Abstract
Autophagy has emerged as an important process of cell metabolism. With continuous in-depth research on autophagy, TFEB has been a key transcription factor regulating autophagy levels in recent years. Studies have established that TFEB regulates autophagy and apoptosis in various diseases. However, the relationship between TFEB and the pathogenesis of endometriosis remains unclear. This study aimed to investigate the effect of TFEB on the mechanism of endometriosis progression. The results showed that TFEB and autophagy-related protein LC3 are highly expressed in ectopic endometrium of patients with endometriosis, overexpression of TFEB in cultured human endometrial stromal cells (HESCs) by lentivirus not only promoted autophagy but also inhibited apoptosis. In addition, the migration and invasion ability of HESCs were enhanced by TFEB overexpression. Furthermore, inhibiting autophagy with specific inhibitors can attenuate migration and invasion of HESCs induced by TFEB. The rat models of endometriosis show that TFEB knockdown can suppress lesion growth in vivo. Our results suggest that autophagy may be involved in the progression mechanism of endometriosis, and the mechanism of autophagy disorder in endometriosis is probably related to TFEB. TFEB may be a key molecule in promoting endometriosis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Endometriosis is a common but challenging gynecological disease with unclear pathogenesis and limited treatment options, affecting 6-10% of women of childbearing age and 20 − 50% of infertile women [1]. It is characterized by the abnormal implantation of the endometrial glands and stroma outside the uterine cavity, especially the ovaries and pelvic peritoneum. Endometriosis is a benign gynecological disease that shares similar characteristics with cancer, such as migration and invasion [2]. At present, the pathogenesis of endometriosis has been extensively studied clinically. Many theories have been put forward, among which the in situ theory includes the Müllerian remnants hypothesis and Coelomic metaplasia or the stem cell differentiation hypothesis. Other researchers put forward the transplantation theory, which includes retrograde menstruation theory and venous dissemination or metaplasia theories. These theories explain the origin of endometriosis [3]. However, the mechanism of how endometrial cells evade intraperitoneal immune surveillance to adhere, implant, proliferate, and differentiate remains unclear.
Autophagy is a conserved process of cell evolution that nutrient depletion, oxidative stress, or other harmful conditions can induce. It begins with the formation of biomembrane bodies which can wrap cytoplasmic macromolecules, aggregated proteins, damaged organelles, or pathogens and then combine with lysosomes to form autophagolysosome, in which the hydrolases can digest the contents and generate nucleotides, amino acids, fatty acids, sugars, and ATP, all of these are eventually recycled into the cytoplasm [4]. LC3 has been widely studied as an autophagosome marker, which is thought to be involved in the final link of autophagosome membrane formation, and SQSTM1(P62), a multifunctional connexin, is a selective substrate for autophagy with a short LC3-interacting region (LIR) that facilitates direct interaction with LC3 and ultimately p62 is specifically degraded by autophagy [5]. Hengwei Liu et al. found that the ectopic endometrium of patients with endometriosis was highly expressed with autophagy-associated protein LC3 [6]. Giulia Allavena confirmed that autophagy was upregulated in ovarian endometrioma by analyzing the expression of LC3 and P62, which may be related to the interaction between p53 and heme oxygenase 1 [7]. Altogether, autophagy may involve in the progression of endometriosis.
The transcription factor EB (TFEB) is the main regulator of lysosomal function and autophagy, belongs to the basic helic-loop-helic-Leucine-zipper (BHLH-ZIP) transcription factor family (MiT family) [8], as a major transcription regulator of the autophagy-lysosomal pathway, TFEB positively regulates the expression of autophagy and lysosomal biogenesis-related genes, thus promoting autophagosome formation, autophagosome-lysosome fusion, and the degradation of autophagy substrates [9, 10]. The association between TFEB and autophagy has been extensively studied in neurodegeneration [11] and renal cell carcinoma [12]. Kuiper et al. have shown that TFEB is expressed in the endometrium [13]. However, the correlation between TFEB and autophagy in endometriosis has yet to be studied. In this research, we found that upregulation of autophagy in ectopic endometrium was positively associated with TFEB, and TFEB overexpression in human endometrial stromal cells could inhibit apoptosis and promote autophagy, which further affects the migration and invasion of endometrial cells.
Materials and methods
Ethics statement
The endometriosis and normal endometrium tissue collected from humans was approved by the Ethical Committee of the Second Affiliated Hospital of Wenzhou Medical University and Yuying Children’s Hospital. The animal studies were approved by the Animal Use and Care Committee of Wenzhou Medical University and conducted following the guidelines for the Care and Use of Laboratory Animals of the National Institutes of Health.
