Abstract
ARTS (Sept4_i2) is a pro-apoptotic protein and a product of the Sept4 gene. ARTS acts upstream of mitochondria to initiate caspase activation. ARTS induces apoptosis by specifically binding XIAP and allowing de-repression of active caspases required for Mitochondrial Outer Membrane Permeabilzation (MOMP). Moreover, ARTS promotes apoptosis by inducing ubiquitin-mediated degradation of both major anti-apoptotic proteins XIAP and Bcl-2. In the resolution phase of inflammation, the infiltrating leukocytes, which execute the acute innate response, undergo apoptosis and are subsequently cleared by phagocytic macrophages (i.e. efferocytosis). In this course, macrophages undergo reprogramming from inflammatory, to anti-inflammatory, and eventually to resolving macrophages that leave the injury sites. Since engulfment of apoptotic leukocytes is a key signaling step in macrophage reprogramming and resolution of inflammation, we hypothesized that a failed apoptosis in leukocytes in vivo would result in an impaired resolution process. To test this hypothesis, we utilized the Sept4/ARTS−/− mice, which exhibit resistance to apoptosis in many cell types. During zymosan A-induced peritonitis, Sept4/ARTS−/− mice exhibited impaired resolution of inflammation, characterized by reduced neutrophil apoptosis, macrophage efferocytosis and expression of pro-resolving mediators. This was associated with increased pro-inflammatory cytokines and reduced anti-inflammatory cytokines, secreted by resolution-phase macrophages. Moreover, ARTS overexpression in leukocytes in vitro promoted an anti-inflammatory behavior. Overall, our results suggest that ARTS is a key master-regulator necessary for neutrophil apoptosis, macrophage efferocytosis and reprogramming to the pro-resolving phenotype during the resolution of inflammation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptosis or programmed cell death is an essential process for normal development and tissue homeostasis. Abnormal regulation of this process is associated with a wide variety of human diseases, including immunological and developmental disorders, neurodegeneration and cancer [1,2,3]. Apoptosis can be initiated by both extrinsic and intrinsic signals centered in and from the mitochondria [4, 5]. Both pathways are executed by activating caspases (cysteine-aspartic proteases) through cleavage from their inactive zymogens [6,7,8]. Apoptotic caspases are organized into “initiator caspases” (caspase-2, -8, -9, and -10) and effector caspases (caspase-3, -7, and -6) [8,9,10]. Caspases 8 and 10 are cleaved primarily in response to extrinsic signals, while caspase-9 is activated in the intrinsic mitochondrial pathway. These enzymes act in a cascade that culminates in cleavage of multiple cellular proteins, resulting in the death of the cell [7]. In living cells, caspases are kept in check by inhibitors of apoptosis (IAP) proteins [11, 12]. The best studied IAP is XIAP, which is the only IAP that can directly binds and inhibit caspases [13,14,15]. Notably, XIAP contains a RING domain responsible for E3-ligase activity [16,17,18,19]. IAPs are negatively regulated by IAP-antagonist proteins, such as Smac (second mitochondrial-derived activator of caspases/Diablo,OMI/HTRA2, XAF1 and ARTS (Apoptosis Related protein in the TGF-β Signaling pathway) [20,21,22,23,24,25,26,27]. ARTS (Sept4_i2) (Apoptosis-Related protein in the TGFβ Signaling pathway) is a splice variant derived from the Sept4 (Septin 4) gene, and the only splice variant that functions as a pro-apoptotic protein [28]. Although ARTS was originally discovered in cells induced for apoptosis by TGF-β [24], it was later found to act downstream of all apoptosis stimuli tested, such as treatment with STS (Staurosporine), Etoposide, Arabinoside (Ara-c), Nocadosole, UV radiation, TNF-α, etc [24, 27, 29,30,31]. ARTS acts upstream of mitochondria to initiate caspase activation [29, 32, 33]. ARTS is localized at the outer membrane of the mitochondria [29]. Upon apoptotic stimuli, ARTS rapidly translocates to the cytosol in a caspase-independent manner and antagonizes XIAP [27, 29]. ARTS binds directly to the XIAP/BIR3 domain and promotes the auto-ubiquitylation and degradation of XIAP, in addition to serving as an adaptor bringing the E3-ligase Siah to stimulate the degradation of XIAP [27, 29, 34]. Moreover, ARTS acts as a scaffold by bringing XIAP with its E3-ligase activity, into close proximity with Bcl-2, promoting UPS-mediated-degradation of Bcl-2 [35]. Thus, ARTS functions as a dual antagonist of both XIAP and Bcl-2 to initiate Mitochondrial outer membrane permeabilization (MOMP) and apoptosis. Furthermore, the translocation of ARTS from the MOM to the cytosol precedes MOMP and the release of Cyto c and Smac, and is required for it [29, 35]. Human and mice studies have shown that ARTS functions as a potent tumor suppressor protein. ARTS expression is lost in more than 70% of Acute Lymphoblastic Leukemia (ALL) patients [31], in 50% of lymphoma patients [36], and in a significant fraction of hepatocellular carcinoma (HCC) patients (S. Larisch and H. Steller, unpublished data). Studies using Sept4/ARTSnull mice showed that ARTS is a physiological antagonist of XIAP in vivo. In particular, Sept4/ARTSnull mice have increased numbers of hematopoietic stem and progenitor cells (HSPCs) which are resistant to apoptosis [36]. Whole-body deletion of Sept4/ARTS equipts the Intestinal stem cells (ISCs) niche with increased resistance against apoptosis [37]. In addition, Sept4/ARTS deficient mice have elevated numbers of hair follicle stem cells (HFSCs) that are protected against apoptosis and display marked improvement in wound healing and regeneration of hair follicles [38]. These mice exhibit spontaneous accelerated tumor development and elevated XIAP levels [36,37,38,39]. This suggests that the pro-apoptotic function of ARTS as an XIAP antagonist and its function in stem cells may serve to inhibit the emergence of cancer [39]. Moreover, the resistance of Sept4/ARTSnull HSPCs to apoptosis and the cell-autonomous lymphoproliferation is suppressed by the loss of XIAP function in Sept4/ARTS/XIAP double-knockout mice [36]. These results demonstrate the important physiological role of ARTS in regulating apoptosis and tumor suppressor in vivo through its role as a specific XIAP-antagonist [33].
The active termination of an inflammatory process is termed the resolution of inflammation; distinct molecular and cellular events are triggered to ensure the eventual complete resolution that is essential to restore tissue homeostasis [40, 41]. To achieve that homeostasis, polymorphonuclear cells (PMNs), typically short-lived neutrophils that infiltrate sites of inflammation to fight pathogens, must be eliminated. Thus, apoptosis of PMNs and the subsequent clearance by macrophages plays a major role in initiating the resolution of the acute inflammatory response [42]. Removal of these cells occurs rapidly and without induction of a pro-inflammatory response. The engulfment of apoptotic cells, termed efferocytosis, drives the reprogramming of the engulfing macrophages to anti-inflammatory/reparative macrophages, which secrete lower levels of pro-inflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-α (TNF α), and higher levels of anti-inflammatory cytokines, such as transforming growth factor-β (TGF β), and in some cases IL-10 [42,43,44]. This reprogramming is also known as type I (M1, pro-inflammatory) to Type II (M2c, anti-inflammatory/deactivated) macrophage conversion. Reprogramming to M2 macrophages inhibits the expression of cyclooxygenase-2 (COX2), matrix metalloproteinase-9 (MMP-9) and inducible nitric oxide synthase (iNOS), which are key enzymes in some inflammatory cytotoxic response [42,43,44]. Concomitantly, M2 macrophages stimulate the expression of arginase-1, which competes with iNOS for the same substrates; reactive NO production is prevented thereof [45, 46]. Recent reports further characterized another step in macrophage reprogramming – the conversion to pro-resolving macrophages with unique properties, such as the ability to release immunomodulatory lipid mediators [40, 41, 43, 47]. As compared to peritoneal M1 and M2 macrophages, pro-resolving macrophages are identified by a lower surface expression of CD11b and F4/80, reduced expression of the respective M1 and M2 markers iNOS and arginase 1, and increased expression of the enzyme implicated in the synthesis of specialized pro-resolving lipid mediators, 12/15-lipoxygenase (LO) [40, 48, 49]. Eosinophils also take part in the resolution of inflammation by the production of similar “stop signal” mediators [50, 51]. The resolution sequel ends with the departure of the pro-resolving macrophages through the lymphatic system [40, 43, 48]. A failure to complete resolution of acute inflammation may result in chronic inflammation, impaired wound healing that culminates in debilitating tissue fibrosis and organ dysfunction [40, 52,53,54].
