Abstract
Strong 14-3-3 zeta protein expression plays an important role in tumorigenesis, including in the maintenance of cell growth, resistance increase, and the prevention of apoptosis. In this study, we focus on two targets: (1) the expression of 14-3-3 zeta in the different grades of human astrocytoma (II–IV), (2) suppression of 14-3-3 zeta protein expression in glioblastoma derived astrocytes by 14-3-3 zeta shRNA lentiviral particles. The tissues of human astrocytoma were provided from 30 patients (ten of each grade of astrocytoma). Control tissues were obtained from the peritumoral brain zone of those patients with glioblastoma. The protein and mRNA expression levels of each astrocytoma grade were assessed via western blotting and RT-PCR, respectively. Results indicated that 14-3-3 zeta was significantly expressed in glioblastoma multiforme (grade IV) and 14-3-3 zeta expression levels enhanced according to the increase of astrocytoma malignancy. In the cellular study for knock down of the 14-3-3 zeta protein, surgical biopsy of glioblastoma was used to isolate primary astrocyte. Astrocytes were transduced with 14-3-3 zeta shRNA or non-targeted shRNA lentiviral particles. Furthermore, reduction of the 14-3-3 zeta protein expression in the astrocytes evaluated through qRT-PCR and western blot after transduction of 14-3-3 zeta shRNA lentiviral particles. Moreover, apoptosis properties, including DNA fragmentation and ratio increase of Bax/Bcl-2 were observed in astrocytes following reduction of 14-3-3 zeta protein expression. Further observation indicated that the mitochondrial pathway through release of cytochorome c and caspase-3 activity was involved in the apoptosis induction. Hence, this study demonstrates a key role of the 14-3-3 zeta protein in tumorigenesis but also indicates that 14-3-3 zeta can be considered as a target for the astrocytoma treatment specially glioblastoma.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Astrocytomas are the most common brain tumors that are classified into four grades based on rate of the malignancy including pilocytic astrocytoma (grade I), diffuse astrocytoma (grade II), anaplastic astrocytoma (grade III) and Glioblastoma multiforme (grade IV) according to WHO’s classification in 2016 [1]. Grade 4 astrocytoma, also known as glioblastoma multiforme, is the most malignant type of primary brain tumor. Clinical therapy of glioblastoma is poor in adults and children owing to the failure to transfer chemotherapeutic agents across the blood–brain barrier [2]. Furthermore, metastasis plays a key role in the recurrence and rapid progression of glioblastoma. Also, this type of primary brain tumor is resistant to conventional therapies, including surgery, chemotherapy, radiotherapy, and multidisciplinary treatments [3]. Patients with glioblastomas may survive for about 12 months from the time of diagnosis. Therefore, new and suitable approaches are necessary for understanding the molecular biology of cancer and identifying novel solutions for delivering the tumoricidal agents [4]. Recent studies have reported that the protein 14-3-3 plays an important role in tumorigenesis [5]. Note that 14-3-3 proteins are 28–33 kDa acidic polypeptides that are widely expressed in eukaryotic cells. The 14-3-3 protein has seven isoforms—namely beta, gamma, epsilon, eta, sigma, theta, and zeta—which are expressed by seven specific genes in mammals [6]. There are different isoforms of the 14-3-3 protein in various tissues—for example, in the intestines and liver—but the highest level of 14-3-3 proteins is present in the brain [5]. This protein family plays important roles in intracellular signaling, transcriptional regulation, cellular differentiation and proliferation, cytoskeletal organization, stress signaling, and apoptosis. The functions of the 14-3-3 proteins were performed by binding phospho-serine/threonine motifs on the target protein [7, 8]. It has been observed that expression of the 14-3-3 proteins increased in some human diseases, specifically in some types of cancer. In addition, it is possible to increase expression of the 14-3-3 zeta protein in stomach cancer [9], breast cancer [10], oral squamous cell carcinomas [11] and glioblastoma multiforme [12]. This indicated that 14-3-3 zeta protein lead to primary tumorigenesis in the central nervous system. Overexpression of 14-3-3 may reduce tumor cell apoptosis and this function is useful for survival and proliferation of tumor cells [13]. So, novel therapeutic approaches could be designed to tackle glioma treatment via inhibition of the 14-3-3 protein action. This would mean triggering apoptosis, inhibiting tumor growth, and stopping the formation of new tumors. To evaluate this hypothesis, we designed a method that delivers the 14-3-3 zeta shRNA lentiviral particles in primary astrocytes isolated from human glioblastoma in order to decrease the gene expression of 14-3-3 zeta in these cells. This method has indicated that cells may act to efficiently suppress 14-3-3 zeta protein expression through mRNA fragmentation into astrocyte cytoplasm [14]. Finally, down-regulation of the 14-3-3 zeta protein increases the sensitivity to apoptosis in the primary astrocytes. Small hairpin RNA (shRNA) has therapeutic potential via specific gene silencing [15].