Human tissue collection
Abdominal ectopic endometrial tissue and corresponding eutopic endometrial tissue were obtained from premenopausal women who underwent laparoscopic ovarian endometriosis cyst resection and hysteroscopy in the Second Affiliated Hospital of Wenzhou Medical University. Normal endometrial tissue was obtained from women who underwent laparoscopy and hysteroscopy for tubal infertility. All participants were between 35 and 45 years old and had not used hormone drugs 3 months before surgery. All specimens were collected in the late stage of secretion and confirmed by histopathology.
Culture of human endometrial stromal cells(HESCs)
The collected endometrial tissues were washed three times by PBS, minced into pieces with sterile scissors, and then treated with 2 mg/ml (0.1%) collagenase II for 4 h at 37 °C. Stromal cells were isolated from epithelial cells and fragments using 150 and 37.4 μm screens, respectively. After incubation overnight, stromal cells stuck to the wall, the debris was removed from the medium, and the stromal cells were washed with PBS. HESCs were cultured with DMEM/F12 containing 10% fetal bovine serum and 1% penicillin/streptomycin antibiotics and incubated in a 37 °C incubator with 5% CO2, then replaced with the complete medium every other day.
Lentivirus transfection
Lenti-TFEB, Lenti-shTFEB, and Lenti-NC from GeneChem (Shanghai, China) were used for lentivirus transfection. The cell was transfected at 30–50% confluence. About > 95% of the cells survived 24 h later and then changed the culture medium, and the cells continued incubating for a further 3 days for follow-up experiments.
Protein extraction and western blot analysis
The endometrial tissue we collected and the cultured cells were washed with PBS and lysed with a radioimmunoprecipitation analysis (RIPA) buffer (Beyotime, Shanghai, China) that contained protease inhibitors. The tissue was cut up and mixed with the lysate for grinding, or the cells were scraped into the lysate, then sat on ice for 30 min, centrifuged for 15 min with 12000rmp at 4 °C, and the supernatant was taken. Protein concentration was determined by a BCA protein detection kit (Beyotime, Shanghai, China). The same amount of protein was mixed with 5 × sample buffer and boiled at 100℃ for 10 min. The same volume of the sample was loaded into 12.5% sodium dodecyl sulfate-polyacrylamide gel electrophoresis gels (PAGE, gel thickness 1 mm) for electrophoresis and then transferred to polyvinylidene fluoride (PVDF 0.45 μm) membrane (Bio-Rad, California, USA). Next, the membrane was blocked with 5% skim milk for 2 h, rinsed 3 times with TBST, then incubated with the required primary antibody at 4 °C overnight. On the second day, the membranes were washed with TBST 3 times and incubated with secondary antibodies at room temperature for 2 h. Chemiluminescence and reagent (Invitrogen, Carlsbad, USA) were used to observe the blots. The gray of these protein blots was quantified with Image Lab 3.0 software (BioRad, California, USA).
Immunocytochemical staining
The tissues fixed with paraformaldehyde were dehydrated and embedded in paraffin, then the wax block was sliced and dewaxed with xylene and hydrated with alcohol. Next, the endogenous peroxidase was blocked with 3% hydrogen peroxide, and 0.4% pepsin was added and incubated at 37 °C for 20 min for antigen extraction. Afterward, the slices were sealed with 10% goat serum at 37 °C for 30 min. Add primary antibody against TFEB (1:1000; Wuhan, China) and LC3 (1:1000; Abcam, Cambridge, UK) and incubate overnight at 4 °C. After rewarming, the sections were washed three times with PBS and incubated with horseradish peroxidase combined secondary antibody at 37 °C. For 30 min, then stained with DAB (Beyotime, Shanghai, China) and observed under the microscope. Hematoxylin was used for nuclear staining, and the slice was counterstained in hematoxylin before dehydration and fixation. Three visual fields were selected in each group to analyze the positive cell rate.
Transwell migration and invasion assays
A Transwell 24-well plate and 8 μm diameter filter (Corning Costar, Tewksbury, MA, USA) were used for migration and invasion experiments. Unlike the migration assay, the invasion assay required the addition of 40μL of working Matrigel, diluted by FBS-free DMEM in 1:3 (Becton, Dickinson and Company, USA) to the chamber and solidification at 37 °C for at least 5 h. The following steps are the same. 20,000 cells in 200μL free medium suspended cells were inoculated into the insert, and 700 μl medium containing 20% fetal bovine serum was filled into the lower chamber. The cells were cultured at 37 °C for 24 h and evaluated for migration potential. In the invasion experiment, the invasion ability of cells was evaluated after 48 h. The cells were fixed with polymethanol for 20 min and then stained with crystal violet (1:1000 dissolved in PBS) for 10 min. The cells under the membrane were observed under an inverted microscope of × 200.