Apoptotic PMNs are key effectors in the resolution of inflammation, through their immunomodulatory properties on ingesting macrophages [55]. These anti-inflammatory mechanisms include inhibition of phagocytic activity in macrophages [40] and release of sphingosine-1-phosphate (S1P) that is capable of suppressing inflammatory macrophages [56, 57]. Another efficient mechanism is the sequestration of chemokines that normally attract inflammatory leukocytes to the inflamed efferent sites, thereby reducing their bioavailability. This is pursued by expression of certain chemokine receptors upon apoptosis—CCR5 and the atypical decoy receptor D6/ACKR2 [58, 59]. D6/ACKR2 exerts different signaling from classical chemokine receptors and hence continuously recycles to the cell surface in a ligand-independent manner [60]. Both receptors are unable to elicit a chemotactic activity in apoptotic leukocytes [58,59,60,61], albeit we have shown recently that D6-bound CCL5 on these cells is able to promote the reprogramming of resolution phase macrophages [62]. Taken together, mechanisms triggered by both macrophages and the phagocytosed apoptotic corpses allow a self-limiting and short-lived inflammatory response. In this study, we hypothesized that apoptosis-resistant PMNs would possibly impair resolution of inflammation in vivo. For that purpose, we utilized the Sept4/ARTS−/− mice, which were shown to exhibit reduced response / resistance to apoptotic stimuli. Here, we report that the Sept4/ARTS−/− mice exhibit impaired resolution of inflammation, which is characterized by reduced apoptosis in PMN, and consequently reduced efferocytosis and hampered macrophage reprogramming to pro-resolving phenotypes with anti-inflammatory cytokine production.
Materials and methods
Mice
Sept4/ARTS−/− mice were generously provided by Prof. Steller, Rockefeller University, New York city. Interbreeding with C57BL/6 mice gave rise to ARTS+/+ (WT), ARTS+/− and ARTS−/− offspring (6–8-week-old; protocol approved by the Committee of Ethics, University of Haifa).
Isolation of resident macrophages
To isolate bone marrow (BM) macrophages, abdomen and hind legs of Sept4/ARTS+/+ or Sept4/ARTS−/− mice were sterilized with 70% ethanol. Pelvic and femoral bones were dissected and excess muscle from legs was removed. Each bone end was cut off and BM was flushed with 1 ml of PBS until bone cavity appeared white. Then, cells were centrifuged at 1200 rpm for 7 min, and the supernatant was discarded. Cell pellet was resuspended in 1% BSA in PBS and macrophages thereof were isolated using EasySep PE selection magnetic beads directed against F4/80, following the manufacturer’s instructions (StemCell Technologies). To isolate spleen macrophages, spleens were dissected from the abdominal cavity and meshed against a plastic greed in 1 ml of PBS. The recovered specimen were filtered through 0.45 µm sterile filter, after which the collected cells were centrifuged at 1200 rpm for 7 min. The supernatant was discarded and pellet was treated with 1 ml of RBC lysis buffer for 2 min on ice. Pellet was washed with 10 ml of 1X PBS and then macrophages were isolated as above.
Zymosan A peritonitis
Age-matched WT, Sept4/ARTS+/− or Sept4/ARTS−/− mice were injected intra-peritoneally with zymosan A (Sigma-Aldrich, 1 mg/ml in sterile PBS, 1 ml/mouse). After 24 h or 66 h, mice were euthanized with CO2, and peritoneal exudates were collected by lavaging with 5 ml of sterile saline.
Flow cytometry
For the determination of leukocyte subtypes and expression of surface markers, exudates were stained with FITC-conjugated anti-mouse Gr-1 or Ly6G (0.5 µg/106 cells), PE-conjugated anti-mouse F4/80 (clone CI:A3-1; 0.2 µg/106 cells), Pacific Blue- or PerCP-conjugated rat anti-mouse Ly6C, APC-conjugated anti-mouse Tim4 or PerCP-conjugated anti-mouse CD11b (clone M1/70;1; 0.2 µg/106 cells); all from BioLegend. Gating strategy to distinguish cell types is depicted in Fig. S1. Propidium Iodide (PI) and Annexin V (2 µl per sample) (BioLegend) were used to identify necrotic and apoptotic cells, respectively. After washing the cells twice with 1% BSA in PBS, the cells were analyzed using FACSCalibur or FACSCantoII (BD Biosciences). Data analysis was performed using the FlowJo software. In addition, eosinophils were identified, and their level of degranulation was determined, using side scatter.
Isolation of murine peritoneal macrophages
Cells were recovered from peritoneal exudates 66 h following zymosan A challenge. Macrophages were labeled with PE-conjugated rat anti-F4/80 and isolated using EasySep-PE selection magnetic beads according to the manufacturer’s instructions (Stem-Cell Technologies). The PE negative fraction was designated Macrophage-depleted leukocytes (MDLs).
Neutrophil apoptosis
Peritoneal PMNs from WT or Sept4/ARTS−/− mice at 24 h PPI were isolated using PE-conjugated Gr-1 antibodies with PE selection magnetic beads (StemCell Technologies). PMNs were incubated (106 cells/1 ml of culture media) for 24 h and then washed with PBS and resuspended (2 × 105/100 μl) in PBS. The cells were stained with AnnexinV-FITC and PI MEBCYTO Apoptosis Kit (MBL Laboratories) and analyzed for live, early and late apoptotic cells by flow cytometry using FACSCanto II.
Apoptotic cell engulfment
Jurkat (human CD4+ T cell leukemia cell line) cells were incubated (106 cells/ml) with 1 µM staurosporine (Sigma) for 4 h to trigger apoptosis. In some experiments, apoptotic cells were then washed with serum-free media and incubated (10 × 106 cells/ml) with 10 mM CypHer5E Mono NHS Ester (GE Healthcare) for 30 min. Before incubation with macrophages, the apoptotic cells were washed twice with culture medium. Macrophages were recovered from male WT or Sept4/ARTS−/− mice 48 h or 66 h post peritonitis for the ex vivo and in vivo assay of apoptotic cell uptake, respectively. Macrophages were incubated for 4 h with labeled apoptotic cells for ex vivo evaluation or immediately stained for in vivo evaluation of uptake. Unbound cells were washed and macrophages from both assays were transferred to poly-prep slides (Sigma-Aldrich) and fixed with 2% paraformaldehyde. Then, cells were stained with Hoechst 33342 (Invitrogen) and Phalloidin-FITC (Biotium) at room temperature for 20 min and enumerated under a fluorescent microscope (Zeiss). Two areas of two cover slips, each containing at least 50 (overall 200) macrophages were analyzed, and the average number of PMN or Jurkat cells engulfed per macrophage, as well as the number of macrophages with cutoff numbers of engulfed PMN were calculated. Phagocytic index was calculated as previously described [63].
Cell culture
Murine peritoneal macrophages were obtained from mice 66 h post peritonitis. Jurkat cells were used as a model for apoptotic PMNs and THP-1 cells (human acute monocytic leukemia cell line) were used as model for macrophages. All cells were cultured in RPMI 1640, supplemented with 10% fetal bovine serum (FBS), 2 µM glutamine, 100 units/ml penicillin and 100 µg/mL streptomycin. Human cell lines were maintained at 37 °C in a 5% CO2 humidified atmosphere, while primary murine cells were maintained at 37 °C in a 7.5% CO2 humidified atmosphere. Apoptosis of Jurkat cells was induced using the following methods: (1) Exposure to UV radiation with a 3 ft. 30 watt UV tube (Airstream S-series Class II Biohazard Safety Cabinet "ESCO®") for 5 min; (2) Incubation with 1 µM staurosporine (STS); (3) Transfection with ARTS-containing plasmids as follows—Freshly cultured Jurkat cells (106 cells/ml) were transfected with plasmids encoding c-myc-tagged ARTS vector, according to the jetPEI™ transfection reagent (Polyplus®) protocol as described [64]. THP-1 cells (0.5 × 106 cells /ml) were differentiated to macrophages by incubation with phorbol 12-myristate 13-acetate (PMA, 50 ng/ml) for 72 h. Co-culture—transfected Jurkat cells (0.16, 1.5, or 5 million cells) were added to differentiated THP-1 macrophages at 1:3, 3:1, or 10:1 ratios.