Materials and methods
Experimental protocol
There were three collections of experiments: (1) preparation of the human astrocytoma tissue and evaluation of the 14-3-3 zeta expression in grades II–IV of human astrocytoma. (2) Isolation and culture of the astrocytes from human glioblastoma tissue (3) transduction of the astrocytes with produced lentivirus containing 14-3-3 zeta shRNA and control shRNA lentiviral particles and assessment of the 14-3-3 zeta expression into astrocytes. Cells were classified into three groups: Group A, control group (untransduced astrocytes); Group B, lentiviral particles containing reporter gene of green fluorescence protein (GFP) group (astrocytes were transduced with control shRNA lentiviral particles); Group C, 14-3-3 zeta shRNA lentiviral particles-transduced astrocytes.
Human astrocytoma tissues preparation
This study was approved by the local ethic committee of the Shahid Beheshti University of Medical Sciences, Tehran, Iran before we can start our study. An informed consent form was signed by patients for participation in this study. All samples were obtained from patients with astrocytoma admitted to the Shohada Tajrish Hospital between 2016 and 2017. Asrocytoma tissues were gathered in the condition of tumor-related neurosurgical operation. Primitive therapy of these patients was surgery without chemotherapy or radiation treatment. Tissues were from 30 patients (12 females and 18 males; median age, 45 years; ten sample of Grade II, Grade III and Grade IV, respectively). According to study of Lemée et al. [16], glioblastoma peritumoral brain zone from 10 patients was obtained as control tissue. Astrocytoma tissues were frozen immediately in liquid nitrogen (− 180 °C) after surgery. The histopathological diagnosis and determination of the malignant grade of the astrocytoma tissues were by three neuropathologists separately based on the classification criteria of astrocytoma tumors by world health organization in 2016 [1]. Histological abnormalities were not observed in the pathology report for the control brain tissues (glioblastoma peritumoral brain zone). All samples were assessed by RT-PCR and western blotting.
Isolation and culture of astrocye cells from human glioblastoma multiforme tumor
According to results of astrocytoma tissues, we considered glioblastoma for the cellular study owing to the highest expression levels of 14-3-3 zeta. Sample was provided from patients with glioblastoma under surgical resection. Glioblastoma multiforme was approved using pathological test. The isolation of primary astrocyte from glioblastoma was achieved according to a previously explained protocol by Hashemi et al. [17, 18]. Sample was digested with 0.05% trypsin–EDTA for 10 min at 37 °C water bath. After centrifugation at 180×g for 5 min, cells re-suspended in DMEM/F12 medium containing 1% (100 U/ml) antibiotic/antimycotic and 2% FBS and incubated in 5% CO2 at 37 °C. The amount of FBS was gradually increased from 2 to 10% in culture medium for 2 weeks. Astrocytic antigen of S100-beta was evaluated using immunocytochemistry.
Immunocytochemistry
Astrocytes (density of 5 × 104 per well) were seeded in a 24-well culture plate. After 24 h, three main stages including cell fixation with 4% paraformaldehyde in PBS for 20 min, permeabilization of the cell membrane with 0.2% triton X-100 for 15 min, blocking with 10% albumin bovine serum for 1 h were performed, respectively. The cells were incubated with primary antibody of rabbit anti-S100-beta (1:100; cat number: HPA015768, Sigma-Aldrich, USA), rabbit polyclonal 14-3-3 zeta primary antibody (1:200; cat number: sc-1019, Santa Cruz Biotechnology Inc., USA) overnight at 4 °C. Cells were incubated with secondary antibody of fluorescein isothiocyanate (FITC)-conjugated goat anti-rabbit (1:200; cat number: ab6717, Abcam, USA) for 2 h at room temperature in the dark. Each well was washed three times with phosphate-buffered saline (PBS) at every stage except the blocking stage. Finally, protein expression was evaluated using an inverted fluorescence microscopy.