TUNEL staining
The endometrial cells were fixed with paraformaldehyde, then stained with a cell death detection kit (Meilunio, Dalian, China) at 37 °C for 30 min, following the instructions. Nuclei were stained blue with DAPI. Finally, the TUNEL-positive cells were observed under a fluorescence microscope.
Cell count kit-8
The cells were seeded in a 96-well plate at a density of 1 × 103 cells in 100ul free medium per well; 10 μl CCK-8 working fluid was added to the wells at 0, 24, 48, and 72 h, and then absorbance was measured at 450 nm by a microplate reader after 1 h incubation at 37 °C.
Animal model
6 to 8 weeks-old SD female rats were selected for adaptive feeding for 1 week, and then Estradiol valerate was an intramuscular injection to each rat every day for 3 days. The rats were anesthetized with an intraperitoneal injection of 10% chloral hydrate, making a 1.5-2 cm longitudinal incision on the hypogastrium. Bilateral uterine horns and ovaries were found, the right uterus was separated and cut, and the broken end was ligated. The tissues were cut lengthwise, washed with saline, trimmed to a size of 0.5 cm*0.5 cm, fixed on the abdominal cavity wall with abundant blood vessels by diagonal suture, and washed with saline. Finally, the abdominal cavity was closed layer by layer. After surgery, the rats were divided into estrogen, estrogen + LV-NC, and estrogen + LV-shTFEB group. Intra-foci injection of 10μL Lentivirus was performed at 0,7, 14 days post endometriosis surgery. Estrogen + LV-NC groups accepted 10 μL LV- NC while the estrogen + LV-shTFEB group was injected with LV-shTFEB. All rats accepted 0.1 ml estrogen by intramuscular injection every other day and were sacrificed 3 weeks after surgery.
Results
The TFEB and LC3 protein levels increased in ectopic and eutopic endometrium in human and rat models
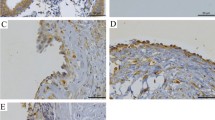
To ascertain the relationship between autophagy and TFEB in endometriosis, Western blot was used to detect TFEB protein expression in 3 pairs of fresh ectopic endometrium, eutopic endometrium, and normal endometrium. The results showed that the expression levels of TFEB and autophagy-associated protein LC3 in ectopic and eutopic endometrium were higher than in normal endometrial tissues (Fig. 1B and C) and were more obvious in ectopic lesions, while the expression of p62 was opposite. Moreover, the expression of TFEB and autophagy marker LC3 showed a similar trend by immunohistochemical staining. The same results were observed in the endometriosis of the rat model (Fig. 2A, B, and C). This result shows that autophagy is upregulated in endometriosis, and TFEB may be an important molecule regulating autophagy.
The TFEB level in ectopic and eutopic endometrium of patients with endometriosis and normal endometrium; (A) Representative immunohistochemical images of TFEB and LC3 protein localization in normal endometrium, eutopic endometrium, and ectopic endometrium of human; (B, C) The proteins expression of TFEB, Lc3 and p62 verified by representative Western blots in normal endometrium, eutopic endometrium and ectopic endometrium of human. The data are expressed as the means ± s.d (n = 3) (*P < 0.05;**P < 0.01;***P < 0.001)
The TFEB level in normal endometrium, eutopic endometrium, and ectopic endometrium of rat model; (A) Representative immunohistochemical images of TFEB and LC3 protein localization in normal endometrium, eutopic endometrium, and ectopic endometrium of rat models; (B, C) The proteins expression of TFEB, Lc3 and p62 verified by representative Western blots in normal endometrium, eutopic endometrium and ectopic endometrium of a rat model. The data are expressed as the means ± s.d(n = 3) (*P < 0.05;**P < 0.01;***P < 0.001)
TFEB overexpression induces autophagy in endometrial cells
To further explore the role of TFEB in autophagy in endometriosis, we transfected endometrial cells with TFEB overexpression lentivirus in vitro. Compared with the LV-NC group and control group, increased TFEB and LC3 were observed in the LV-TFEB group, while the expression of P62 was the opposite (Fig. 3A and B). Migration and invasion experiments were carried out to investigate the effect of TFEB on cell viability. As shown in Fig. 3C, overexpression of TFEB enhanced the migration and invasion ability of endometrial cells. In conclusion, these results suggest that TFEB can induce autophagy and promote migration and invasion in human endometrial cells.