Cytokine secretion
Murine peritoneal macrophages (106 cells/well) were activated by LPS (1 µg/ml; Sigma Aldrich) for 16 h, while human differentiated THP-1 macrophages (106 cells/ well) were activated by LPS (500 ng/ml) for 24 h. The cell-free supernatants were collected and the levels of TNF-α, IL-1β, IL-6, IL-10 and IL-12 were determined by standard ELISA kits, according to the manufacturer’s instructions (R&D systems, Minneapolis; BioLegend).
Western Blot
Cells were collected, centrifuged, washed with PBS and lysed in RIPA buffer containing Protease Inhibitors Cocktail (PIC, Sigma-Aldrich). Samples were run by SDS-PAGE (10%) and transferred to a PVDF membrane. The membranes were blotted with antibodies against the following antigens: β-actin, c-Myc, GAPDH and tubulin (Santa-Cruz); arginase-1, iNOS (Abcam); D6 (Abnova); COX2, 12/15-LO, sphingosine kinase (SphK) (Cayman Chemicals); MMP-9 and CCR7 (R&D Systems); H2AX or phospho-H2AK (Calbiochem); Caspase-9 and PARP (Cell Signaling). Anti-CCR2 and anti-CCR5 antibodies were a gift from Dr. Mack (Klinikum der Universitat Regensburg, Regensburg, Germany), and the anti-galectin-1 antibody was a gift from Dr. Rabinovich (Universidad de Buenos Aires, Buenos Aires, Argentina). The membranes were reacted with matching secondary antibodies conjugated with Horseradish Peroxidase (HRP, Jackson Immunoresearch laboratories). The membranes were developed with EZ-ECL detection kit (Biological Industries, Beit Haemek) and analyzed using Luminescent Image Analyzer LAS-4000 (Fujifilm Corporation) & "Image Reader LAS-4000" software (Fujifilm Corporation). Densitometry analysis of blot lanes was done using ImageJ 1.50.
Statistical analysis
Experiments were performed at least 3 times with 3–4 replicates of each data point. Results were analyzed by the two-tailed Student's t-test, one- or two-way ANOVA, and Pearson correlation. Data are presented as mean ± SEM. Results were considered statistically significant when p < 0.05 (*), 0.01 (**), 0.001 (***).
Results
Sept4/ARTS−/− mice display an aberrant distribution of peritoneal leukocyte populations and macrophage reprogramming
To determine whether Sept4/ARTS deficiency affect leukocyte numbers at homeostatic conditions, we enumerated the differences in myeloid cell populations between unchallenged male Sept4/ARTS−/− mice and their WT controls. We observed an elevation in the number of bone marrow (BM) neutrophils and Ly6Clo monocytes (Fig. S2a, b), which could be ascribed to lower apoptosis rate. There were no differences in Ly6Chi monocytes, macrophages and other BM-resident leukocytes (Fig. S2b, c and data not shown). Analysis of myeloid cell populations in the spleen and peritoneum indicated no difference in the number of neutrophils, monocytes, resident Tim4+ macrophages and other subsets (Fig. S2d–j and data not shown). Overall, there were no major differences between the genotypes in steady state. In order to evaluate whether ARTS-mediated apoptosis in leukocytes affects the resolution of inflammation, we treated male WT, Sept4/ARTS+/− and Sept4/ARTS−/− mice with zymosan A. Zymosan A-induced peritonitis is a well-established model of spontaneously-resolving inflammation [65]. Using this model, we did not observe any difference in the total number of peritoneal leukocytes (Fig. 1a). At 24 h post-injection, neutrophil recruitment is normally at its peak, while at 66 h post-injection, apoptotic neutrophil efferocytosis and clearance are normally observed [55, 59, 65, 66]. As compared to WT mice, Sept4/ARTS−/− mice displayed apoptosis and clearance rates rather than a larger fraction of peritoneal neutrophils at 66 h post induction, but not at 24 h post induction (Fig. 1b). This result suggests that Sept4/ARTS−/− mice exhibited a lower neutrophil apoptosis and clearance rates rather than an impaired neutrophil recruitment. Of note, the observation that heterozygous Sept4/ARTS+/− mice display a partially aberrant phenotype implies a haploinsufficiency of ARTS in completing neutrophil apoptosis. As expected, the fraction of peritoneal macrophages increased with time (Fig. 1c). While at 24 h post induction we observed no difference between genotypes, at 66 h post induction, Sept4/ARTS−/− mice displayed a significantly smaller fraction of macrophages in comparison to WT mice (Fig. 1c). Notably, the eosinophil fraction remained unaffected (Fig. 1d). Female Sept4/ARTS−/− mice displayed a larger fraction of neutrophils as well, together with smaller fractions of macrophages and eosinophils (Fig. S3a-c). Next, we further characterized the monocyte/macrophage sub-populations. Ly6C expression on monocytes/macrophages is associated with an inflammatory phenotype [67]. In line, and unlike unchallenged mice (Fig. S2h), we detected an increased Ly6C expression and a larger fraction of peritoneal Ly6Chi monocyte/macrophages in ARTS−/− mice (Fig. 1e–f). Macrophage reprogramming during resolution of inflammation is also associated with a reduction in F4/80 and CD11b surface expression [48]. Indeed, we observed a higher expression of F4/80 and CD11b in peritoneal macrophages of Sept4/ARTS−/− mice in comparison to their WT counterparts (Fig. 1g–j). In addition, Sept4/ARTS−/− mice displayed a larger fraction of CD11bhigh macrophages and smaller fraction of CD11blow macrophages as compared to WT mice (Fig. 1h–i). The CD11bhigh subset of both Sept4/ARTS+/− mice and Sept4/ARTS−/− mice demonstrated an increased CD11b expression (Fig. 1-j). These observations suggest a hampered reprogramming of resolution phase macrophages to the pro-resolving F4/80lowCD11blow phenotype [48]. In contrast, female Sept4/ARTS−/− mice displayed a rather different phenotype: a reduced F4/80 expression (Fig. S3d) and no difference in CD11b subsets or expression (Fig. S3e-g). These findings suggest that hampered macrophage reprogramming during peritonitis is more evident in males than females. Hence, we decided to continue our analysis only with Sept4/ARTS−/− males. Together, Sept4/ARTS−/− mice retained higher numbers of peritoneal neutrophils at the expense of macrophages, which also failed to acquire a pro-resolving phenotype.
Sept4/ARTS−/− mice display an aberrant distribution of peritoneal leukocyte populations and macrophage reprogramming. Peritoneal exudate cells from male Sept4/ARTS+/+ (WT), Sept4/ARTS+/− (ARTS+/−) or Sept4/ARTS−/− (ARTS−/−) mice were collected 24 h or 66 h post zymosan A injection (1 mg/mouse). a Total number of peritoneal cells were counted 24 h post induction, n = 6–8. b–j The cells were immunostained for leukocyte markers F4/80, Ly6C, Gr-1 (or Ly6G) and CD11b and analyzed by flow cytometry. a–c The average percentages of three leukocyte populations: F4/80−GR-1+(Ly6G+) neutrophils (a), F4/80+GR-1−CD11b+ macrophages (b) and F4/80+SSChi eosinophils (c), n = 6–9. d, e The average mean fluorescence intensity (MFI) of Ly6C expression on monocytes/macrophages (d) and the average percentage of Ly6Chi monocytes/macrophages (e), n = 6. (f) The average MFI of F4/80 expression on macrophages, n = 8–9. g–h Macrophage sub-types were further gated according to CD11b expression, n = 7–9. i The average MFI of CD11b expression within the CD11bhigh macrophage subset, n = 8–9. Altogether, the data summarize 2–3 independent experiments and are presented as mean ± SEM. Analysis by two-tailed Student’s t-test; statistical significance is relative to the respective data from WT mice, * p < 0.05, ** p < 0.01, *** p < 0.001. Analysis by one-way ANOVA was statistically significant for all the results, except for (c)
Peritoneal Sept4/ARTS−/− leukocytes are apoptosis-resistant
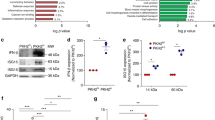
The higher numbers of peritoneal neutrophils in Sept4/ARTS−/− mice imply a failure in the execution of apoptosis in these cells during peritonitis. Not only neutrophils, but also macrophages undergo some degree of apoptosis during the resolution of inflammation [68]. Bearing in mind that all cell types in Sept4/ARTS−/− mice are ARTS-deficient, we examined whether their respective leukocytes are apoptosis-resistant. BM-resident macrophages of unchallenged Sept4/ARTS−/− mice expressed lower levels of cleaved PARP and caspase-9 (Fig. S4a), supporting diminished apoptosis in these cells. Using our peritonitis model, we compared the apoptotic properties of ARTS−/− macrophages, macrophage-depleted leukocytes (MDLs, enriched with PMNs) and neutrophils (incubated ex vivo) to their WT counterparts. Indeed, we observed reduced expression of active (cleaved) caspase-9, H2AX/phospho-H2AX and full-length/cleaved PARP, all of which are classical apoptotic markers (Fig. 2a–c). Caspase-9 is the initiator in the caspase-dependent pathway [69], whereas H2AX (a histone) and PARP (a polymerase) are markers for DNA damage [70]. ARTS-deficient MDLs expressed reduced levels of active caspase-9 and PARP/cPARP at 66 h post peritonitis induction, whereas Sept4/ARTS−/− neutrophils expressed reduced levels of these proteins after incubation ex vivo for 4 or 24 h (Fig. 2b, c). Furthermore, once peritoneal Sept4/ARTS−/− macrophages and neutrophils were isolated and cultured for 24–48 h, they demonstrated an increased survival as compared to their WT counterparts (Fig. 2d, e). Notably, increased survival of these cells was not observed immediately after isolation (Fig. 2d, e). Of note, both cultured peritoneal Sept4/ARTS−/− macrophages and neutrophils showed a delay in late apoptosis rate, whereas only Sept4/ARTS−/− neutrophils showed a delay in early apoptosis rate (Fig. S4b–e). These findings indicate that ARTS−/− macrophages and neutrophils retained their apoptotic-resistance ex vivo.