Transduction of astrocytes with lentivirus containing 14-3-3 zeta shRNA
Astrocytes were seeded with density of 1 × 106 per well on a six-well dish and incubated at 37 °C, 5% CO2 for 24 h. Cells were 50% confluent on the day of transduction. Culture medium of the cell was changed with culture medium containing polybrene (5 µg/ml, sc-134220). Two coding regions from the human 14-3-3 zeta gene were selected as shRNA target sequences. We had two 14-3-3 zeta shRNA-carrying lentiviral vectors: LV-14-3-3 zeta shRNA1 and LV-14-3-3 zeta shRNA2. Control shRNA lentiviral was used as a negative control. The shRNA sequences were 14-3-3 zeta: 5′-AGCCTGCATGAAGTCTGTAACTGAGCAAG-3′, 5′-ACTACCGTTACTTGGCTGAGGTTGCCGCT-3′ and Control. GFP: 5′-CACAAGCTGGAGTACAACTACAACAGCCA-3′. Cells were transduced with five multiplicity of infection (MOI) LV-14-3-3 zeta shRNA1 and LV-14-3-3 zeta shRNA2 particles (TL308321V, Origene, China) or control shRNA lentiviral particles (TR30021V, Origene, China) and incubated at 37 °C, 5% CO2 overnight. Then, the culture medium replaced with 2 ml of complete medium (without polybrene) and the cells were incubated for 24 h. Culture Medium changed with fresh medium containing puromycin dihydrochloride (5 µg/ml, sc-108071) and incubated for 72 h for selection of the stable cells expressing the 14-3-3 zeta shRNA. Transduction efficiency was observed for GFP expression in astrocytes by inverted fluorescent microscope.
Tumoricidal effect of shRNA 14-3-3 zeta in astrocytes
Tumoricidal property of astrocyte-control shRNA lentiviral particles or astrocyte-14-3-3 zeta shRNA lentiviral particles was assessed in this stage. Astrocytes were cultured with a total seeding density of 4 × 103 in each well of 96-well cell culture plate for 7 days. Survival rate of cell was evaluated by MTT test after 7 days.
Apoptosis assay
TUNEL staining was achieved according to various groups in the experimental design. Astrocyte-control shRNA lentiviral particles or astrocyte-14-3-3 zeta shRNA lentiviral particles were cultured for 7 days. Following, identification of the apoptotic cells was assessed using in situ cell death detection kit (Roche, Mannheim, Germany) terminal uridine deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining. Test was done according to the manufacturer’s protocol. Briefly, cells were fixed by 4% paraformaldehyde for 10 min. Later stage, cells were permeabilized with 0.2% triton X-100 for 2 min on ice, and finally cells incubated with a mixture of TUNEL reaction for 1 h. Cells were exposed with 5% ethanol for positive apoptosis control. Cells were induced only with label solution for negative apoptosis control. TUNEL-positive cells were observed under a fluorescent microscope (Olympus AX-70; Tokyo, Japan).
Quantitative real-time RT-PCR
Astrocytes (density of 1 × 106 per well) were seeded in a six-well culture plate for 7 days. After 7 days, TriPure Isolation Reagent (Roche, Germany) was used to isolate total RNA of frozen astrocytoma tissues and cultured cells. For first-strand cDNA synthsis, 1 µg of each RNA sample was added to reverse transcriptase (Takara, Japan) with Random Hexamer, Oligo DT and RNase inhibitor and reaction was programmed using a thermal cycler for 5 min at 85 °C and 15 min at 37 °C. RT-PCR was achieved using SYBR Premix Ex Taq II (Tli Plus) (Takara, Japan) according to the manufacturer’s instructions through the qRT-PCR detection system (Applied Biosystem, one step, RT-RCP Germany). The primer sequences of the PCR product for BAX was forward primer (F) 5′-CAAACTGGTGCTCAAGGC-3′ and reverse primer (R) 5′-CACAAAGATGGTCACGGTC-3′ and for Bcl-2 was (F) 5′-GTACTTAAAAAATACAACATCACAG-3′ and (R) 5′-CTTGATTCTGGTGTTTCCC-3′, for 14-3-3 zeta was (F) 5′-AGCTGGTTCAGAAGGCCAAA-3′ and (R) 5′-AAGATGACCTACGGGCTCCT-3′ and for housekeeping Hypoxanthine Phosphoribosyltransferase1 (HPRT1) was (F) 5′-CCTGGCGTCGTGATTAGTGA-3′ and (R) 5′-AAGACGTTCAGTCC TGTCCAT-3′. All qRT-PCR reactions were assessed in triplicate. Gene expression levels were calculated according to the ∆∆Ct method.
