further downstream in the estuaries. Having rarely been exposed to episodic drops in salinity in the downriver area, oysters transferred to the upriver site may not have the capacity to resume pumping activities at these lower salinities (Loosanoff 1953). A prolonged period (March–November) of low salinity (<10 ‰) in upriver areas of the Chesapeake Bay has resulted in heavy mortalities of adult oysters (Engle 1946).
While oysters in Eastern Canada have rarely been transferred to the upriver area, it should be mentioned that some growers are presently transferring or relaying wild oysters from the upriver area to their aquaculture site located downriver. Regulatory agencies support this relay fishery, whereby oyster growers are permitted to harvest wild market-size oysters upriver, where they are freely available but nevertheless susceptible to bacterial (E. coli) contamination from sewage effluent, a human health concern. Their transfer to aquaculture sites downriver allows a prescribed depuration to take place. It also contributes to increasing the salt content of the meats (improved taste). There is nevertheless anecdotal evidence of high mortalities in the transferred stocks, presumably related to transfer shock [Paul Cormier (NB Aquaculture, Fisheries and Agriculture), personal communication, July 23, 2014]. Investigating the impact of this relay fishery on oyster productivity is necessary to guide industry.
In this study, the first objective was to evaluate the benefits of incorporating an upriver site in the production cycle of an oyster operation. Oysters were taken from downriver aquaculture sites and were transferred upriver, where they were monitored for shell and meat growth during a 5-month period. The second objective was to evaluate the impact of the relay fishery on oyster productivity. Wild upriver oysters were transferred downriver and monitored over the same time period.
Materials and methods
Study area and experimental design
The experiment was conducted at two sites (upriver, downriver) located 20 km apart in the Richibucto River in eastern New Brunswick, Canada (Fig. 1). The underlying bedrock of the Richibouctou watershed consists of soft sandstone and siltstone and is located within vast bogs (Turcotte-Lanteigne and Ferguson 2008). On May 25, 2009, seed oysters (d1, d2) (same genetic stock) originating from two downriver areas of similar environmental conditions were transferred to the upriver site. The d1 oysters originated from a wild collection within the same estuary (N 46°40′54.434″; W 64°51′57.529″). The d2 oysters were also a wild collection, but from an adjacent estuary (Bouctouche Bay) (N 46°31′26.928″; W 64°43′56.911″). The initial shell height (mean ± SE) was significantly different between the sources (d1 24.0 ± 0.3 mm; d2 28.0 ± 0.3 mm). At both study sites (~3 m depth), oysters were held in cages (60 cm × 120 cm × 10 cm) constructed of plastic-coated wire mesh and divided into 32 compartments, each holding an individual oyster (Fig. 2). Cages were suspended in the upper water column near the surface. A total of 64 d1 and 64 d2 oysters were equally distributed among 4 cages at both sites. Cages were deployed for 155 days, from May 25 to October 28, 2009.
Additionally, ~4-year-old oysters (UPR, DNR) originating from upriver and downriver areas were reciprocally transferred. The UPR oysters were hand-collected from a wild population adjacent to the upriver site (N 46°35′11.227″; W 64°58′51.376″). The DNR oysters (wild seed from Bouctouche, NB) were obtained from a Richibucto Harbor aquaculture site downriver (N 46°41′42.231″; W 64°49′50.243″). The initial shell height (mean ± SE) was significantly different between the sources (UPR 62.5 ± 0.8 mm; DNR 69.9 ± 0.4 mm). Oysters were held in cages, as described above (Fig. 2). A total of 64 UPR and 64 DNR oysters were equally distributed among 4 cages at each site. Cages were deployed for 155 days, from May 25 to October 28, 2009.
Environmental parameters
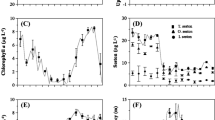
At each site, salinity was determined from electrical conductivity measurements taken every 3 h using in situ YSI 6560 probes (YSI, Yellow Springs, Ohio). Rate of flow (or discharge) data was obtained from Environment Canada’s (EC) Water Survey of Canada (1964–2010; station no. 01BS001). To compute the water discharge (m3 s−1), EC established a water level–discharge relationship from multiple measurements (channel depth, width, flow velocity) taken at different water levels in the river. Water temperature was measured every 3 h for the duration of the study using in situ Minilog-TR probes (AMIRIX Systems Inc., Halifax, NS). Relative fluorescence was measured using in situ YSI 6025 chlorophyll sensors. Relative fluorescence units (RFU) were corrected for instrument drift and converted into a 0–1 scale.
