Abstract
Transglutaminase 2 (TG2) is a multi-functional protein that has both protein cross-linking and guanosine 5′-triphosphate (GTP) hydrolysis activities. The activities of this protein are controlled by many cellular factors, including calcium (Ca2+) and GTP, and have been implicated in several physiological activities, including apoptosis, angiogenesis, wound healing, cellular differentiation, neuronal regeneration, and bone development. TG2 is linked to many human diseases such as inflammatory disease, celiac disease, neurodegenerative disease, diabetes, tissue fibrosis, and various cancers and is one of the most dynamic enzymes in terms of its functions, structures, and regulatory mechanisms. The aim of this review was to summarize the functional, structural, and regulatory diversity of TG2, with a particular focus on the structure of TG2.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Transglutaminase 2 (TG2), also known as tissue transglutaminase, is a multi-functional protein containing both protein cross-linking and guanosine 5′-triphosphate (GTP) hydrolysis activities [1, 2]. This protein can also function as a protein disulfide isomerase [3], protein kinase [4, 5], and as a scaffolding factor [6] (Fig. 1a). Protein cross-linking activity by transamidation, one of the main functions of TG2, is regulated positively by calcium (Ca2+) and negatively by GTP (Fig. 1a) [7]. TG2 is ubiquitously found in the cell, while other TG isoenzymes activated at specific cell types (for example TG1 (skin), TG3 (skin), TG4 (prostate), TG5 (skin), TG6 (unclear), TG7 (testis and lung)) [2].
Functional diversity of transglutaminase 2 (TG2). a TG2 is multifunctional protein, possessing transamidation activity (crosslinking, deamination, and polyamine incorporation), GTP hydrolysis activity, protein disulfide isomerase (PDI) activity, protein kinase activity, and scaffolding activity. The transamidation activity of TG2 is regulated positively by Ca2+ and negatively by GTP. b Transamidation reaction mechanism of TG2. Reaction is started by a nucleophilic attack by the thiol group at catalytically active cysteine on TG2. Second nucleophilic attack from the amine donor transforms the thioester intermediate to complete the reaction
TG2 is localized both intracellularly and extracellularly, and in some cell types, such as neuroblastoma cells, the enzyme is also found in the nucleus [8]. In the cytosol, TG2 functions as a signal transfer molecule that transmits a receptor signal to an effector by binding GTP and hydrolyzing it to guanosine diphosphate (GDP) and inorganic phosphate (Pi) [9]. When secreted outside the cell, TG2 functions as a cross-linking enzyme in the matrix in a Ca2+-dependent manner [10]. TG2 activity has been implicated in various physiological activities, including apoptosis [11,12,13], angiogenesis [14, 15], wound healing [15, 16], cellular differentiation [17, 18], neuronal regeneration [19], and bone development [20, 21]. This protein has also been linked to many human diseases such as inflammatory disease [22], celiac disease [23], neurodegenerative disease [24, 25], diabetes [26], tissue fibrosis [27], and cancers [28,29,30].
Several reports show that TG2 is downregulated in aggressive tumors and metastases [29, 31]. Transfection and expression of recombinant TG2 in several tumor cells led to significant reduction in tumor incidence and progression [14, 32]. On the other hands, elevated TG2 expression in many types of cancer cells, including pancreatic and breast cancer cells, was reported [33, 34]. Several mutations on TG2 that impair its activities have been found in the early stage of type 2 diabetes [26]. Overexpression of TG2 in the pathogenic stage of neurodegenerative diseases, such as Alzheimer’s disease (AD), causes cross-linking of several AD-related proteins, including tau, Aβ, and α-synuclein. Cross-linking of these proteins causes their accumulation, which has been linked to long-term chronic stimulation and neurodegeneration in AD [35,36,37,38]. TG2 is a biologically and therapeutically important target for the treatment of the aforementioned diseases. Several small molecules and peptidomimetics that can inhibit TG2 activities have been developed and used for treatment.
The aim of this review is to summarize the functional, structural, and regulatory diversity of TG2, with a particular focus on its structure. We will discuss various functions, dynamic conformational changes, and regulatory mechanisms that control the activities of multi-functional TG2. This review might provide a better understanding of TG2’s functions and activity control mechanisms, and could lead to the identification of new inhibitor target sites to develop the next generation of TG2 inhibitors.