Western blotting
Astrocytes (density of 1 × 106 per well) were seeded in a six-well culture plate for 7 days. Frozen astrocytoma tissues and cultured cells were homogenized with RIPA lysis buffer. 20 µl of lysate containing 30 µg protein loaded in 12% SDS–polyacrylamide gel electrophoresis (PAGE). Stages of electroblotting by wet blotting system and blocking of membrane in 5% non-fat milk in TBST for an hour was done respectively. Membrane incubated with rabbit polyclonal 14-3-3 zeta primary antibody (1:200; cat number: sc-1019, Santa Cruz Biotechnology Inc., USA), rabbit polyclonal β-actin primary antibody (1:200; cat number: sc-10731, Santa Cruz Biotechnology Inc., USA), rabbit polyclonal Bax primary antibody (1:200; cat number: sc-493, Santa Cruz Biotechnology Inc., USA), rabbit polyclonal Bcl-2 primary antibody (1:200; cat number: sc-492, Santa Cruz Biotechnology Inc., USA) and rabbit polyclonal activated caspase 3 primary antibody (1:200; cat number: sc-98785, Santa Cruz Biotechnology Inc., USA) overnight at 4 °C. After three washes with TBS-T, the membrane incubated with HRP conjugated anti-goat IgG and HRP-conjugated anti-rabbit IgG (1:10,000; cat number: sc-2004, Santa Cruz Biotechnology Inc., USA) for 1 h in room temperature in the dark. After three washes with TBS, protein was appeared using chemiluminescence kit (Amersham Biosciences, Orsay, France) on an X-ray film after 10 s exposure.
Primary astrocytes mitochondrial function
Primary astrocytes mitochondrial isolation
The cultured cells were scraped and homogenized by homogenizer, and then cell mitochondria were gathered by differential centrifugation [19]. The first centrifugation was performed at 600×g, 5 min at 4 °C, then the supernatant was removed and the second centrifugation done at 10,000×g, 5 min at 4 °C. After aspirating the supernatant, the mitochondrial pellet was combined in the isolation medium and the final centrifugation was performed at 10,000×g, 5 min 4 °C. The final mitochondrial pellets were suspended in respiration buffer (0.32 mM sucrose, 10 mM Tris, 20 mM Mops, 50 µM EGTA, 0.5 mM MgCl2, 0.1 mM KH2PO4 and 5 mM sodium succinate), MMP assay buffer (220 mM sucrose, 68 mM d-mannitol, 10 mM KCl, 5 mM KH2PO4, 2 mM MgCl2, 50 µM EGTA, 5 mM sodium succinate, 10 mM HEPES, 2 µM Rotenone) and swelling buffer (70 mM sucrose, 230 mM mannitol, 3 mM HEPES, 2 mM Tris–phosphate, 5 mM succinate and 1 µM of rotenone). Protein concentrations were determined via the Coomassie blue protein-binding protocol.
Measurement of primary astrocytes mitochondrial radical oxidative species level
Astrocyte mitochondrial hydrogen peroxide and superoxide levels were evaluated using dichloro-dihydro-fluorescein diacetate (DCFH-DA) and dihydroethidum (DHE), respectively. Briefly, isolated mitochondria in respiration buffer were incubated with DCFH-DA and DHE (final concentration, 10 µM) for 10 min. Finally, dichlorofluorescin (DCF) and DHE fluorescence intensity that produced in the mitochondria is proportional to hydrogen peroxide and superoxide levels and was measured using a fluorescence spectrophotometer at an excitation and emission wavelength of 488 and 540 nm, respectively.
Measurement of primary astrocytes mitochondrial membrane potential
The mitochondrial membrane potential (MMP) was assessed using the mitochondria-specific cell-permeant cationic fluorescence dye rhodamine 123. rhodamine 123 easily absorbed and accumulated in the mitochondrial membrane. A suspension of mitochondrial fractions was prepared in an MMP buffer which contains 220 mM sucrose, 68 mM d-mannitol, 10 mM KCl, 5 mM KH2PO4, 2 mM MgCl2, 50 µM EGTA, 5 mM sodium succinate, 10 mM HEPES, 2 µM Rotenone and was further treated with 10 µM rhodamine 123 for 60 min. Using a fluorescence spectrophotometer, the fluorescence intensity was read with excitation of 490 nm and emission of 520 nm.
Measurement of primary astrocytes mitochondrial swelling
In order to estimate the mitochondrial swelling, the mitochondrial fractions were suspended in a swelling buffer which contains 70 mM sucrose, 5 mM succinate, 2 mM Tris-phosphate, 3 mM HEPES, 230 mM mannitol and 1 µM rotenone. Changes in light scattering recorded at 540 nm [17]. The absorbance was read spectrophotometrically every 10 min using an ELISA reader (Biotek 5, USA). The intensity of absorbance is reversely proportional to the mitochondrial swelling rate.
Determination of cytochrome c release
Cytochrome c release from astrocyte’s mitochondria was assessed using a standard ELISA kit (R and D Systems Inc. Minneapolis, MN, USA). The 96-well microplate provided in the kit was precoated with a cytochrome c specific monoclonal antibody. Initially, 75 µl of Horseradish peroxidase (HRP) conjugated monoclonal antibody specific for cytochrome c and 50 µl of the sample, standard or control were added into each well and mixed gently prior to 2 h incubation. Then, each well was aspirated once and washed four times to remove any residual liquid followed by adding 100 µl of the substrate solution (Hydrogen peroxidase plus Tetramethylbenzidine) into the wells prior to 30 min of incubation. Finally, 100 µl of the stop solution was added and the absorbance was read using a microplate reader at 450 nm.