Overexpression of TFEB induces autophagy. (A, B) The representative Western blot results of TFEB, p62, and LC3 proteins in HECs transfected with negative Control (NC) plasmid and TFEB overexpression plasmid; (C, D) Representative photos of HEC migration and invasion transfected with negative Control (NC) plasmid and TFEB overexpression plasmid. The number of cells migrating and invading the inferior cavity was quantitatively evaluated
TFEB overexpression inhibits endometrial cell apoptosis
Autophagy can alleviate cell death by selectively inhibiting Pro-apoptotic proteins in the cytoplasm [14]. Consistent with this conclusion, we found that the expression of anti-apoptotic protein Bcl-2 was increased in the LV-TFEB group compared with the control and LV-NC group, while the pro-apoptotic protein Bax was decreased (Fig. 4A and B); Furthermore, CCK8 assay showed the viability of endometrial cell increased after TFEB transaction (Fig. 4C). The role of TFEB in regulating apoptosis was further confirmed by Tunel staining, all of these results indicate that TFEB transfection inhibited the apoptosis of endometrial cells.
TFEB overexpression inhibits apoptosis in HESCs; (A, B) The representative Western blot results of Bcl-2 and Bax proteins in HESCs transfected with negative Control (NC) plasmid and TFEB overexpression plasmid are as above. (C) CCK-8 was used to detect cell vitality in HESCs transfected with negative Control (NC) plasmid and TFEB overexpression plasmid. (D, E) TUNEL staining assay was performed to detect the level of cell apoptosis
TFEB-induced autophagy, migration, and invasion of endometrial cells are attenuated by chloroquine
Chloroquine is a lysosomal cavity alkalizer that can inhibit autophagy by blocking autophagosome-lysosomal fusion. The expression of autophagy-related protein LC3II is regulated by autophagosome formation and lysosomal degradation. Studies have shown that TFEB not only promotes the synthesis of autophagosomes but also promotes the biogenesis and function of lysosomes, thus accelerating the degradation of autophagosomes [15]. Both increased autophagosome formation and decreased autophagosome degradation can induce LC3 protein expression elevated [16], so we evaluated autophagy flux with chloroquine, as shown in Fig. 5A and B. The accumulation of LC3II and P62 protein increased with chloroquine blocking of autophagosome-lysosomal fusion in Control + CQ compared with the control group, this effect was enhanced in Lc3 when TFEB overexpressed, but decreased in P62; Furthermore, the ability of migration and invasion of endometrial cells weakened by CQ could be reversed by TFEB overexpression partly(Fig. 5C and D). The above results showed that TFEB-induced autophagy-related protein expression was not achieved by blocking autophagy flow but by promoting the formation of autophagosomes.
TFEB-induced autophagy, migration, and invasion of endometrial cells are attenuated by Chloroquine; (A, B) The protein expression of LC3II and P62 in HESCs as treated above; (C) Representative photographs of migration and invasion in HESCs as treated above. (D) The number of cells migrating and invading the inferior cavity was quantitatively evaluated
The protective effect of TFEB-induced in endometrial cells under oxidative stress was reversed by CQ
Studies have shown that overexpression of TFEB can promote autophagy flux and reduce apoptosis induced by oxidative stress, while autophagy inhibitors reverse the protective effect of TFEB [17]. Our study confirmed the protective effect of TFEB overexpression on TBHP-induced apoptosis through Western blot and TUNEL. The anti-apoptotic protein Bcl-2 was higher in the LV-TFEB group than in the control group, while the pro-apoptotic protein Bax was the opposite. Endometrial cells were then co-cultured with CQ, and results showed that the expression of Bcl-2 in the LV-TFEB + CQ group was significantly lower than that of LV-TFEB. (Fig. 6A and B), consistent with the Western blot results, the percentage of TUNEL-positive cells in the LV-TFEB + CQ group was higher than in the LV-TFEB group (Fig. 6C), suggesting that the patency of autophagy flux is essential for TFEB-mediated inhibition of apoptosis.
The effect of TFEB on endometriosis lesion growth in vivo animal experiment
To investigate the potential role of TFEB in vivo, we injected LV-shTFEB around the endometriosis lesion in rats (Fig. 7). The volumes of endometriosis lesions in the LV-shTFEB + estrogen group were significantly smaller than estrogen and estrogen + LV-NC groups at one month after surgery. In addition, we applied a Western blot of LC3-II to detect the TFEB-induced autophagy in vivo. The lentivirus-mediated TFEB knockdown could decrease the LC3-II accumulation in the ectopic endometrium of rat models. These data implied that TFEB is a potential target to promote the progression of endometriosis.