Functional diversity of TG2
Transamidation activity
Among the many activities of TG2, post-translational modification of target proteins by cross-linking, deamination, and amine incorporation, are the main functions of TG2. TG2 can deamidate the γ-carboxamide group of specific glutamine residues on a protein during the cross-linking process with an ε-amino group of a lysine residue. The cysteine thiol at the active site of TG2 is used as a nucleophile for the transamidation process (Fig. 1b). The thiol group attacks the carboxamide of a glutamine residue to form a thioester intermediate. This intermediate is then attacked by the ε-amino group of a lysine residue, producing a stable isopeptide bond that cross-links the two substrates (Fig. 1b). TG2 can also convert glutamine to glutamic acid via a deamination reaction, which removes the γ-carboxamide group of specific glutamine residues, replacing it with water. In vitro, this deamination activity was shown by the deamination of gliadin A, which is a component of wheat and a main factor of celiac disease. The transamidation activity of TG2 is positively regulated by Ca2+ and negatively by GTP [7].
GTPase activity
Under low Ca2+ concentration conditions, TG2 maintains a closed form and acts as a GTPase, indicating that it functions as a G protein, participating in signaling processes [39]. The GTPase activity of TG2 is involved in α1-adrenergic receptormediated transmembrane signaling with phospholipase Cδ in the liver and mediates the proliferation of hepatic cells [40].
Protein disulfide isomerase activity
The protein disulfide isomerase (PDI) activity of TG2 was initially reported in a study of the denaturation and renaturation of RNase A [3]. Reduced, denatured RNase A could be transformed into the native active form by TG2. The PDI activity of TG2 is not affected by the presence of Ca2+ and GTP. The cysteine residue in the active site is not important for the PDI activity of TG2, indicating that the domain responsible for the PDI activity is not related to the active site of the transaminase activity. More recently, another substrate for the PDI activity of TG2, adenine nucleotide translocator 1 (ANT1), was identified [41]. ANT1 is a bifunctional protein involved in ADP/ATP exchange and is one of the components of the mitochondrial membrane permeability transition pore [42, 43]. Mitochondrial pore formation by ANT1 is induced by many pro-apoptotic agents such as Ca2+ and atractyloside. Upon apoptosis stimulation, ANT1 becomes a substrate for TG2’s cross-linking and PDI activities [41]. These activities of TG2 on ANT1 cause oligomerization of ANT1 on the mitochondrial membrane, leading to apoptosis [41]. Thus, the PDI activity of TG2 is implicated in mitochondrial-dependent apoptosis.
Kinase activity
The protein kinase activity of TG2 was initially reported in a functional study of the insulin-like growth factor-binding protein-3 (IGFBP-3) kinase in T47D breast cancer cells [44]. In that study, TG2 on the cell membrane was observed to phosphorylate IGFBP-3 and IGFBP-5 at multiple serine residues, and Ca2+ could inhibit this kinase activity [44]. Additional substrates for the kinase activity of TG2 have been identified. These include P53 [5]; histones H1, H2A, H2B, H3, and H4 [4]; and the retinoblastoma protein (Rb) [45]. Phosphorylation of P53 by TG2 decreases its binding with Mdm2, while phosphorylation of Rb by TG2 destabilizes complex formation between Rb and E2F1, indicating that TG2 might act as a kinase to promote the function of certain tumor suppressor proteins. This hypothesis correlates with the reported positive function of TG2 in the suppression of certain cancers [29, 31, 32]. The most recent TG2 substrate identification study, using a candidate-coated chip, showed that the cytosolic proteins G6PD, CYCS, and CAPN1, the transmembrane protein CDH1, and the extracellular protein MMP-3, were substrates for TG2 kinase [46]. G6PD is involved in the pentose phosphate pathway and the protective mechanism against oxidative stress [46]. CYCS and CAPN1 are involved in TG2-mediated apoptosis [47]. TG2 on the plasma membrane functions as an extracellular stabilizing factor; therefore, identification of the transmembrane protein CDH1 and the extracellular protein MMP-3 as TG2 kinase substrates is not surprising. The involvement of these secreted proteins as TG2 kinase substrates suggests that TG2 kinase regulates the breakdown of the extracellular matrix and cancer metastasis.
Scaffold activity
The scaffolding function of TG2 was initially suggested by TG2’s ability to form a ternary complex with fibronectin and collagen, which promotes cell adhesion to fibronectin via direct interaction with integrins [6]. This activity of secreted TG2 on the extracellular matrix (ECM) acts in a cross-linking activityindependent manner, although the cross-linking activity of TG2 is well characterized in the process of ECM turnover [48].