Statistical analysis
All data are demonstrated as mean ± standard error of the mean. All tests are repeated in the three independent experiments. The statistical analysis of data was carried out using SPSS 20 statistical software using paired t-test and one-way analysis of variance (ANOVA) followed by Tukey’s test. P values less than 0.05 were regarded statistically significant difference.
Results
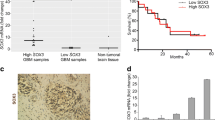
The expression levels of 14-3-3 zeta detected in the human astrocytoma
In this study, the expression levels of 14-3-3 zeta in the different grades of astrocytoma were indicated by western blotting and qRT-PCR. There were same results in the mRNA and protein expression levels of 14-3-3 zeta regarding to malignancy grades. mRNA and protein expression levels of 14-3-3 zeta were increased with the grade malignancy increase of astrocytoma. Increased expression of 14-3-3 zeta was observed in the glioblastoma (grade IV) than control tissue and astrocytoma of low grade (I and II). In next part of the study, we selected glioblastoma (grade IV) owing to over-expression of 14-3-3 zeta and following protein expression of 14-3-3 zeta was controlled through 14-3-3 zeta shRNA lentiviral particles in the glioblastoma derived primary astrocytes (Fig. 1a, b).
Morphology and confirmation of astrocytes isolated from human glioblastoma multiforme tumor
Astrocytic cells were isolated and cultured from a sample of the human glioblastoma. The cells were observed to be of two types: epithelioid (large, with an abundance of cytoplasm and interconnecting processes) and fibroblastic (slender, elongated, and spindle-shaped) (Fig. 2a). The immunofluorescence technique demonstrated that the majority of cells expressed S100-beta protein, suggesting high purity of the astrocyte in the culture (Fig. 2b). Expression of the 14-3-3 zeta protein was evaluated using immunocytochemistry in astrocytes, indicating that the 14-3-3 zeta protein was highly expressed in astrocytes isolated from glioblastoma (Fig. 2c).
Morphology and antigens expression of S100-beta and 14-3-3 zeta in astrocytes derived from human glioblastoma multiforme. Astrocytes isolated from human glioblastoma after 5 days culture (a). Astrocytes were positive to glial marker of S100-beta (b). Astrocytes expressed malignant marker of 14-3-3 zeta (c) (scale bar 150 µm)
Efficiency of 14-3-3 zeta shRNA lentiviral particles transduction in astrocytes
Expression level of GFP in the cells was regarded as a marker for the transduction efficiency. Transduction efficiency was observed high in astrocytes according to GFP expression rate. Moreover, transduction efficiency was enhanced through selection of puromicin-resistant astrocytes. The results demonstrated that 14-3-3 zeta shRNA lentiviral particles transduced to astrocytes, suggesting that the astrocytes carried 14-3-3 zeta shRNA. Also, puromycin was added to the culture medium to increase the purity of astrocytes transducted to the 14-3-3 zeta shRNA lentiviral particles (Fig. 3).
Tumoricidal effect of 14-3-3 zeta shRNA in astrocytes
In order to test our strategy that 14-3-3 zeta-targeting shRNA can be affected astrocytes survival, we transduced astrocytes with 14-3-3 zeta shRNA and following this up with an MTT analysis. Transduction of the 14-3-3 zeta shRNA1 and 14-3-3 zeta shRNA2 into astrocytes led to a significant decrease in the survival rate compared to the astrocytes containing control shRNA lentiviral particles group. However, no significant difference was observed between astrocytes containing control shRNA lentiviral particles group and un-transduced astrocytes (control group). These results suggest that 14-3-3 zeta shRNA inhibited astrocyte viability (Fig. 4).
Tumoricidal effect of astrocytes depends on 14-3-3 zeta shRNA lentiviral particles. Survival rate of astrocytes decreased when transduced to 14-3-3 zeta shRNA. Viability of astrocytes was evaluated as percent absorbance of control cells. **P < 0.01, ***P < 0.001, significant intergroup differences versus control
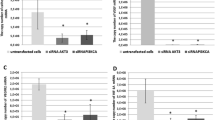
Protein and mRNA expression levels of 14-3-3 zeta
The mRNA and protein expression of 14-3-3 zeta was assessed by qRT-PCR and western blot, respectively. Suppression of 14-3-3 zeta mRNA and protein expression using 14-3-3 zeta shRNA1 and 14-3-3 zeta shRNA2 also observed markedly relative to the control in astrocytes. However, 14-3-3 zeta shRNA1 was demonstrated significant decrease in the 14-3-3 zeta expression. Therefore, 14-3-3 zeta shRNA1 was selected for subsequent experiments of study. Results have confirmed that shRNA 14-3-3 zeta can be silenced 14-3-3 zeta protein into astrocyte (Fig. 5).