Discussion
Endometriosis is closely related to hormones, inflammation, oxidative stress, and environmental factors [18]. Ectopic endometrium is histologically and functionally similar to eutopic endometrium, with periodic bleeding in response to hormonal changes. The blood in cysts has a large amount of free or non-protein-bound iron, which can produce excess ROS through autoxidation or the Fenton reaction [19]. To re-establish homeostasis and resistance to stress under stressful conditions, cells must initiate a series of intracellular signaling pathways. Autophagy plays an important role in maintaining the stability of the intracellular environment, which can be induced by oxidative stress. Many pathological conditions, such as cancer [20] and neurodegenerative diseases [21], are associated with the dysfunction of this process.
TFEB has been identified as a master regulator of autophagic flux via inducing lysosome biogenesis and promoting autophagosome formation and its fusion with the lysosome. It has been found that TFEB is activated under oxidative stress induced by tert-butyl peroxide or hydrogen peroxide in nematodes [22, 23]. The activity and subcellular localization of TFEB are related to the phosphorylation level under conditions of amino acid satiety, Rag GTPases-Ragulator complex recruited TFEB to lysosomal membranes, and mammalian target of rapamycin complex 1 (mTORC1) phosphorylates TFEB at serine 211, the Phosphorylated TFEB is sequestered by chaperones of the 14-3-3 family, which prevent its translocation to the nucleus [24]. While under starvation conditions, inactivation of mTORC1 allows nuclear translocation of TFEB to mediate cellular adaptation to stress. Fang et al. also confirmed that arsenic trioxide could promote ROS production, induce TFEB nuclear shift and inhibit PI3K/AKT/mTOR pathway. These effects will promote macrophage autophagy and reduce atherosclerotic lesions [25]. Allavena et al. found that the LC3-II and LC3-II/LC3-I ratios in ovarian endometrial cysts were significantly higher than in normal endometrial, while p62 was significantly lower in ovarian endometrial cysts than that in normal endometrial [7]. Ying Ding et al.‘s study shows that Beclin-1 expression of ovarian granule cells was increased in patients with ovarian endometrioma [26]. All of these confirmed that autophagy is elevated in endometriosis lesions. However, whether TFEB is involved in regulating autophagy in endometriosis remains unclear.
Similar to the conclusions of the above researchers, the results of Western blots and immunohistochemical staining in our research showed that TFEB and LC3 expression were elevated both in human and rats’ ectopic endometriosis, while p62 was decreased, which means autophagy level is upregulation in endometriosis and TFEB may be related to the regulation of autophagy; To clarify further whether TFEB plays a role in endometrial autophagy, we transfected endometrial cells with lentivirus overexpressed TFEB in vitro, the results showed that TFEB overexpression significantly promoted the expression of the autophagy-related protein in endometrial cells. Lingling Zhu et al. also found in acute kidney injury that overexpression of TFEB activates transcription of autophagy and lysosomal-related genes, including Becn1, CtsB, Lamp, and Lc3b [27]. These results suggest that the activation of autophagy in endometrial cells is associated with TFEB. However, the expression level of autophagy in endometriosis has been controversial. Choi et al. have confirmed that aberrant mTOR activation in ovarian endometrioma cysts leads to the downregulation of autophagy in ectopic endometrium [28], contrary to our results. These contrasting results can be interpreted as a complex signaling network involved in the regulation of autophagy. As a matter of fact, in addition to the canonical PI3K-AKT-mTOR signaling, autophagy and TFEB can also be activated through atypical signaling pathways. Recent studies suggest that ROS can directly activate lysosomal MCOLN1 to induce lysosomal Ca2 + release and establish a Ca2 + microdomain near the lysosome, leading to calcineurin activation, which induces TFEB dephosphorylation, separated with 14-3-3 proteins, then translocated into the nucleus, and finally induced autophagy [29]. In addition, Wang et al. also showed that ROS could directly oxidize the C212 site of TFEB without inhibiting the activity of MTORC1, directly promoting the rapid nuclear translocation of TFEB [30]. These studies suggest that elevated TFEB and autophagy activity may result from the activation of other atypical signaling pathways, which requires further experimental verification.
. Autophagy and apoptosis are the main factors determining cell fate. More and more evidence shows that apoptosis and autophagy have antagonistic effects. Autophagy helps human cancer cells survive through apoptotic resistance, and inhibition of autophagy leads to caspase-dependent apoptotic cell death [31]. In the t(6;11) translocation renal cell carcinomas, researchers found that TFEB could promote anti-apoptosis protein BCL-2 expression by upregulating its promoter activity [32]. The same results were found in our study. Overexpression of TFEB induced autophagy activation of endometrial cells and inhibited apoptosis. To some extent, this can explain why endometrium implanted outside the uterine cavity can survive and lead to the progression of endometriosis. Although a benign lesion, it can infiltrate and invade distant tissues, similar to a malignant tumor. Migration and invasion experiments were performed to study the effect of TFEB on its malignant behavior. We showed that overexpressing TFEB in endometrial cells showed obvious migration and invasion ability. Autophagy involves the structural changes of the subcellular membrane, the formation and fusion of autophagosomes with lysosomes, and finally, the degradation of substances and the release of energy by acidic hydrolases. This process is called “autophagy flux“ [33]. CQ is a lysosomal cavity Alkalizer, which can inhibit the downstream autophagy flux. In our study, we found that the accumulation of LC3II protein in endometrial cells treated by chloroquine combined with overexpressed TFEB is more than that in other groups, indicating that the increase of LC3II induced by TFEB is the result of autophagy activation rather than lysosomal degradation blockage. Furthermore, we found that TFEB can rescue cell apoptosis and reverse the decline in migration and invasion abilities to a certain extent, which is induced by CQ, indicating that the patency of autophagic flux is equally important for the survival and behavioral effects of ectopic endometrial cells.