Productivity parameters
Shell height [SH (anterior–posterior)] and width [SW (dorsoventral)], whole wet weight (W) and approximate shell surface area (S) of each oyster was measured prior to deployment (May 25) and when retrieved (October 28). SH and SW were measured to the nearest 0.01 mm using an electronic caliper. W was measured to the nearest 0.01 g. The S of each shell was determined to the nearest 0.01 cm2 using University of Texas Health Science Center of San Antonio (UTHSCSA) Image Tool Software. The overall growth (SH, SW, W, S) of individual oysters was calculated as the final minus the initial measurement, an approach that is consistent with the linear growth reported for the size range under investigation (Doiron and Maillet 2010). Mortality (M) was assessed by counting live/dead oysters in each cage at the end of the study.
Sixteen oysters per cage were selected (two middle rows) for the condition Index (CI). The CI was calculated using the organic tissue weight (dried tissue weight minus ash weight) and its relationship to dry shell weight as follows (Landry et al. 2001):
The dried tissue weight was obtained by oven-drying the meat for at least 24 h at 70 °C and weighing resultant mass. The ash weight was obtained by heating the oyster meat at 500 °C for 6–7 h, weighing the resulting ash. The dried shell weight was measured following air-drying the shell at room temperature for 24 h. The dry shell weight, dry tissue and ash weights were measured to the nearest 0.0001 g.
Dynamic energy budget model simulations
In order to test the influence on oyster (adult) growth of the different temperature regimes at the two sites, a numerical ecophysiological model was set up using the dynamic energy budget (DEB) theory (Kooijman 2000). The model describes how an individual oyster utilizes the energy it acquires from food ingestion and assimilation to fulfill the requirements of the growth/maturation and maintenance of its somatic and reproductive tissues. Given that environmental conditions (water temperature and food concentrations, here chlorophyll a was used as a proxy) are known for the period studied, the model can reproduce the individual growth over that time period. Model equations are detailed in Pouvreau et al. (2006), and the set of parameters specific to C. virginica was retrieved from Filgueira et al. (2014).
At each site, growth observations were pooled for both stocks and two models were built, one for each site. Each model was based on the environmental conditions of the respective site and was calibrated against the pooled growth observations by tuning the only site-specific parameter, i.e., the half-saturation coefficient that scales the food ingestion to available food at each site.
Finally, to test whether water temperature alone could explain growth differences between the two sites, each model was run one more time using the temperature forcing of the opposite site, such that for each site, oyster growth could be predicted based on the water temperature of the other site. The growth (in terms of shell height) at the end of the experiment was then compared to the ‘non-inverted’ temperature scenario.
Statistical data analysis
Separate statistical analyses were carried out for different outcomes: SH, SW, W, S, CI and M.
A linear mixed model with random cage effects was used due to the hierarchical (nested) structure of the experimental design (Dohoo et al. 2009). The statistical design had a split-plot character because two oyster sources were reared within the same cages, which in turn were located at two sites. Thus, the cages corresponded to wholeplots, and individual oysters corresponded to subplots; furthermore, site and source were the wholeplot and subplots factors, respectively. The linear mixed models had site, source and its interaction, as well as overall cage rows and columns as fixed effects. In addition to the random cage effects, cage-specific row and column effects were also analyzed.
The assumptions of normality and homoscedasticity of linear mixed models were evaluated by residuals plots based on the standardized results. The deviation of residuals from normality was assessed using the Anderson–Darling normality test. Whenever model assumptions could not be met, an optimal power transformation was obtained by Box–Cox analysis (Dohoo et al. 2009). A value of 10 was added to the SW data (market-sized oysters) prior to log transformation, based on trial and error and inspection of residuals.