Peritoneal Sept4/ARTS−/− leukocytes are apoptosis-resistant and display a hyperinflammatory phenotype with loss of pro-resolving properties. Peritoneal exudate cells from male Sept4/ARTS+/+ (WT), Sept4/ARTS+/− (ARTS+/−) or Sept4/ARTS−/− (ARTS−/−) mice were collected from unchallenged mice or 4, 24 or 66 h post zymosan A injection (1 mg/mouse). a–c, f–g Peritoneal F4/80+ macrophages were isolated and separated from F4/80− leukocytes (i.e. macrophage-depleted cells, MDLs). Cell pellets were lysed, run by SDS-PAGE and blotted with antibodies against the indicated proteins, with GAPDH or actin serving as controls. The membranes were then reacted with matching HRP-conjugated secondary antibodies and developed. a–c, f Representative blot images. g Densitometry analysis. d Peritoneal F4/80+ macrophages were isolated 66 h post zymosan A injection. Then, they were cultured or not for 48 h, followed by staining with Annexin V and PI and analysis by flow cytometry, n = 4. c–d Peritoneal Ly6G+ neutrophils were isolated 24 h post zymosan A injection. Then, they were cultured for 4 or 24 h, followed by staining with Annexin V and PI and analysis by flow cytometry, n = 3–4. d, e Peritoneal macrophages or neutrophils were cultured for 0, 4, 24 or 48 h; followed by staining with Annexin V and PI and analysis by flow cytometry, n = 3–4. h Cell-free peritoneal exudates were analyzed for TGF-β levels using a standard ELISA. Analysis by one-way ANOVA confirmed statistical significance. Altogether, the data summarize 2–4 experiments and are presented as mean ± SEM. Analysis by two-tailed Student’s t-test; statistical significance is relative to the respective data from WT mice, * p < 0.05, ** p < 0.01, *** p < 0.001
Sept4/ARTS−/− macrophages display a hyperinflammatory phenotype with loss of pro-resolving properties
An increase in CD11bhigh macrophages at the expense of CD11blow macrophages, as observed in Sept4/ARTS−/− mice undergoing peritonitis, may signify a shift towards a hyperinflammatory phenotype [48]. Therefore, we examined the inflammatory properties of isolated peritoneal macrophages. Compared to WT macrophages, Sept4/ARTS−/− and Sept4/ARTS+/− macrophages expressed increased amounts of iNOS, COX2 and MMP-9 (Fig. 2a), all of which are enzymes that characterize ongoing inflammation [43, 71, 72]. Strikingly, Sept4/ARTS−/− macrophages also expressed reduced amounts of the pro-resolving mediators galectin-1 and 12/15-LO (Fig. 2a, f–g) [46, 66, 73]. ARTS−/− macrophages also expressed lower CCR7 (Fig. 2a), which is essential in the chemoattraction of M1 and M2 macrophages [74]. Arginase-1, which is normally expressed in anti-inflammatory macrophages, but diminishes in the pro-resolving CD11blow phenotype [45, 48], was increased in Sept4/ARTS−/− macrophages (Fig. 2f–g). Together, these results underscore a hampered reprogramming to pro-resolving CD11blow macrophages. Likewise, compared to WT MDLs, Sept4/ARTS−/− MDLs expressed reduced amounts of pro-resolving effectors, including SphK, and chemokine receptors with scavenging capacity, like CCR5 and the silent receptor D6 (Fig. 2c) [57,58,59, 62]. The regulation of these scavenging chemokine receptors was specific as CCR2, an inflammatory chemokine receptor, was upregulated on Sept4/ARTS+/− and Sept4/ARTS−/− MDLs (Fig. 2b). In support of a blockage in acquiring a pro-resolving phenotype, we detected reduced concentrations of TGF-β in the peritoneal exudates of ARTS+/− and ARTS−/− mice as compared to WT mice (Fig. 2h). TGF-β is a key cytokine upregulated during the resolution of peritonitis and an essential mediator of resolution of inflammation [75, 76]. Of note, the reduction in TGF-β concentrations was more substantial in Sept4/ARTS−/− mice as compared to Sept4/ARTS+/− mice (Fig. 2h). Efferocytosis is a key step in resolution of inflammation [40,41,42, 55]. Measurement of apoptotic cell efferocytosis can be done by counting the number of engulfed cells per macrophage. To determine whether ARTS regulates efferocytosis during the resolution of peritonitis, we isolated WT and Sept4/ARTS−/− macrophages 66 h post induction. We then measured apoptotic cell uptake by counting the number of engulfed cells (i.e., mouse PMNs) per macrophage. In this in vivo analysis, we revealed that Sept4/ARTS−/− mice exhibited impaired efferocytosis as indicated by a lower average number of engulfed cells per macrophage, a lower percentage of phagocytic macrophages, and a lower phagocytic efficiency index (Fig. 3a–d). To confirm a reduced phagocytic efficiency of Sept4/ARTS−/− macrophages in comparison to WT macrophages, we isolated macrophages at an earlier time point of 48 h post induction and incubated them with pre-labelled apoptotic Jurkat T cells. In this ex vivo assay, we confirmed that Sept4/ARTS−/− macrophages had an impaired ability of apoptotic cell uptake as indicated by a lower average number of apoptotic cells per macrophage, a lower percentage of phagocytic macrophages, and a lower phagocytic efficiency index (Fig. 3e–h). Eosinophils play a role in regulating inflammation and its resolution by storing preformed cytokines, chemokines and growth factors, available for immediate release [50, 51]. Peritoneal Sept4/ARTS−/− eosinophils demonstrated higher granularity, analyzed by flow cytometry, when compared to their WT counterparts (Fig. S4f). Thus, eosinophil degranulation seems to be hampered in ARTS−/− mice. Considering the pro-resolving role of eosinophils in peritonitis [50, 51], these findings are in accord with the hyperinflammatory state of these mice. Altogether, based on our peritonitis model, Sept4/ARTS−/− macrophages manifested a hyperinflammatory phenotype, which was associated with loss of pro-resolving properties and an impaired efferocytosis.