In conclusion, we first testify that the expression of TFEB is increased in human endometriosis and rat endometriosis. Overexpression of TFEB enhances autophagy, inhibits apoptosis, and promotes endometrial cell migration and invasion. In addition, our in vivo studies have shown that TFEB knockdown can inhibit the progression of endometriosis, providing basic evidence that TFEB may be an important molecule promoting endometriosis.
Limitations
This study has several limitations: (a) The sample size of the study was relatively small; (b) The expression of autophagy in different stages of the menstrual cycle and endometriosis in other parts has not been studied; (c) Previous studies have found that estrogen also plays an important role in autophagy. In our animal experiments, estrogen was used for modeling, but the influence of estrogen itself on autophagy was not further analyzed; Therefore, future research is needed to gain a deeper insight and more comprehensive exploring of these issues.
Data availability
The data that support the findings of this study are available on resquest from the corresponding author.
Abbreviations
- CQ:
-
Chloroquine
- HESCs:
-
Human endometrial stromal cells
- mTORC1:
-
Mammalian target of rapamycin complex 1
- TFEB:
-
Transcription factor EB
References
Liu Y, Wang J, Zhang X (2022) An update on the multifaceted role of NF-kappaB in endometriosis. Int J Biol Sci 18(11):4400–4413 Epub 2022/07/23. https://doi.org/10.7150/ijbs.72707
Kvaskoff M, Mu F, Terry KL, Harris HR, Poole EM, Farland L et al (2015) Endometriosis: a high-risk population for major chronic diseases? Hum Reprod Update 21(4):500–516 Epub 2015/03/15. https://doi.org/10.1093/humupd/dmv013
Lagana AS, Garzon S, Gotte M, Vigano P, Franchi M, Ghezzi F et al (2019) The pathogenesis of endometriosis: molecular and cell biology insights. Int J Mol Sci 20(22). Epub 2019/11/14. https://doi.org/10.3390/ijms20225615. PubMed PMID: 31717614; PubMed Central PMCID: PMCPMC6888544
Li X, He S, Ma B (2020) Autophagy and autophagy-related proteins in cancer. Mol Cancer 19(1):12 Epub 2020/01/24. https://doi.org/10.1186/s12943-020-1138-4
Shibutani ST, Saitoh T, Nowag H, Munz C, Yoshimori T (2015) Autophagy and autophagy-related proteins in the immune system. Nat Immunol 16(10):1014–1024 Epub 2015/09/19. https://doi.org/10.1038/ni.3273
Liu H, Zhang Z, Xiong W, Zhang L, Xiong Y, Li N et al (2017) Hypoxia-inducible factor-1alpha promotes endometrial stromal cells migration and invasion by upregulating autophagy in endometriosis. Reproduction 153(6):809–820 Epub 2017/03/30. https://doi.org/10.1530/REP-16-0643
Allavena G, Carrarelli P, Del Bello B, Luisi S, Petraglia F, Maellaro E (2015) Autophagy is upregulated in ovarian endometriosis: a possible interplay with p53 and heme oxygenase-1. Fertil Steril 103(5):1244–1251 .007. PubMed PMID: 25772769
Settembre C, Di Malta C, Polito VA, Garcia Arencibia M, Vetrini F, Erdin S et al (2011) TFEB links autophagy to lysosomal biogenesis. Science 332(6036):1429–1433 Epub 2011/05/28. https://doi.org/10.1126/science.1204592
Napolitano G, Ballabio A (2016) TFEB at a glance. J Cell Sci 129(13):2475–2481 Epub 2016/06/03. 10.1242
Chen M, Dai Y, Liu S, Fan Y, Ding Z, Li D (2021) TFEB biology and agonists at a glance. Cells 10(2). https://doi.org/10.3390/cells10020333PubMed PMID: 33562649; PubMed Central PMCID: PMCPMC7914707 Epub 2021/02/11
Cortes CJ, La Spada AR (2019) TFEB dysregulation as a driver of autophagy dysfunction in neurodegenerative disease: molecular mechanisms, cellular processes, and emerging therapeutic opportunities. Neurobiol Dis 122:83–93 Epub 2018/06/01. https://doi.org/10.1016/j.nbd.2018.05.012