The mRNA expression and protein of 14-3-3 zeta. Knock down effect of 14-3-3 zeta in astrocytes transduced with 14-3-3 zeta targeting shRNA lentiviral particles or transduced with non-targeting shRNA was evaluated via A qPCR analysis and B bands indicate a astrocyte, b astrocyte-control shRNA lentiviral particles, c astrocyte-14-3-3 zeta shRNA1, d astrocyte-14-3-3 zeta shRNA2, respectively. *P < 0.05, **P < 0.01, ***P < 0.001, differences versus control
Apoptosis induction in astrocytes by 14-3-3 zeta shRNA1
To confirm the apoptosis induction in astrocytes containing 14-3-3 zeta shRNA1 or astrocyte-control shRNA lentiviral particles, apoptosis was carried out using a TUNEL kit. Apoptotic cells were observed in the group of astrocyte containing 14-3-3 zeta shRNA1. However, apoptosis was not observed in the astrocytes containing control shRNA lentiviral particles group. As a result, 14-3-3 zeta shRNA1 seemed to reduce intracellular 14-3-3 zeta expression following induced apoptosis in the astrocytes (Fig. 6).
Gene and protein expression levels of apoptotic markers in astrocytes
To determine the molecular mechanism that underlines the apoptosis in astrocytes transduced to 14-3-3 zeta shRNA1 lentiviral particles, we looked at apoptosis regulator proteins, including Bcl-2, Bax, and caspase 3, that play roles in the integration of cellular survival and apoptosis signal on the mitochondria function. We hypothesized that the 14-3-3 zeta protein levels might have increased tumor cell survival and that, in turn, 14-3-3 zeta shRNA1 could reduce cell survival and induce apoptosis. To test this hypothesis, we evaluated the mRNA and protein expression levels of survival (Bcl-2) and apoptotic (Bax, caspase3) factors in this family. The results indicated that the mRNA and protein expression of Bcl-2 reduced but the mRNA and protein expression of Bax and protein expression of activated caspase 3 increased in the astrocytes containing 14-3-3 zeta shRNA1 group compared to the astrocytes containing control shRNA lentiviral particles group (Fig. 7A, B).
The mRNA and proteins expression of anti-apoptotic and apoptotic in the astrocytes. A Quantitative analysis of Bax/Bcl-2 ratio in the various groups. B Using western blot analysis, apoptotic proteins rate were evaluated in cell culture. The densities of Bax, Bcl-2 (B1), and activated caspase-3 (B2). Bands indicate a astrocyte, b astrocyte-control shRNA lentiviral particles, c astrocyte-14-3-3 zeta shRNA1, respectively. ***P < 0.001, differences versus control
Primary astrocyte mitochondrial function
We hypothesized up-regulation of the 14-3-3 zeta expression can inhibit stress-induced apoptosis, whereas down-regulation of 14-3-3 zeta expression seem to induce apoptosis through the mitochondrial pathway. To assess the mitochondrial apoptotic pathway involved in glioblastoma astrocytes transduced to 14-3-3 zeta shRNA1 lentiviral particles, main indicators of mitochondrial apoptotic pathway including ROS production including hydrogen peroxide and superoxide anion, inner membrane potential, swelling and cytochrome c release of the mitochondrial were investigated. The main effect of 14-3-3 zeta shRNA1 through down-regulation of 14-3-3 zeta expression leads to intracellular stress and following mitochondrial hydrogen peroxide and superoxide anion production (Fig. 8a). Conversely, mitochondrial hydrogen peroxide and superoxide anion production was low in the control groups including astrocytes and astrocytes transduced to shRNA control lentiviral particles. Mitochondrial membrane potential (MMP) is an index of the mitochondrial inner membrane condition. The results of this study indicated that 14-3-3 zeta shRNA1 lead to significant increase of collapse of the mitochondrial inner membrane in astrocytes (Fig. 8b). Mitochondrial swelling is a sign of mitochondrial membrane permeability. The main effect of 14-3-3 zeta shRNA1 was significant for mitochondrial swelling in astrocytes (Fig. 8c). Change of the mitochondrial inner membrane potential and swelling leads to Instability of mitochondrial membrane and following cytochrome c release (Fig. 8d). Furthermore, cytochrome c release can trigger cell death signaling cascade including caspases.