Peritoneal Sept4/ARTS−/− macrophages exert impaired efferocytosis. Peritoneal F4/80+ macrophages were isolated from male Sept4/ARTS+/+ (WT) or Sept4/ARTS−/− (ARTS−/−) mice 66 h (a–d) or 48 h (e–h) post zymosan A injection (1 mg/mouse). The isolated cells were stained with Hoechst for nuclear detection and phalloidin-FITC for F-Actin. a–d The macrophages were enumerated for apoptotic PMN uptake in vivo (nuclei in macrophage cytoplasm). e–h Jurkat T cells underwent staurosporine-induced apoptosis, stained with CypHer5E Mono NHS Ester, after which they were incubated with isolated macrophages for 4 h. The macrophages were enumerated for apoptotic cell uptake ex vivo (red-labelled phagocytosed cells). The percentage of phagocytic macrophages engulfing discrete numbers of apoptotic cells (a, c, f) and the average number of apoptotic cells engulfed per macrophage (b, g) were calculated. e Representative images. Arrows point at labelled engulfed cells. (d, h) The phagocytic efficiency index was calculated as previously described [63]. The data is averaged from 4 coverslips generated from 2–3 mice in 2 separate experiments – in each slide at least 50 macrophages were analyzed. The data are presented as mean ± SEM. Analysis by two-tailed Student’s t-test; statistical significance is relative to the respective data from WT mice, * p < 0.05, ** p < 0.01, *** p < 0.001. In c, the Pearson analysis shows no correlation between WT and ARTS−/− macrophages, suggesting a statistically different engulfment capacity
Peritoneal Sept4/ARTS−/− macrophages display a pro-inflammatory cytokine repertoire
Macrophage reprogramming is accompanied by reduced secretion of pro-inflammatory cytokines and increased secretion of anti-inflammatory cytokines upon exposure to bacterial stimuli; this can be measured ex vivo by exposure of macrophages to LPS [48]. We applied this methodology first to isolated BM-, spleen- and peritoneal-resident macrophages of unchallenged WT and Sept4/ARTS−/− mice. While LPS increased cytokine expression regardless of genotype and source of cells, there were notable difference in LPS-induced cytokine secretion between Sept4/ARTS−/− and WT macrophages (Fig. S5). Spleen-derived Sept4/ARTS−/− macrophages demonstrated reduced LPS-induced secretion of pro- and anti-inflammatory cytokines (Fig. S5a–c), whereas BM-resident Sept4/ARTS−/− macrophages secreted similar levels of all cytokines as compared with WT macrophages (Fig. S5d–f). On the contrary, in response to LPS, resident peritoneal Sept4/ARTS−/− macrophages secreted higher levels of pro-inflammatory TNF-α and IL-6 and lower levels of the anti-inflammatory cytokine IL-10 (Fig. S5g–i), suggesting a hyper-inflammatory phenotype. Notably, the differences in cytokine secretion between WT and Sept4/ARTS−/− resident peritoneal macrophages were evident only in LPS-stimulated peritoneal macrophages and not in vehicle-treated ones. Next, we stimulated resolution phase peritoneal macrophages obtained from WT and Sept4/ARTS−/− mice with LPS. Our results indicated a shift towards an inflammatory cytokine repertoire once Sept4/ARTS−/− macrophages were compared to WT controls, regardless of LPS treatment (Fig. 4). Sept4/ARTS−/− macrophages also secreted higher levels of the pro-inflammatory cytokines IL-1β and TNF-α as compared to WT controls (Fig. 4a, b), whereas Sept4/ARTS+/− macrophages secreted higher levels of TNF-α, but not IL-1β (Fig. 4a, b). Conversely, ARTS+/− and ARTS−/− macrophages secreted lower levels of the anti-inflammatory cytokine IL-10 as compared to controls (Fig. 4c). Surprisingly, resolution phase ARTS−/− macrophages secreted lower levels of the cytokines IL-6 and IL-12 in comparison to WT controls (Fig. 4d, e), although these cytokines are considered pro-inflammatory in nature [77, 78]. As expected, LPS per se significantly increased the secretion of IL-1β, TNF-α, IL-6 and IL-12, regardless of macrophage genotype (Fig. 4a, b, d, e). LPS also increased the secretion of IL-10 from WT and ARTS+/− macrophages but was ineffective in Sept4/ARTS−/− macrophages (Fig. 4c). In summary, resolution phase Sept4/ARTS−/− macrophages exhibited a hyperinflammatory phenotype in vivo, consistent with hampered reprogramming by apoptotic PMN uptake, which was mostly retained ex vivo following macrophage isolation.
Peritoneal Sept4/ARTS−/− macrophages display a pro-inflammatory cytokine repertoire. Peritoneal F4/80+ macrophages were isolated from male Sept4/ARTS+/+ (WT), Sept4/ARTS+/− (ARTS+/−) or Sept4/ARTS−/− (ARTS−/−) mice 66 h post zymosan A injection (1 mg/mouse). Then, the macrophages were cultured and activated or not with LPS (1 µg/ml) for 24 h. The cell-free supernatants were collected and analyzed for an array of cytokines, using a standard ELISA. a IL-1β, n = 4–8. b TNF-α, n = 5–9. c IL-10, n = 4–8. d IL-6, n = 3. e IL-12, n = 3. Altogether, the data summarize 3 experiments, including at least 2 technical replicates, and are presented as mean ± SEM. Analysis by two-tailed Student’s t-test; statistical significance is relative to the respective data from WT mice (either with or without LPS, bordered by a dashed line), * p < 0.05, ** p < 0.01, *** p < 0.001. Additionally, the data were statistically significant in all groups when cytokine levels were compared between LPS treatment and no treatment, except for IL-10 levels in the ARTS−/− macrophage group. Analysis by two-way ANOVA was statistically significant for all the results, except for (d). d An interaction between the bars with and without LPS could not be excluded. However, one-way ANOVA was statistically significant both ways in all figures
Overexpression of ARTS in lymphocytes intensifies their immune-silencing properties upon apoptosis
To mimic the process of efferocytosis in vivo and obtain a more direct view of the role played by ARTS in macrophage reprogramming, we used a co-culture model. Co-culturing LPS-activated macrophages with apoptotic leukocytes results in suppression of their inflammatory properties, which among others include reduction in the secretion of pro-inflammatory cytokines [48, 79]. Based on this experimental rationale, we induced apoptosis in human Jurkat cells, using UV irradiation or an incubation with STS, followed by co-culturing with LPS-activated human THP-1 macrophages. As expected, co-culture of macrophages with UV-irradiated or STS-treated Jurkat cells resulted in reduced IL-1β and TNF-α secretion, when compared to LPS-treated macrophages alone or to a co-culture with live Jurkat cells (Fig. 5a, b). Taking into account that ARTS overexpression induces apoptosis [27, 64], we transfected human Jurkat cells with a vector overexpressing ARTS or with a control vector. The transfected cells were co-cultured with LPS-activated human THP-1 macrophages. In a similar manner to the control experiments, co-culture of macrophages with ARTS-transfected Jurkat cells effectively reduced the secretion of IL-1β as compared to control transfected cells (Fig. 5a). We also observed a reduction in TNF-α secretion, but only in higher ratios of apoptotic cells to macrophages (Fig. 5b). In addition to the ability to suppress secretion of pro-inflammatory cytokines, we determined whether ARTS-transfected lymphocytes also increased the expression of pro-resolving effectors, in comparison to Sept4/ARTS−/− leukocytes. We previously reported that CCR5+ apoptotic leukocytes scavenge chemokines that usually attract macrophages to inflamed sites in a peritonitis mouse model [59]. First, we validated that Jurkat cells did increase CCR5 expression following induction of apoptosis by UV irradiation (Fig. 5c). Next, we examined CCR5 expression in ARTS-transfected Jurkat cells as compared to control transfected Jurkat cells. Indeed, ARTS overexpression was sufficient to induce high expression of CCR5 (Fig. 5d). Taken together, the outcome of ARTS overexpression in vitro is consistent with our observations in Sept4/ARTS−/− mice, showing that ARTS expression in apoptotic leukocytes is involved in the cross-talk with macrophages during the resolution of inflammation.
Overexpression of ARTS in leukocytes promotes their immune-silencing properties upon apoptosis. Human Jurkat cells were either exposed to UV irradiation (5 min) or treated with STS (1 μM) to induce apoptosis. Alternatively, the cells were transfected with plasmids encoding c-Myc-tagged ARTS or a control c-Myc vector. a, b After 24 h, live or apoptotic Jurkat cells were co-cultured with THP-1-derived macrophages at the indicated ratios. Next, the co-cultured cells were activated with LPS (500 ng/ml) for additional 24 h. Finally, the cell-free supernatants were collected and analyzed for the content of the cytokines IL-1β (a) and TNF-α (b), using a standard ELISA. Altogether, the data summarizes 4–5 experiments with 3–4 replicates per experiment. The data are presented as mean per experiment ± SEM of all replicates. Analysis by two-tailed Student’s t-test; statistical significance is relative to the respective data from matched-control or mock cells, * p < 0.05, ** p < 0.01, *** p < 0.001. Analysis by one-way ANOVA confirmed statistical significance. c, d UV-irradiated or vector-transfected human Jurkat cells were lysed. The lysates were run by SDS-PAGE and blotted with antibodies against human CCR5, actin (loading control) or c-Myc (transfection control). The membranes were then reacted with matching HRP-conjugated secondary antibodies and developed. Results are representative blot images. c CCR5 expression following UV irradiation at the indicated time points. d CCR5 expression in transfected and untransfected cells
Discussion
Acute inflammation due to bacterial infection or injury is normally characterized by the rapid influx of blood PMNs, typically neutrophils, followed swiftly by recruited monocytes that mature into inflammatory macrophages [40, 41]. Resolution of inflammation can occur only if PMNs are efficiently eliminated by efferocytosis, avoiding local tissue reaction, and the mononuclear cells (macrophages, dendritic cells and lymphocytes) depart, returning to homeostatic numbers and phenotypes [40,41,42,43,44]. Chronic diseases, such as rheumatoid arthritis, diabetes, atherosclerosis, asthma and colitis, are often associated with a hyper-inflammatory phenotype that fails to resolve [54, 80,81,82,83,84,85]. Thus, the resolution phase of inflammatory episodes has to be highly coordinated and regulated, involving distinct immunomodulatory mechanisms, among which macrophage reprogramming and the cross-talk between pro-resolving macrophages and engulfed apoptotic PMNs are key [40, 41]. We and others have shown that apoptotic cells sequester inflammatory chemokines [59, 60], release immunomodulatory factors [46, 47, 57, 66, 86], and that the mere uptake of apoptotic cells suppresses release of pro-inflammatory cytokines from macrophages and promotes the secretion of anti-inflammatory cytokines [43, 48, 79]. It is therefore reasonable to hypothesize that impinging the efferocytic process in macrophages would result in halted reprogramming and a sustained inflammatory condition.