Calio A, Brunelli M, Segala D, Pedron S, Remo A, Ammendola S et al (2020) Comprehensive analysis of 34 MiT family translocation renal cell carcinomas and review of the literature: investigating prognostic markers and therapy targets. Pathology 52(3):297–309 PubMed PMID: 32107074
Kuiper RP, Schepens M, Thijssen J, Schoenmakers EF, van Kessel AG (2004) Regulation of the MiTF/TFE bHLH-LZ transcription factors through restricted spatial expression and alternative splicing of functional domains. Nucleic Acids Res 32(8):2315–2322 Epub 2004/05/01. https://doi.org/10.1093/nar/gkh571
Marino G, Niso-Santano M, Baehrecke EH, Kroemer G (2014) Self-consumption: the interplay of autophagy and apoptosis. Nat Rev Mol Cell Biol 15(2):81–94 Epub 2014/01/10. https://doi.org/10.1038/nrm3735
Zheng G, Pan Z, Zhan Y, Tang Q, Zheng F, Zhou Y et al (2019) TFEB protects nucleus pulposus cells against apoptosis and senescence via restoring autophagic flux. Osteoarthritis Cartilage 27(2):347–357 Epub 2018/11/12. https://doi.org/10.1016/j.joca.2018.10.011
Mizushima N, Yoshimori T (2007) How to interpret LC3 immunoblotting. Autophagy 3(6):542–5. Epub 2007/07/06. https://doi.org/10.4161/auto.4600. PubMed PMID: 17611390
Sun X, Chang R, Tang Y, Luo S, Jiang C, Jia H et al (2021) Transcription factor EB (TFEB)-mediated autophagy protects bovine mammary epithelial cells against H(2)O(2)-induced oxidative damage in vitro. J Anim Sci Biotechnol 12(1):35. https://doi.org/10.1186/s40104-021-00561-7PubMed PMID: 33685494; PubMed Central PMCID: PMCPMC7941962 Epub 2021/03/10
Vercellini P, Vigano P, Somigliana E, Fedele L (2014) Endometriosis: pathogenesis and treatment. Nat Rev Endocrinol 10(5):261–275 Epub 2013/12/25. https://doi.org/10.1038/nrendo.2013.255
Iwabuchi T, Yoshimoto C, Shigetomi H, Kobayashi H (2015) Oxidative stress and antioxidant defense in endometriosis and its malignant transformation. Oxid Med Cell Longev 2015:848595. Epub 2015/07/18 https://doi.org/10.1155/2015/848595
White E (2015) The role for autophagy in cancer. J Clin Invest 125(1):42–46 Epub 2015/02/06. doi: 10.1172/JCI73941. PubMed PMID: 25654549; PubMed Central PMCID: PMCPMC4382247
Stamatakou E, Wrobel L, Hill SM, Puri C, Son SM, Fujimaki M et al (2020) Erratum: author correction: mendelian neurodegenerative disease genes involved in autophagy. Cell Discov 6:40 Epub 2020/06/23. https://doi.org/10.1038/s41421-020-0185-8
Lin XX, Sen I, Janssens GE, Zhou X, Fonslow BR, Edgar D et al (2018) DAF-16/FOXO and HLH-30/TFEB function as combinatorial transcription factors to promote stress resistance and longevity. Nat Commun 9(1):4400. https://doi.org/10.1038/s41467-018-06624-0PubMed PMID: 30353013; PubMed Central PMCID: PMCPMC6199276 Epub 2018/10/26
Ma S, Fang Z, Luo W, Yang Y, Wang C, Zhang Q et al (2016) The C-ETS2-TFEB axis promotes neuron survival under oxidative stress by regulating lysosome activity. Oxid Med Cell Longev 2016:4693703. Epub 2016/05/20 https://doi.org/10.1155/2016/4693703
Zhitomirsky B, Yunaev A, Kreiserman R, Kaplan A, Stark M, Assaraf YG (2018) Lysosomotropic drugs activate TFEB via lysosomal membrane fluidization and consequent inhibition of mTORC1 activity. Cell Death Dis 9(12):1191 Epub 2018/12/14. https://doi.org/10.1038/s41419-018-1227-0
Fang S, Wan X, Zou X, Sun S, Hao X, Liang C et al (2021) Arsenic trioxide induces macrophage autophagy and atheroprotection by regulating ROS-dependent TFEB nuclear translocation and AKT/mTOR pathway. Cell Death Dis 12(1):88 Epub 2021/01/20. https://doi.org/10.1038/s41419-020-03357-1
Ding Y, Zhu Q, He Y, Lu Y, Wang Y, Qi J et al (2021) Induction of autophagy by beclin-1 in granulosa cells contributes to follicular progesterone elevation in ovarian endometriosis. Transl Res 227:15–29 Epub 2020/07/09. https://doi.org/10.1016/j.trsl.2020.06.013