The effect of 14-3-3 zeta knock down on astrocyte mitochondrial factors. a ROS formation, b mitochondrial membrane potential (MMP) collapse, c progressive mitochondrial swelling, d cytochrome c release from astrocyte mitochondria to cytosol. **P < 0.01, ***P < 0.001, significant differences versus control
Discussion
The main purposes within this study were the expression assessment of 14-3-3 zeta in the different grades of human astrocytoma ((II–IV), 2) and decrease of 14-3-3 zeta protein expression in glioblastoma derived astrocytes by 14-3-3 zeta shRNA1 lentiviral particles. To the improve of our knowledge, this is the first study that focused on 14-3-3 zeta protein in primary astrocytes isolated from glioblastoma and apoptotic pathways in this cells following knock down of the 14-3-3 zeta protein. However, different studies have been used homogenous glioma cell lines that are not suitable for brain tumor studies owing to malignant gliomas have a great cellular heterogeneity [20, 21]. We presented that expression levels of 14-3-3 zeta were significantly correlated with grades pathologic whereas, the highest expression level of 14-3-3 zeta was observed in glioblastoma (grade IV). As has been previously reported, we observed 14-3-3 zeta protein within glioblastoma derived astrocytes by immunocytochemistry [21]. We were considered peritumoral brain zone samples as control samle according to study of Lemée et al. [16] that have been reported that peritumoral brain zone samples are the most suitable control sample. Taken together, 14-3-3 zeta can be considered as a potential oncogenesis marker with clinical significance for glioblastoma patients (14-3-3 zeta). Oncogenes disrupt the balance between cell survival and apoptosis through apoptosis inhibition in cancer cells, which increases the resistance of the cancer cells to different treatments [22, 23]. So, a therapeutic approach is formed through identification of cellular oncogenes and apoptosis control in cancer cells. The inhibition of 14-3-3 zeta functions could be performed through the use of a shRNA approach in order to increase of the sensitivity of astrocyte to apoptosis [20]. We hypothesized that 14-3-3 zeta shRNA1 should increase the sensitivity of astrocytes to apoptosis and inhibit the growth and proliferation of astrocytes. Our findings indicated that 14-3-3 zeta can suppress the 14-3-3 zeta protein and induce apoptosis through the mitochondrial apoptotic pathway. It seems that Bax molecules are bound to 14-3-3 proteins in the cell cytoplasm. However, Bax separates from 14-3-3 protein in response to 14-3-3 down-regulation and transfers to the mithochondria periphery, indicating start of the apoptosis induction [24, 25]. Following down-regulation of 14-3-3 zeta, our observations revealed change in membrane potential of mitochondria, ROS production, expression increase of the Bax, permeability increase of mitochondria membrane, release of cytochrome c and activation of the caspase 3. Furthermore, DNA fragmentation, increase of the Bax/Bcl2 ratio, and caspase3 activation are apoptosis properties that we observed in our study parallel to the study of Cao et al. [20]. However, studies indicated that non-target effects problem was mediated by partial sequence complementarity of either the sense or antisense siRNA strands to non-target mRNAs, activation of the interferon response pathways, immune responses and cellular toxicities caused by the nucleotide construct [26].
Conclusion
In conclusion, the results suggest that the up-regulated expression of 14-3-3 zeta protein in astrocytoma according to malignancy grade. The most malignant form of astrosytoma was observed glioblastoma multiforme (grade IV). Furthermore, 14-3-3 zeta shRNA can suppresse 14-3-3 zeta function to sensitize and induce apoptosis in astrocytes isolated from glioblastoma. We also propose that 14-3-3 zeta protein seem to be a promising marker for astrocytoma diagnosis and targets as a novel idea for the treatment of astrocytoma in future.