To examine this hypothesis, we utilized a model of spontaneously-resolving peritonitis in apoptosis-deficient Sept4/ARTS−/− mice, characterized with resistance to cell death [36,37,38]. In steady state conditions, we observed a higher number of neutrophils in the BM of male Sept4/ARTS−/− mice, but not in the peritoneum or the spleen. During the early inflammatory phase (24 h) of peritonitis, similar peritoneal neutrophil and macrophage numbers were observed in WT and Sept4/ARTS−/− mice, although the neutrophils showed partial apoptosis resistance in these mice. On the other hand, at 66 h, peritoneal neutrophils demonstrated higher numbers, increased survival and reduced apoptosis in vivo. These results lead us to reason that peritoneal Sept4/ARTS−/− neutrophils failed to carry out effective apoptosis, and consequently were not efferocytosed by resolution phase macrophages resulting in diminished engulfment-related signaling, impaired reprogramming and sustained inflammation. Indeed, macrophages isolated from Sept4/ARTS−/− mice retained their inflammatory phenotype, associated with higher F4/80, CD11b, Ly6C, iNOS, COX2 and MMP-9 expression. Moreover, these hyperinflammatory macrophages secreted higher levels of the pro-inflammatory cytokines IL-1β and TNF-α and lower levels of the anti-inflammatory cytokines TGF-β and IL-10. A similar response was observed in LPS-treated resident peritoneal, but not BM or splenic macrophages. In this model of zymosan A peritonitis, resident peritoneal macrophages emigrate to remote organs and BM-originating monocyte-derived macrophages constitute most of the resolution-phase macrophage population [48, 87]. Therefore, it is unlikely that sustained presence of resident peritoneal macrophages drives the hyper-inflammatory phenotype of Sept4/ARTS−/− mice; it is rather the defective reprograming of Sept4/ARTS−/− macrophages. Moreover, Sept4/ARTS−/− macrophages did not increase in numbers, favoring a reprogramming blockage over the possibility of enhanced inflammatory response via increased macrophage recruitment or proliferation. In line with the hyper-inflammatory phenotype, Sept4/ARTS−/− macrophages demonstrated an impaired capacity to uptake PMNs in vivo or apoptotic cells ex vivo, which probably leads to halted reprogramming. Notably, ARTS−/− mice did not display a classical enhanced inflammation, since the secretion of pro-inflammatory cytokines, such as IL-6 and IL-12, were unexpectedly reduced from their macrophages. Moreover, our RNA-Seq analysis indicates that satiated resolution-phase macrophages, which are generated by self-limiting efferocytosis, express higher levels of IL-6 and IL-12 in comparison to their phagocytic counterparts [87]. Therefore, blocked reprogramming may reduce the levels of these cytokines. Notably, a similar macrophage phenotype was observed in D6/ACKR2-deficient macrophages during the resolution of peritonitis [58]. These macrophages exhibited defective reprogramming due to hampered signaling from apoptotic PMN, resulting in hampered resolution of inflammation [58, 62]. Of note, macrophages isolated from female Sept4/ARTS−/− mice seemingly secreted higher levels of IL-12 as compared to WT females, while IL-6 levels remained intact (data not shown). Nonetheless, macrophages isolated from Sept4/ARTS−/− females did not display an impaired reprogramming as observed in macrophages isolated from Sept4/ARTS−/− males. This observation is in line with the positive effect of estrogen on resolution of inflammation [88], supporting a more prominent peritonitis in Sept4/ARTS−/− males as compared to Sept4/ARTS−/− females.
In a direct cause and effect, these findings showed that inhibiting effective efferocytosis by modes of inhibiting apoptosis in PMNs blocks macrophage reprogramming. In support, the cyclin-dependent kinase inhibitor, R-roscovitin, induces neutrophil apoptosis and consequently enhances macrophage reprogramming and resolution of inflammation in various acute models [62, 89]. Moreover, TNF-related apoptosis-inducing ligand (TRAIL)-deficient mice also display higher neutrophil numbers due to apoptosis-resistance, consequently leading to an enhanced inflammatory response following an induction of peritonitis [90]. The authors claimed that TRAILnull mice displayed a delayed neutrophil apoptosis together with a delayed resolution of inflammation [90]. We have yet to test whether in Sept4/ARTS−/− mice delayed apoptosis and resolution of inflammation take place rather than their absolute blockage. There might be an unknown ARTS-independent pathway that can trigger a compensatory apoptosis in leukocytes; for example, the apoptosis rate of Sept4/ARTS−/− lymphocytes was similar to their WT counterparts [36]. Still, at a time point of 66 h post-induction, when normally resolution takes place [48, 65], we observed reduced expression of pro-resolving effectors in macrophages isolated from Sept4/ARTS−/− mice, including TGF-β, 12/15-LO, galectin-1 and CCR7. In our study, eosinophils, which also participate in the resolution of inflammation [51], exhibited impaired de-granulation. Follow-up studies will determine whether this aberrant phenotype of macrophages and eosinophils leads to reduced production of specialized pro-resolving lipid mediators. It should be mentioned that effects that were evident in ARTS−/− mice were also evident to some extent in ARTS+/− mice, suggesting haploinsufficiency of ARTS signaling in mediating apoptosis and resolution of inflammation.
One may suggest that ARTS deficiency in macrophages per se halted their reprogramming rather than ARTS-mediated apoptosis in PMNs. We examined this possibility by performing co-culture experiments. Incubating ARTS-overexpressing Jurkat cells together with macrophages resulted in inhibited secretion of pro-inflammatory IL-1β and TNF-α as effectively as incubation with Jurkat cells that underwent classically-induced apoptosis. ARTS-overexpressing Jurkat cells also increased CCR5 expression in a similar manner to classically-induced apoptotic Jurkat cells. These results are consistent with a cell-autonomous mechanism induced by ARTS in PMNs; ARTS deficiency in apoptotic PMNs rather than in macrophages seems to be the mechanism by which macrophages retain their inflammatory properties. In line with these results, Sept4/ARTS−/− PMNs expressed reduced levels of pro-resolving receptors, such as CCR5 and D6, which sequester inflammatory chemokines [59,60,61], and SphK, which synthesizes the anti-inflammatory lipid S1P [56, 57]. Further mechanistic insight is required to determine how ARTS-mediated apoptosis develops in both types of leukocytes. Our findings are of clinical importance, since therapeutic induction of apoptosis in leukocytes, including ARTS activation [91], may facilitate resolution of undesired harmful inflammation.