In the presence of a significant interaction between the various factors, pairwise comparison tests with Bonferroni correction were performed to determine significance between groups. All estimates were reported as least squares mean (LSM) ± standard error (SE); in some instances following back-transformation, the back-transformed value was interpreted as the median, with the SE computed using the delta method (Weisberg 2005). In the log back-transformation, the confidence intervals are presented, instead of SE.
A few extreme outliers were removed after they were found statistically significant by the outlier detection test (based on deletion residuals): W (3 obs.) and CI (1 obs.) for the adult oysters (UPR, DNR); surface area (1 obs.) for the seed oysters (d1, d2). In addition, a number of missing values were due to mortality (32) and missing (18) oysters in the field.
A binary logistic regression analysis of the M data (dead/alive) was carried out with random cage effects because the experimental design was of a hierarchical (nested) structure, as described above.
For all analyses, the significance level was set at P ≤ 0.05. The analyses were carried out using Minitab software (version 15.1.30) (Minitab Inc., State College, PA), STATA software (version 13.0) (StataCorp LP, College Station, TX) and SAS software (version 9.2) (SAS Institute Inc., Cary, NC).
Results
Environment
While relative chlorophyll fluorescence at both sites were the same (upriver vs. downriver, respectively) (0.064 ± 0.008 vs. 0.080 ± 0.011, P = 0.25), the upriver site was characterized by higher temperature (18.1 ± 0.3 vs. 16.7 ± 0.3 °C, P < 0.001) and lower salinity (14.6 ± 0.3 vs. 25.5 ± 0.2 ‰, P < 0.001). At both sites, the tidal influence based on a harmonic analysis explains ~13–14 % of the observed salinity variation (Foreman 1977). Although the influence from the diurnal (and semi-diurnal) constituents is weak, the lunar cycle still has an influence on the observed salinity, particularly at the upriver site. The tide explains the observed periodic lows (Δ5 ‰), occurring at the beginning of July, Aug, September and even October (albeit less obvious) (Fig. 3). The largest diurnal salinity variations due to tides were observed at the upriver site with amplitude ranging from 0.3 to 1.2 ‰ during neap and spring tides, respectively. At the upriver site, salinity reached <10 ‰ in July and October during ebb tide, coinciding with heavy rains (precipitation) based on freshwater discharge data from Environment Canada. The lowest observed salinity (3–4 ‰) occurred in mid-October during a lunar event and when freshwater discharge at a peak (~100 m3 s−1 for 4 consecutive days). Monthly discharge averages for June–September (~11 m3 s−1) were comparable to the historical (1964–2010) monthly averages (10.5 m3 s−1) (Environment Canada 1964–2010, http://www.wsc.ec.gc.ca).
Mortality rates
The binary logistic regression analysis indicated a significant difference in mortality between seed sources (P = 0.05), but not between sites (P = 0.22). The d2 oyster seeds have a lower risk of dying (odds ratio = 0.39) than d1 seeds, regardless of site. The mortality rates in adult oysters were significantly lower (P = 0.03; odds ratio = 0.11) at the upriver site, regardless of source. Live and dead oyster counts are summarized in Table 1 (seed) and Table 2 (adults). Mortality rates were below 15 % (seed) and 8 % (adults).
Oyster productivity
Oyster seed (d1, d2) transferred upriver grew as well as seed that remained downriver. No significant difference in growth as measured by shell height, width, surface area, CI or wet weight was detected between rearing sites, regardless of source (Table 3). Nor was there any difference between rearing sites for final dry shell and organic meat weights.
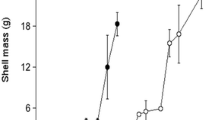
In contrast, the upriver environment positively affected the shell growth of adult oysters (Table 4). For example, in terms of shell height, DNR oysters that were transferred upriver grew by 2.7 ± 0.5 mm, whereas those that remained downriver grew by 1.8 ± 0.5 mm. However, there was no effect on wet weight (P = 0.54) and CI (P = 0.15), and DNR oysters had similar final organic meat weights, 0.71 ± 0.04 g upriver and 0.62 ± 0.04 downriver. For UPR stocks, the transfer of adults to the downriver area seemed unfavorable representing a difference of ~2.2 mm in shell height and ~2.1 mm in shell width compared to their counterpart that remained upriver. Moreover, a significant reduction in meat content (organic weight) was observed at the downriver site. UPR oysters had a final meat (organic) weight of 0.74 ± 0.04 g upriver, compared to only 0.48 ± 0.04 g downriver.