Zhu L, Yuan Y, Yuan L, Li L, Liu F, Liu J et al (2020) Activation of TFEB-mediated autophagy by trehalose attenuates mitochondrial dysfunction in cisplatin-induced acute kidney injury. Theranostics 10(13):5829–5844 Epub 2020/06/03. https://doi.org/10.7150/thno.44051
Choi J, Jo M, Lee E, Kim HJ, Choi D (2014) Differential induction of autophagy by mTOR is associated with abnormal apoptosis in ovarian endometriotic cysts. Mol Hum Reprod 20(4):309–317 Epub 2013/12/10. https://doi.org/10.1093/molehr/gat091
Zhang X, Cheng X, Yu L, Yang J, Calvo R, Patnaik S et al (2016) MCOLN1 is a ROS sensor in lysosomes that regulates autophagy. Nat Commun 7:12109 Epub 2016/07/01. https://doi.org/10.1038/ncomms12109
Wang H, Wang N, Xu D, Ma Q, Chen Y, Xu S et al (2020) Oxidation of multiple MiT/TFE transcription factors links oxidative stress to transcriptional control of autophagy and lysosome biogenesis. Autophagy 16(9):1683–1696 PubMed PMID: 31826695; PubMed Central PMCID: PMCPMC8386635
Chen Y, Henson ES, Xiao W, Huang D, McMillan-Ward EM, Israels SJ et al (2016) Tyrosine kinase receptor EGFR regulates the switch in cancer cells between cell survival and cell death induced by autophagy in hypoxia. Autophagy 12(6):1029–1046 PubMed PMID: 27166522; PubMed Central PMCID: PMCPMC4922445
Zhan HQ, Qin R, Li YL, Liu MM, Gan L (2021) TFEB promotes BCL-2 expression by upregulating its promoter activity in the t(6;11) translocation renal cell carcinomas. Am J Transl Res 13(8):8804–8818 Epub 2021/09/21. PubMed PMID: 34539996; PubMed Central PMCID: PMCPMC8430107
Xie C, Shi Y, Chen Z, Zhou X, Luo P, Hong C et al (2021) Apigenin alleviates intervertebral disc degeneration via restoring autophagy flux in nucleus pulposus cells. Front Cell Dev Biol 9:787278 Epub 2022/02/01. https://doi.org/10.3389/fcell.2021.787278
Acknowledgements
The authors thank the staff of the Second Affiliated Hospital of Wenzhou Medical.
Funding
This study was financially supported by the Natural Science Foundation of Zhejiang Province [grant No. LY20H040005]. The Public Welfare Science and Technology Project of Wenzhou City [grant No. Y20210021]. The Medical Science and Technology Project of Zhejiang Province [grant No.2023KY145]. The Natural Science Foundation of Zhejiang Province [grant No. LY23H040003]. The Medical Science and Technology Project of Zhejiang Province [grant No. 2022KY212]. The Zhejiang Province Traditional Chinese Medicine Science and Technology Project [grantNo.2023ZL510]. The Public Welfare Science and Technology Project of Wenzhou City [grant No. Y2020087].
Author information
Authors and Affiliations
Contributions
Zhao Yu and Qiong Zhang designed the experiments and revised manuscript; Chen Qiuyu and Sennan Zhu conducted experiments and wrote the main manuscript; Yu Meng Qi and Zhou Yi analysed data; Sun Jindan and Du Wenzhuo prepared Figs. 1 and 2; Chen Ziqi, Tao Jiayu and Feng Xiao prepared 3, 4, 5, 6 and 7.
Corresponding authors
Ethics declarations
Statements and declarations
The research results reported in this article are all original research results of myself or all members of our research group.
Competing interests
The authors declare no competing interests.
Ethical approval
This study was approved by the Ethics Committee of Wenzhou Medical University Affiliated Second Hospital and Yuying Children’s Hospital. (Ethical code LCKY209-132)
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Chen, Q., Zhou, Y., Yu, M. et al. Transcription factor EB-mediated autophagy affects cell migration and inhibits apoptosis to promote endometriosis. Apoptosis 29, 757–767 (2024). https://doi.org/10.1007/s10495-024-01939-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-024-01939-4