References
Fuchs Y, Steller H (2011) Programmed cell death in animal development and disease. Cell 147:742–758
Meier P, Finch A, Evan G (2000) Apoptosis in development. Nature 407:796–801
Thompson CB (1995) Apoptosis in the pathogenesis and treatment of disease. Science 267:1456–1462
Kerr JF, Wyllie AH, Currie AR (1972) Apoptosis: a basic biological phenomenon with wide-ranging implications in tissue kinetics. Br J Cancer 26:239–257
Buttke TM, Sandstrom PA (1994) Oxidative stress as a mediator of apoptosis. Immunol Today 15:7–10
Donepudi M, Grutter MG (2002) Structure and zymogen activation of caspases. Biophys Chem 101–102:145–153
Thornberry NA, Lazebnik Y (1998) Caspases: enemies within. Science 281:1312–1316
McComb S, Chan PK, Guinot A et al (2019) Efficient apoptosis requires feedback amplification of upstream apoptotic signals by effector caspase-3 or -7. Sci Adv 5:eaau9433
Ramirez MLG, Salvesen GS (2018) A primer on caspase mechanisms. Semin Cell Dev Biol 82:79–85
Tait SW, Green DR (2010) Mitochondria and cell death: outer membrane permeabilization and beyond. Nat Rev Mol Cell Biol 11:621–632
Eckelman BP, Salvesen GS (2006) The human anti-apoptotic proteins cIAP1 and cIAP2 bind but do not inhibit caspases. J Biol Chem 281:3254–3260
Salvesen GS, Duckett CS (2002) IAP proteins: blocking the road to death's door. Nat Rev Mol Cell Biol 3:401–410
Deveraux QL, Reed JC (1999) IAP family proteins-suppressors of apoptosis. Genes Dev 13:239–252
Deveraux QL, Leo E, Stennicke HR, Welsh K, Salvesen GS, Reed JC (1999) Cleavage of human inhibitor of apoptosis protein XIAP results in fragments with distinct specificities for caspases. EMBO J 18:5242–5251
Suzuki Y, Nakabayashi Y, Nakata K, Reed JC, Takahashi R (2001) X-linked inhibitor of apoptosis protein (XIAP) inhibits caspase-3 and -7 in distinct modes. J Biol Chem 276:27058–27063
Gyrd-Hansen M, Darding M, Miasari M et al (2008) IAPs contain an evolutionarily conserved ubiquitin-binding domain that regulates NF-kappaB as well as cell survival and oncogenesis. Nat Cell Biol 10:1309–1317
Schile AJ, Garcia-Fernandez M, Steller H (2008) Regulation of apoptosis by XIAP ubiquitin-ligase activity. Genes Dev 22:2256–2266
Rajalingam K, Dikic I (2009) Inhibitors of apoptosis catch ubiquitin. Biochem J 417:e1–3
Vucic D, Stennicke HR, Pisabarro MT, Salvesen GS, Dixit VM (2000) ML-IAP, a novel inhibitor of apoptosis that is preferentially expressed in human melanomas. Curr Biol 10:1359–1366
Du C, Fang M, Li Y, Li L, Wang X (2000) Smac, a mitochondrial protein that promotes cytochrome c-dependent caspase activation by eliminating IAP inhibition. Cell 102:33–42
Verhagen AM, Ekert PG, Pakusch M et al (2000) Identification of DIABLO, a mammalian protein that promotes apoptosis by binding to and antagonizing IAP proteins. Cell 102:43–53
Hegde R, Srinivasula SM, Zhang Z et al (2002) Identification of Omi/HtrA2 as a mitochondrial apoptotic serine protease that disrupts inhibitor of apoptosis protein-caspase interaction. J Biol Chem 277:432–438
Yin W, Cheepala S, Clifford JL (2006) Identification of a novel splice variant of X-linked inhibitor of apoptosis-associated factor 1. Biochem Biophys Res Commun 339:1148–1154
Larisch S, Yi Y, Lotan R et al (2000) A novel mitochondrial septin-like protein, ARTS, mediates apoptosis dependent on its P-loop motif. Nat Cell Biol 2:915–921
van Loo G, van Gurp M, Depuydt B et al (2002) The serine protease Omi/HtrA2 is released from mitochondria during apoptosis. Omi interacts with caspase-inhibitor XIAP and induces enhanced caspase activity. Cell Death Differ 9:20–26
Leaman DW, Chawla-Sarkar M, Vyas K et al (2002) Identification of X-linked inhibitor of apoptosis-associated factor-1 as an interferon-stimulated gene that augments TRAIL Apo2L-induced apoptosis. J Biol Chem 277:28504–28511
Gottfried Y, Rotem A, Lotan R, Steller H, Larisch S (2004) The mitochondrial ARTS protein promotes apoptosis through targeting XIAP. EMBO J 23:1627–1635
Mandel-Gutfreund Y, Kosti I, Larisch S (2011) ARTS, the unusual septin: structural and functional aspects. Biol Chem 392:783–790
Edison N, Zuri D, Maniv I et al (2012) The IAP-antagonist ARTS initiates caspase activation upstream of cytochrome C and SMAC/Diablo. Cell Death Differ 19:356–368
Lotan R, Rotem A, Gonen H et al (2005) Regulation of the proapoptotic ARTS protein by ubiquitin-mediated degradation. J Biol Chem 280:25802–25810
Elhasid R, Sahar D, Merling A et al (2004) Mitochondrial pro-apoptotic ARTS protein is lost in the majority of acute lymphoblastic leukemia patients. Oncogene 23:5468–5475
Edison N, Curtz Y, Paland N et al (2017) Degradation of Bcl-2 by XIAP and ARTS promotes apoptosis. Cell Rep 21:442–454
Abbas R, Larisch S (2020) Targeting XIAP for Promoting Cancer Cell Death-The Story of ARTS and SMAC. Cells 9(3):663
Garrison JB, Correa RG, Gerlic M et al (2011) ARTS and Siah collaborate in a pathway for XIAP degradation. Mol Cell 41:107–116
Edison N, Curtz Y, Paland N et al (2017) Degradation of Bcl-2 by XIAP and ARTS Promotes Apoptosis. Cell Reports 21:442–454
Garcia-Fernandez M, Kissel H, Brown S et al (2010) Sept4/ARTS is required for stem cell apoptosis and tumor suppression. Genes Dev 24:2282–2293
Koren E, Yosefzon Y, Ankawa R et al (2018) ARTS mediates apoptosis and regeneration of the intestinal stem cell niche. Nat Commun 9:4582
Fuchs Y, Brown S, Gorenc T, Rodriguez J, Fuchs E, Steller H (2013) Sept4/ARTS regulates stem cell apoptosis and skin regeneration. Science 341:286–289
Koren E, Fuchs Y (2019) The ARTS of Cell Death. J Cell Death 12:1179066019836967
Ariel A, Timor O (2013) Hanging in the balance: endogenous anti-inflammatory mechanisms in tissue repair and fibrosis. J Pathol 229:250–263
Headland SE, Norling LV (2015) The resolution of inflammation: Principles and challenges. Semin Immunol 27:149–160
Greenlee-Wacker MC (2016) Clearance of apoptotic neutrophils and resolution of inflammation. Immunol Rev 273:357–370
Ariel A, Serhan CN (2012) New Lives Given by Cell Death: Macrophage Differentiation Following Their Encounter with Apoptotic Leukocytes during the Resolution of Inflammation. Front Immunol 3:4
Elliott MR, Koster KM, Murphy PS (2017) Efferocytosis Signaling in the Regulation of Macrophage Inflammatory Responses. J Immunol 198:1387–1394
Campbell L, Saville CR, Murray PJ, Cruickshank SM, Hardman MJ (2013) Local arginase 1 activity is required for cutaneous wound healing. J Invest Dermatol 133:2461–2470
Freire-de-Lima CG, Xiao YQ, Gardai SJ, Bratton DL, Schiemann WP, Henson PM (2006) Apoptotic cells, through transforming growth factor-beta, coordinately induce anti-inflammatory and suppress pro-inflammatory eicosanoid and NO synthesis in murine macrophages. J Biol Chem 281:38376–38384
Dalli J, Serhan CN (2017) Pro-resolving mediators in regulating and conferring macrophage function. Front Immunol 8:1400
Schif-Zuck S, Gross N, Assi S, Rostoker R, Serhan CN, Ariel A (2011) Saturated-efferocytosis generates pro-resolving CD11b low macrophages: modulation by resolvins and glucocorticoids. Eur J Immunol 41:366–379
Lin N, Shay JES, Xie H et al (2018) Myeloid Cell Hypoxia-Inducible Factors Promote Resolution of Inflammation in Experimental Colitis. Front Immunol 9:2565
Yamada T, Tani Y, Nakanishi H, Taguchi R, Arita M, Arai H (2011) Eosinophils promote resolution of acute peritonitis by producing proresolving mediators in mice. FASEB J 25:561–568
Isobe Y, Kato T, Arita M (2012) Emerging roles of eosinophils and eosinophil-derived lipid mediators in the resolution of inflammation. Front Immunol 3:270
Ueha S, Shand FH, Matsushima K (2012) Cellular and molecular mechanisms of chronic inflammation-associated organ fibrosis. Front Immunol 3:71