The DEB model simulations indicated that transferring oysters from one site to the temperature regime of the other site does not induce any growth difference [<0.08 % difference in individual growth (in terms of shell height) in the different temperature regimes for both transfer experiments, i.e., upriver to downriver temperature (0.25 mm) and downriver to upriver temperature (0.50 mm)]. According to these model results, the difference in water temperature between the two sites is not a valid explanation for the observed difference in adult oyster growth.
Discussion
Mortality
Despite spending 5 months (May–October 2009) exposed to the ecological conditions found at the upriver site, seed and adult oysters, including those originating from downriver, exhibited mortality rates comparable to those reported by industry (Doiron 2008). Ongoing wild oyster health monitoring in the vicinity of the study sites has not detected any diseases of concern (Mary Stephenson, Fisheries and Oceans, pers. comm. November 12, 2014). The average mortality rate was below 15 % (seed) and 8 % (adults). More importantly, in adult oysters, the mortality rate upriver was significantly lower than the mortality rate downriver. This result suggests that in Atlantic Canada both seed and mature crop may be transferred upriver for disease protection from late May until October with no apparent concern for survival. This conclusion is further supported by numerous reports showing the species’ tolerance to low salinities. Loosanoff (1953) and Wells (1961) reported 7.5 and 7.0 ‰, respectively, as the species’ lower salinity threshold for survival. However, the duration of low-salinity exposure is also an important factor affecting survival. For instance, a 6-month (April–October) exposure to salinities in the range of 3–9 ‰ ultimately resulted in massive (50–95 %) mortality in Chesapeake Bay (Engle 1946). In our study, oysters were exposed to salinities below 10 ‰ for only four consecutive days in July and nine consecutive days in October. Moreover, low temperatures, particularly in October (<10 °C), presumably lowered the oysters’ metabolic activity (Loosanoff 1953; Wells 1961; Pollack et al. 2011). Consequently, if the oysters were closed and operating anaerobically during the freshets, the accumulation of toxic metabolic waste within the tissues would have been minimized.
It is noteworthy that the freshets recorded upriver were caused by the combined effects of ebb tides and freshwater discharges. In keeping with this information, oysters in Eastern Canada may have greater difficulty tolerating spring freshets than autumnal freshets. In spring, the melting of snow adds substantial quantities of freshwater into the estuarine systems. In the Richibucto River, for instance, the monthly freshwater discharge averages 84 m3 s−1 in April compared to 17 m3 s−1 in October (Environment Canada 1964–2010, http://www.wsc.ec.gc.ca). These differential discharges are consistent with the Bataller et al. (1999) report, which indicated that the salinity in the upriver area of the Richibucto was approximately 10 ‰ lower in spring than autumn. Moreover, in spring, the freshwater discharge starts in late March and extends over 1–2 months, therefore coinciding with the spring awakening of oysters (Comeau et al. 2012; Comeau 2014) and presumably the onset of aerobic metabolism/oxygen intake. Exactly how spring freshets impact the survival of oysters in Eastern Canada remains to be investigated. In the interim, since freshwater kills are well documented (Andrews et al. 1959; Fisher et al. 1989; La Peyre et al. 2009) and disease outbreaks occur at high water temperature (Shumway 1996), it may be advisable to avoid transferring oysters upriver until after spring freshets.
Based on climate change predictions, precipitations in New Brunswick will increase by 10 % in 2050 and 15 % in 2080 (Vasseur and Catto 2008); however, it will be scattered uniformly throughout the year. Therefore, oysters reared upriver from May through October should not experience a substantial change in salinity levels.
Growth performance
Oyster shell growth observed at the upriver site lends further support to incorporating an upriver site in the production cycle. Seed oysters transferred upriver grew as well as those that remained downriver, both in terms of shell length and meat content. These results are consistent with Shaw (1966), whereby seed oysters (25 mm shell height) from a low-salinity area (8–16 ‰) of Chesapeake Bay (Maryland) transferred to both low (8–16 ‰) and high (17–35 ‰) salinity areas showed no significant differences in growth rates after two seasonal cycles.