References
Louis DN, Perry A, Reifenberger G, Deimling AV, Branger DF, Cavenee WK et al (2016) The 2016 World Health Organization classification of tumors of the central nervous system: a summary. Acta Neuropathol 131:803–820
Kleihues P, Louis DN, Scheithauer BW, Rorke LB, Reifenberger G, Burger PC, Cavenee WK (2002) The WHO classification of tumors of the nervous system. J Neuropathol Exp Neurol 61:215–225
Giese A, Bjerkvig R, Berens ME, Westphal M (2003) Cost of migration: invasion of malignant gliomas and implications for treatment. J Clin Oncol 21:1624–1636
Wen PY, Kesari S (2008) Malignant gliomas in adults. N Engl J Med 359:492–507
Cao WD, Zhang X, Zhang JN, Yang ZJ, Zhen HN, Cheng G et al (2006) Immunocytochemical detection of 14-3-3 in primary nervous system tumors. J Neuro-Oncol 77:125–130
Van Hemert MJ, Steensma HY, van Heusden GP (2001) 14-3-3 proteins: key regulators of cell division, signaling and apoptosis. Bioessays 23:936–946
Tzivion G, Avruch J (2002) 14-3-3 proteins: active cofactors in cellular regulation by serine/threonine phosphorylation. J Biol Chem 277:3061–3064
Van Heusden GP (2005) 14-3-3 proteins: regulators of numerous eukaryotic proteins. IUBMB Life 57:623–629
Nishimura Y, Komatsu S, Ichikawa D, Nagata H, Hirajima S, Takeshita H, Kawaguchi T, Arita T et al (2013) Overexpression of YWHAZ relates to tumor cell proliferation and malignant outcome of gastric carcinoma. Br J Cancer 108:1324–1331
Neal CL, Yao J, Yang W, Zhou X, Nguyen NT, Lu J, Danes CG, Guo H et al (2009) 14-3-3 zeta overexpression defines high risk for breast cancer recurrence and promotes cancer cell survival. Cancer Res 69:3425–3432
Matta A, Masui O, Siu KW, Ralhan R (2016) Identification of 14-3-3 zeta associated protein networks in oral cancer. Proteomics 16:1079–1089
Cao L, Cao W, Zhang W, Lin H, Yang X, Zhen H, Cheng J, Dong W et al (2008) Identification of 14-3-3 protein isoforms in human astrocytoma by immunohistochemistry. Neurosci Lett 432:94–99
Rapp UR, Fischer A, Rennefahrt UE, Hekman M, Albert S (2007) BAD association with membranes is regulated by Raf kinases and association with 14-3-3 proteins. Adv Enzyme Regul 47:281–285
Wolvetang EJ, Pera MF, Zuckerman KS (2007) Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun 363:610–615
Xu Y, Linde A, Larsson O, Thormeyer D, Elmen J, Wahlestedt C, Liang Z (2004) Functional comparison of single- and double-stranded siRNAs in mammalian cells. Biochem Biophys Res Commun 316:680–687
Lemée JM, Com E, Clavreul A, Avril T, Quillien V, de Tayrac M et al (2013) Proteomic analysis of glioblastomas: what is the best brain control sample? J Proteom 24:165–173
Hashemi M, Hadjighassem MR (2017) Preparation of primary astrocyte culture derived from human glioblastoma multiforme specimen. Bio-protocol 7:1–9
Hashemi M, Fallah A, Aghayan HR, Arjmand B, Yazdani N, Verdi j, Ghodsi SM, Miri SM, Hadjighassem M (2016) A new approach in gene therapy of glioblastoma multiforme: human olfactory ensheathing cells as a novel carrier for suicide gene delivery. Mol Neurobiol 53:5118–5128
Frezza C, Cipolat S, Scorrano L (2007) Organelle isolation: functional mitochondria from mouse liver, muscle and cultured filroblasts. Nat Protoc 2:287–295
Cao W, Yang X, Zhou J, Teng Z, Cao L, Zhang X, Fei Z (2010) Targeting 14-3-3 protein, difopein induces apoptosis of human glioma cells and suppresses tumor growth in mice. Apoptosis 15:230–241
Yang X, Cao W, Lin H, Zhang W, Lin W, Cao L et al (2009) Isoform-specific expression of 14-3-3 proteins in human astrocytoma. J Neurol Sci 276:54–59
Fulda S (2009) Apoptosis pathways and neuroblastoma therapy. Curr Pharm Des 15:430–435
Fulda S (2009) Tumor resistance to apoptosis. Int J Cancer 124:511–515
Nomura M, Shimizu S, Sugiyama T, Narita M, Ito T, Matsuda H, Tsujimoto Y (2003) 14-3-3 interacts directly with and negatively regulates pro-apoptotic Bax. J Biol Chem 278:2058–2065
Lee YK, Hur W, Lee SW, Hong SW, Kim SW, Choi JE (2014) Knockdown of 14-3-3ζ enhances radiosensitivity and radio-induced apoptosis in CD133+ liver cancer stem cells. Exp Mol Med 46:e77
Hannus M, Beitzinger M, Engelmann J, Weickert M, Spang R, Hannus S et al (2014) siPools: highly complex but accurately defined siRNA pools eliminate off-target effects. Nucleic Acids Res 42:8049–8061
Funding
This study was funded by functional neurosurgery research center, Shahid Beheshti University of medical sciences (Grant Number: 11974).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in study according to the Ethical Commission of the Shahid Beheshti University of Medical Sciences. Ethical code of study is IR.SBMU.RETECH.REC.1396.744.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Hashemi, M., Zali, A., Hashemi, J. et al. Down-regulation of 14-3-3 zeta sensitizes human glioblastoma cells to apoptosis induction. Apoptosis 23, 616–625 (2018). https://doi.org/10.1007/s10495-018-1476-5
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-018-1476-5