Gieseck RL 3rd, Wilson MS, Wynn TA (2018) Type 2 immunity in tissue repair and fibrosis. Nat Rev Immunol 18:62–76
Krzyszczyk P, Schloss R, Palmer A, Berthiaume F (2018) The role of macrophages in acute and chronic wound healing and interventions to promote pro-wound healing phenotypes. Front Physiol 9:419
Bratton DL, Henson PM (2011) Neutrophil clearance: when the party is over, clean-up begins. Trends Immunol 32:350–357
Hughes JE, Srinivasan S, Lynch KR, Proia RL, Ferdek P, Hedrick CC (2008) Sphingosine-1-phosphate induces an antiinflammatory phenotype in macrophages. Circ Res 102:950–958
Weigert A, Johann AM, von Knethen A, Schmidt H, Geisslinger G, Brune B (2006) Apoptotic cells promote macrophage survival by releasing the antiapoptotic mediator sphingosine-1-phosphate. Blood 108:1635–1642
Pashover-Schallinger E, Aswad M, Schif-Zuck S, Shapiro H, Singer P, Ariel A (2012) The atypical chemokine receptor D6 controls macrophage efferocytosis and cytokine secretion during the resolution of inflammation. FASEB J 26:3891–3900
Ariel A, Fredman G, Sun YP et al (2006) Apoptotic neutrophils and T cells sequester chemokines during immune response resolution through modulation of CCR5 expression. Nat Immunol 7:1209–1216
Graham GJ, McKimmie CS (2006) Chemokine scavenging by D6: a movable feast? Trends Immunol 27:381–386
Martinez de la Torre Y, Locati M, Buracchi C et al (2005) Increased inflammation in mice deficient for the chemokine decoy receptor D6. Eur J Immunol 35:1342–1346
Aswad M, Assi S, Schif-Zuck S, Ariel A (2017) CCL5 promotes resolution-phase macrophage reprogramming in concert with the atypical chemokine receptor D6 and apoptotic polymorphonuclear cells. J Immunol 199:1393–1404
Lumbroso D, Soboh S, Maimon A, Schif-Zuck S, Ariel A, Burstyn-Cohen T (2018) Macrophage-derived protein S facilitates apoptotic polymorphonuclear cell clearance by resolution phase macrophages and supports their reprogramming. Front Immunol 9:358
Bornstein B, Gottfried Y, Edison N et al (2011) ARTS binds to a distinct domain in XIAP-BIR3 and promotes apoptosis by a mechanism that is different from other IAP-antagonists. Apoptosis 16:869–881
Cash JL, White GE, Greaves DR (2009) Zymosan-induced peritonitis as a simple experimental system for the study of inflammation. Methods Enzymol 461:379–396
Rostoker R, Yaseen H, Schif-Zuck S, Lichtenstein RG, Rabinovich GA, Ariel A (2013) Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat 107:85–94
Yang J, Zhang L, Yu C, Yang XF, Wang H (2014) Monocyte and macrophage differentiation: circulation inflammatory monocyte as biomarker for inflammatory diseases. Biomark Res 2:1
Kolaczkowska E, Koziol A, Plytycz B, Arnold B (2010) Inflammatory macrophages, and not only neutrophils, die by apoptosis during acute peritonitis. Immunobiology 215:492–504
Li P, Zhou L, Zhao T et al (2017) Caspase-9: structure, mechanisms and clinical application. Oncotarget 8:23996–24008
Plesca D, Mazumder S, Almasan A (2008) DNA damage response and apoptosis. Methods Enzymol 446:107–122
Weinberg JB (2000) Nitric oxide synthase 2 and cyclooxygenase 2 interactions in inflammation. Immunol Res 22:319–341
Vandooren J, Van den Steen PE, Opdenakker G (2013) Biochemistry and molecular biology of gelatinase B or matrix metalloproteinase-9 (MMP-9): the next decade. Crit Rev Biochem Mol Biol 48:222–272
Ackermann JA, Hofheinz K, Zaiss MM, Kronke G (2017) The double-edged role of 12/15-lipoxygenase during inflammation and immunity. Biochim Biophys Acta Mol Cell Biol Lipids 1862:371–381
Xuan W, Qu Q, Zheng B, Xiong S, Fan GH (2015) The chemotaxis of M1 and M2 macrophages is regulated by different chemokines. J Leukoc Biol 97:61–69
Huynh ML, Fadok VA, Henson PM (2002) Phosphatidylserine-dependent ingestion of apoptotic cells promotes TGF-beta1 secretion and the resolution of inflammation. J Clin Invest 109:41–50
Bannenberg GL, Chiang N, Ariel A et al (2005) Molecular circuits of resolution: formation and actions of resolvins and protectins. J Immunol 174:4345–4355
McLoughlin RM, Witowski J, Robson RL et al (2003) Interplay between IFN-gamma and IL-6 signaling governs neutrophil trafficking and apoptosis during acute inflammation. J Clin Invest 112:598–607
Filardy AA, Pires DR, Nunes MP et al (2010) Proinflammatory clearance of apoptotic neutrophils induces an IL-12(low)IL-10(high) regulatory phenotype in macrophages. J Immunol 185:2044–2050
Voll RE, Herrmann M, Roth EA, Stach C, Kalden JR, Girkontaite I (1997) Immunosuppressive effects of apoptotic cells. Nature 390:350–351
Abdolmaleki F, Farahani N, Gheibi Hayat SM et al (2018) The Role of Efferocytosis in Autoimmune Diseases. Front Immunol 9:1645
Thomas D, Apovian C (2017) Macrophage functions in lean and obese adipose tissue. Metabolism 72:120–143
Prame Kumar K, Nicholls AJ, Wong CHY (2018) Partners in crime: neutrophils and monocytes/macrophages in inflammation and disease. Cell Tissue Res 371:551–565
Yurdagul A Jr, Doran AC, Cai B, Fredman G, Tabas IA (2017) Mechanisms and Consequences of Defective Efferocytosis in Atherosclerosis. Front Cardiovasc Med 4:86
Grabiec AM, Hussell T (2016) The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin Immunopathol 38:409–423
Kuhl AA, Erben U, Kredel LI, Siegmund B (2015) Diversity of Intestinal Macrophages in Inflammatory Bowel Diseases. Front Immunol 6:613
Lutaty A, Soboh S, Schif-Zuck S et al (2018) A 17-kDa Fragment of Lactoferrin Associates With the Termination of Inflammation and Peptides Within Promote Resolution. Front Immunol 9:644
Kumaran Satyanarayanan S, El Kebir D, Soboh S et al (2019) IFN-beta is a macrophage-derived effector cytokine facilitating the resolution of bacterial inflammation. Nat Commun 10:3471
Villa A, Rizzi N, Vegeto E, Ciana P, Maggi A (2015) Estrogen accelerates the resolution of inflammation in macrophagic cells. Sci Rep 5:15224
Rossi AG, Sawatzky DA, Walker A et al (2006) Cyclin-dependent kinase inhibitors enhance the resolution of inflammation by promoting inflammatory cell apoptosis. Nat Med 12:1056–1064
McGrath EE, Marriott HM, Lawrie A et al (2011) TNF-related apoptosis-inducing ligand (TRAIL) regulates inflammatory neutrophil apoptosis and enhances resolution of inflammation. J Leukoc Biol 90:855–865
Edison N, Reingewertz TH, Gottfried Y et al (2012) Peptides mimicking the unique ARTS-XIAP binding site promote apoptotic cell death in cultured cancer cells. Clin Cancer Res 18:2569–2578
Acknowledgements
We thank Kfir Lapid, Ph.D. (Berlin, Germany) for the professional manuscript writing and editing services. The study was supported by grants from the Israel Science Foundation (Grant No. 678/13 (to A.A) and 822/12 (to S.L), the Rosetetress Trust (to A.A) and The Wolsfon Charitable Trust (to A.A and to S.L), and by a generous grant award from the Hymen Milgrom Trust (to S.L). O.Z.T is a recipient of a presidential scholarship from the University of Haifa.
Author information
Authors and Affiliations
Contributions
NM, ZZZ, PK, OZT, and SSZ performed experiments and analyzed the results. SSZ assisted in planning the experiments. SL and AA planned the experiments, analyzed the data and wrote the manuscript.
Corresponding authors
Ethics declarations
Competing interests:
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Maimon, N., Zamir, Z.Z., Kalkar, P. et al. The pro-apoptotic ARTS protein induces neutrophil apoptosis, efferocytosis, and macrophage reprogramming to promote resolution of inflammation. Apoptosis 25, 558–573 (2020). https://doi.org/10.1007/s10495-020-01615-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-020-01615-3