For mature stocks, the transfer to the upriver area was associated with a significant but modest enhanced growth, corresponding to a gain of ~0.9 mm in shell height and ~1.8 mm in shell width. The exact reason for this outcome is unknown. Although shell erosion is common in exposed sites (Mallet et al. 2009, 2013), we found no indications of damaged new shell growth downriver. High level of calcium carbonate in the river system is unlikely given the presence of bogs and the bedrock composition within the watershed (Turcotte-Lanteigne and Ferguson 2008). It is well known that oysters grow faster in warmer waters (Ingle and Dawson 1952; Shumway 1996). However, according to our DEB model, the 1.4 °C increment in water temperature at the upriver site cannot explain the difference in shell growth among groups of adult oysters, irrespective of food density (quantity or quality). Presumably, any influence of food (or wave/wind exposure) on shell growth (erosion) would have been detected in seed oysters, which was not the case. On the other hand, it is possible that the higher shell growth upriver was linked to gametogenesis, trading off storage (lipids utilized in gametogenesis) for shell growth after the transfer to the upriver site. Such trade-offs have been observed in other species, e.g., freshwater snails will delay reproductive effort in the presence of predators (water bugs), in order to form larger shells (Hoverman et al. 2005; Hoverman and Relyea 2007). Butler (1949) had previously reported gametogenesis inhibition in C. virginica at salinities <6 ‰. Loosanoff (1953) also demonstrated that oysters spawned lightly at 7.5 ‰. In our study, salinity at the upriver site fluctuated between 8 and 20 ‰ prior to the expected spawning period in July (Mayrand et al. 2013), while salinity was higher and more stable downriver (25–30 ‰). The higher survival rates observed in adult oysters at the upriver site relative to the downriver site could be linked to low spawning effort. Therefore, it is possible that transfers along the river impacted physiological processes in adult oysters, such as gametogenesis and shell formation.
Wild upriver oysters
In our study, wild oysters originating from upriver had 31–44 % better shell growth than cultured oysters originating from downriver, regardless of their study site position along the river. The underlying cause for this result is unknown. The genetic pool would not have been reduced as cultured stocks originated from wild spat fall collection. The observed differences may have been a function of compensatory growth from a lifetime of living on upriver beds. Compensatory growth is a period of rapid growth relative to age in animals, such as after a period of food deprivation, and usually manifests itself as short-term accelerated growth (Eldridge and Eversole 1982; Kraeuter et al. 2007). In our study, simply raising upriver oysters off-bottom would have improved water flow and increased food availability. A sessile lifestyle in a heterogeneous environment (i.e., upriver area) may have also led to the development of traits that improve their relative fitness later in life (Ernande et al. 2004; Sanford and Kelly 2011). Through the production of variable phenotypes, from a single plastic genotype, oysters have the flexibility (morphological, physiological and/or behavioral) to respond to changes in environmental conditions (Berger and Kharazova 1997; Hamdoun et al. 2003; Drent et al. 2004).
Interestingly, considerable tissue (organic) loss was observed in wild oysters transferred downriver compared to those that remained upriver (0.48 vs. 0.74 g), supporting anecdotal evidence from relay fisheries. Since tissue measurements made no distinction between somatic and gonadic tissues, it is difficult to discern what actually occurred. For instance, wild oysters from upriver, having not developed and grown in downriver conditions, may have been faced with an ‘unplanned’ spawning event after the transfer downriver. Since the upriver site is not conducive to gametogenic development which is normally initiated in the fall, it could be argued that the observed tissue loss was the result of increased energy demands (i.e., vitellogenesis) after the transfer downriver (Honkoop 2003; Bayne 2004). Honkoop (2003) showed in Sydney rock oyster (Saccostrea glomerata) that the cost of reproductive effort (production, maintenance) was 84 % greater than those of somatic tissues.
Conclusion
After 5 months at the upriver site, aquaculture-derived oysters survived at levels comparable to that of the industry. Moreover, seed and mature oysters originating from downriver grew as well upriver as those that remained downriver, suggesting that transferring them upriver for part of their seasonal growth cycle (summer–autumn period) would serve in providing protection from diseases and predators lurking in the downriver area. The transfer to the upriver area seemed even more favorable for mature stocks, representing a gain of ~0.9 mm in shell height and ~1.8 mm in shell width compared to their counterpart that remained downriver. Based on these findings, it would be advantageous for oyster growers to transfer oysters (seed and mature crop) upriver for part of the seasonal cycle (May–October), as it would serve to augment shell growth while maintaining meat content, and providing protection from diseases and predation.
Mature oysters collected upriver had 31–44 % better shell growth than aquaculture oysters collected downriver, regardless of their study site position along the river. Nevertheless, it seems that ecological conditions downriver led to an expensive energetic cost in upriver-originated oysters transferred downriver as considerable meat content loss was observed (0.48 ± 0.04 vs. 0.74 ± 0.04 g). In light of these findings, collecting wild adult oysters from upriver and relaying them to an aquaculture site downriver is not recommended. These results also suggest that downriver oysters may exhibit meat loss once returned to the downriver environment.
References
Albright BW, Abbe GR, McCollough CB, Barker LS, Dungan CF (2007) Growth and mortality of dermo-disease-free juvenile oysters (Crassostrea virginica) at three salinity regimes in an enzootic area of Chesapeake Bay. J Shellfish Res 26:451–463
Andrews JD, Haven D, Quayle DB (1959) Freshwater kill of oysters (Crassostrea virginica) in James River, Virginia. Proc Natl Shellfish Assoc 49:29–49
Bataller EE, Boghen AD, Burt MDB (1999) Comparative growth of the eastern oyster Crassostrea virginica (Gmelin) reared at low and high salinities in New Brunswick, Canada. J Shellfish Res 18:107–114
Bayne BL (2004) Phenotypic flexibility and physiological tradeoffs in the feeding and growth of marine bivalve molluscs. Integr Comp Biol 44:425–432
Berger VJ, Kharazova AD (1997) Mechanisms of salinity adaptations in marine molluscs. Hydrobiologia 355:115–126
Boettcher KJ, Smolowitz R, Lewis EJ, Allam B, Dicherson H, Ford S, Huq A, Reece K, Rivara G, Woodley CM (2006) Juvenile oyster disease (JOD) in Crassostrea virginica: synthesis of knowledge and recommendations. J Shellfish Res 25:683–686
Bushek D, Ford SE, Burt I (2012) Long-term patterns of an estuarine pathogen along a salinity gradient. J Mar Res 70:225–251
Butler PA (1949) Gametogenesis in the oyster under conditions of depressed salinity. Biol Bull 96:263–269
Chanley PE (1958) Survival of some juvenile bivalves in water of low salinity. Proc Natl Shellfish Assoc 48:52–65
Chu FL, La Peyre JF, Burreson CS (1993) Perkinsus marinus infection and potential defense-related activities in eastern oysters, Crassostrea virginica: salinity effects. J Invertebr Pathol 62:226–232
Comeau LA (2014) Spring awakening temperature and survival of sediment-covered eastern oysters Crassostrea virginica. Aquaculture 430:188–194
Comeau LA, Mayrand E, Mallet A (2012) Winter quiescence and spring awakening of the eastern oyster Crassostrea virginica at its northernmost distribution limit. J Mar Biol 159:2269–2279
Dohoo I, Martin W, Stryhn H (2009) Veterinary epidemiologic research. VER Inc., Charlottetown
Doiron S (2008) Manuel de référence de l’ostréiculteur. Ministère de l’agriculture et de l’aquaculture, NB
Doiron S, Maillet MJ (2010) Rapport d’activités 2009: Évaluation de la croissance des huîtres en suspension le long de la côte-est du Nouveau-Brunswick. Ministère de l’agriculture et de l’aquaculture, NB
Drent J, Luttikhuizen PC, Piersma T (2004) Morphological dynamics in the foraging apparatus of a deposit feeding marine bivalve: phenotypic plasticity and heritable effects. Funct Ecol 18:349–356
Eierman LE, Hare MP (2013) Survival of oyster larvae in different salinities depends on source population within an estuary. J Exp Mar Biol Ecol 449:61–68
Eldridge PJ, Eversole AG (1982) Compensatory growth and mortality of the hard clam, Mercenaria mercenaria (Linnaeus, 1758). Veliger 24:276–278
Elston RA, Ford SE (2011) Shellfish diseases and health management. In: Shumway SE (ed) Shellfish aquaculture and the environment. Wiley, Oxford, pp 359–394
Engle JB (1946) Commercial aspects of the upper Chesapeake Bay oyster bars in light of recent oyster mortalities. In: Proceedings of the National Shellfish Association, pp 42–46
Environment Canada (1964–2010) Water surveys of Canada: water level and streamflow statistics. Station no. 01BS001. http://www.wsc.ec.gc.ca. Cited 20 July 2014
Ernande B, Boudry P, Clobert J, Haures J (2004) Plasticity in resource allocation based life history traits in the Pacific oyster, Crassostrea gigas. I. Spatial variation in food abundance. J Evol Biol 17:342–356
Ewart JW, Ford SE (1993) History and impact of MSX and dermo diseases on oyster stocks in the northeast region. Northeastern Regional Aquaculture Center, University of Massachusetts, Massachusetts
Filgueira R, Guyondet T, Comeau LA, Grant J (2014) A fully-spatial ecosystem-DEB model of oyster (Crassostrea virginica) carrying capacity in the Richibucto Estuary, Eastern Canada. J Mar Syst 136:42–54
Fisher WS, Chintala MM, Moline MA (1989) Annual variation of estuarine and oceanic oyster Crassostrea virginica Gmelin hemocyte capacity. J Exp Mar Biol Ecol 127:105–120
Ford SE, Haskin HH (1988) Management strategies for MSX (Haplosporidium nelsoni) disease in eastern oysters. Am Fish Soc Spec Publ 18:249–256
Ford SE, Smolowitz R (2007) Infection dynamics of an oyster parasite in its newly expanded range. Mar Biol 151:119–133
Foreman MG (1977) Manual for tidal heights analysis and previsions. Pac Mar Sci Rep 77–10:101
Hamdoun AM, Cheney DP, Cherr GN (2003) Phenotypic plasticity of HSP70 and HSP70 gene expression in the Pacific oyster (Crassostrea gigas): implications for thermal limits and induction of thermal tolerance. Biol Bull 205:160–169
Honkoop PJC (2003) Physiological costs of reproduction in the Sydney rock oyster Saccostrea glomerata. Oecologia 135:176–183
Hoverman JT, Relyea RA (2007) How flexible is phenotypic plasticity? Developmental windows for trait induction and reversal. Ecology 88:693–705
Hoverman JT, Auld JR, Relyea RA (2005) Putting prey back together again: integrating predator-induced behavior, morphology, and life history. Oecologia 144:481–491
Ingle RM, Dawson CE Jr (1952) Growth of the American oyster, Crassostrea virginica (Gmelin) in Florida waters. Bull Mar Sci 2:393–404
Kooijman SALM (2000) Dynamic energy and mass budgets in biological systems. Cambridge University Press, Cambridge
Kraeuter JN, Ford S, Cummings M (2007) Oyster growth analysis: a comparison of methods. J Shellfish Res 26:479–491
La Peyre MK, Gossman B, La Peyre JF (2009) Defining optimal freshwater flow for oyster production: effects of freshet rate and magnitude of change and duration on eastern oysters and Perkinsus marinus infection. Estuaries Coasts 32:522–534
Landry T, Hardy M, Ouellette M & MacNair NG (2001) Monitoring the life cycle of the northern quahaug, Mercenaria mercenaria, in Prince Edward Island. Canadian Technical Report Fisheries Aquatic Sciences 2355
Loosanoff VL (1953) Behavior of oysters in water of low salinities. Proc Natl Shellfish Assoc 43:135–151
Mallet AL, Carver C, Hardy M (2009) The effect of floating bag management strategies on biofouling, oyster growth and biodeposition levels. Aquaculture 287:315–323
Mallet AL, Carver CE, Doiron S, Thériault MH (2013) Growth performance of eastern oysters Crassostrea viriginica in Atlantic Canada: effect of the culture gear. Aquaculture 1:396–399
Mayrand E, Sonier T, Comeau LA (2013) Hot water immersion lowers survival, shell growth rate and lysosomal membrane stability of oysters Crassostrea virginica (Gmelin). Aquac Res. doi:10.1111/are.12364
McGladdery SE, Zurbrigg RE (2006) Canada’s approach to aquatic animal biosecurity: experience and evolution. In: Scarfe DA, Lee C-S, O’Bryen PJ (eds) Aquaculture biosecurity: prevention, control, and eradication of aquatic animal disease. Blackwell Publishing, Ames
Medcof JC, Needler AWH (1941) The influence of temperature and salinity on the condition of oysters (Ostrea virginica). J Fish Res Board Can 5:253–257
Méthé D, Léger M (1994) Assessment of oyster spat growth and collection in Bouctouche Bay, New Brunswick, Canada 1987 to 1993. New Brunswick Department of Fisheries and Aquaculture, Bouctouche
North EW, King DM, Xu J, Hood RR, Newell RIE, Paynter K, Kellogg ML, Liddel MK, Boesch DF (2010) Linking optimization and ecological models in a decision support tool for oyster restoration and management. Ecol Appl 20:851–866
Paynter KT, Burreson EM (1991) Effects of Perkinsus marinus infection in the eastern oyster, Crassostrea virginica: II. Disease development and impact on growth at different salinities. J Shellfish Res 10:425–431
Pollack JB, Kim H, Morgan EK, Montagna PA (2011) Role of flood disturbance in natural oyster (Crassostrea virginica) population maintenance in an estuary in South Texas, USA. Estuaries Coasts 34:187–197
Pouvreau S, Bourles Y, Lefebvre S, Gangnery A, Alunno-Bruscia M (2006) Application of a dynamic energy budget model to the Pacific oyster, Crassostrea gigas, reared under various environmental conditions. J Sea Res 56:156–167
Ragone LM, Burreson EM (1993) Effect of salinity on infection progression and pathogenicity of Perkinsus marinus in the eastern oyster, Crassostrea virginica (Gmelin). J Shellfish Res 12:1–7
Sanford E, Kelly MW (2011) Local adaptation in marine invertebrates. Annu Rev Mar Sci 3:509–535
Shaw WN (1966) The growth and mortality of seed oysters, Crassostrea virginica, from Broad Creek, Chesapeake Bay, Maryland, in high-and low-salinity waters. Proc Natl Shellfish Assoc 56:59–63
Shumway SE (1996) Natural environment factors. In: Kennedy VS, Newell RIE, Eble AF (eds) The eastern oyster Crassostrea virginica. Maryland Sea Grant College, College Park, pp 467–503
Turcotte-Lanteigne A, Ferguson E (2008) Ecosystem overview of the Richibucto watershed in New Brunswick. Can Manuscr Rep Fish Aquat Sci 2847:182
Vasseur L, Catto NR (2008) Canada Atlantique. In: Lemmen DS, Warren FJ, Lacroix J, Bush E (eds) Vivre avec les changements climatiques au Canada, 2007th edn. Gouvernement du Canada, Ottawa, pp 119–170
Wallace DH (1966) Oysters in the estuarine environment. Am Fish Soc Spec Publ 3:68–73
Weisberg S (2005) Applied linear regression, 3rd edn. Wiley, New Jersey
Wells HW (1961) The fauna of oyster beds, with special reference to the salinity factors. Ecol Monogr 31:239–266
Acknowledgments
This project was funded by the Aquaculture Collaborative Research and Development Program (ACRDP) in partnership with the Elsipogtog First Nation (Project No. MG–08–01–006). The authors would like to thank Rémi Sonier for his expertise in the field and Dr John Martell for his critical review of the manuscript. Special thanks as well to our industry partner, the Elsipogtog First Nation represented by Mr. Blayne Peters for much needed assistance and guidance in the field.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Méthé, D., Comeau, L.A., Stryhn, H. et al. Survival and growth performance of Crassostrea virginica along an estuarine gradient. Aquacult Int 23, 1089–1103 (2015). https://doi.org/10.1007/s10499-014-9866-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10499-014-9866-3