Structural diversity of transglutaminase 2
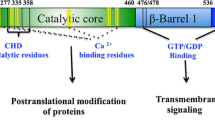
Human TG2 is the most ubiquitous isoform belonging to the TG family. Fulllength human TG2 comprises 687 amino acids and has four distinct domains: the N-terminal β-sandwich domain, the catalytic domain containing a Cys–His–Asp catalytic triad, and two C-terminal β-barrel domains (β-barrel1 and β-barrel2) (Fig. 2a) [49]. The N-terminal β-sandwich domain and catalytic domain are required for the enzymatic activity of TG2, while the C-terminal β-barrel domains are not [50]. Well-known interacting proteins for extracellular stabilization, such as fibronectin and integrin, bind to the N-terminal β-sandwich domain. A unique GTP-binding site is located in a cleft between the catalytic core and the first β-barrel.
Structural domain boundary and dynamic three-dimensional structures of transglutaminase 2 (TG2). a The four distinct domains are indicated by colored boxes with amino acid positions (below). The catalytic triad comprising C277, H335, and D358, is shown in red. b The ribbon structure of the compact, closed form (inactive form) and the expanded, opened form (active form) are shown. A large conformational change upon irreversible inhibitor binding leads to exposure of the active site (marked by red-dotted circle). The chains from the N- to the C-termini are colored from blue to red. (Color figure online)
To date, nine crystal structures of TG2 complexed with GDP [49], adenosine triphosphate (ATP) [51], GTP [52], two autoantibody Feb fragments [53, 54], and four covalently bound inhibitors (three are not published but deposited on PDB) [55], respectively, have been elucidated. Structural studies showed that TG2 undergoes an extraordinarily large conformational change upon activation (Fig. 2b). GTP, GDP, and ATP inactivate TG2 by promoting a transition to the compact conformation (closed form), while Ca2+ and the irreversible inhibitor (Ac-P(DON)LPF-NH2 (gluten peptide mimetic)) activate TG2 by promoting an extended ellipsoid structure (open form) (Fig. 2b). The large conformational change detected for the structure of the covalently bound inhibitor/TG2 complex, from the compact to extended form, is a unique feature of TG2 (Fig. 2b). It is thought that intracellular TG2 is maintained in the closed conformation because of the high concentration of GTP. However, chemical and physical injury, and circumstances in which Ca2+ is increased, trigger the rapid transition of TG2 into the open conformation, which is the active form for transamidase activity [55]. In the closed state, the catalytic triad, comprising C227, H335, and D358, is buried in TG2 (Fig. 3a). Once it is activated by the large conformational change, the extended β-barrel domains cause exposure of the catalytic nucleophile (C277) on the surface of TG2 (Fig. 3b, c). An active site study with a covalently attached inhibitor showed that the active site and substrate-binding pocket of TG2 are relatively flat at one side, although opposite side is well-defined, compared with those of other families of enzymes (Fig. 3d). This one side open-shaped active site might be necessary to bind various substrates for its multiple activities. This property of TG2 is one of the reasons why it has been difficult to find chemicals that inhibit TG2 activity specifically for pharmaceutical purposes.
Extended structure-mediated activation mechanism of transglutaminase 2 (TG2). a The closed, inactive form of TG2. The catalytic triad, comprising C277, H335, and D358, is buried in TG2. b The extended, active form of TG2. In the opened state, the catalytic nucleophile (C277) is exposed on the surface of TG2. c The location of the active site in the opened and closed forms of TG2. Red-rectangles indicate the active site. Gray ribbon structure and blue surface structure indicated opened and closed form of TG2, respectively. d The binding pocket of a covalently attached inhibitor. The structural study of the complex of TG2 with the covalently attached inhibitor showed that the substrate-binding pocket is relatively flat at one side, although opposite side is well-defined, compared with other enzyme in this families. (Color figure online)
Structural comparison with other transglutaminases
In mammals, nine different TG isoenzymes have been identified at the genomic level and characterized. Among the characterized proteins, three crystal structures have been determined, including Factor XIII, TG3, and TG2 [49, 56, 57]. Factor XIII, which is activated by thrombin-dependent proteolysis, is involved in blood clotting via stabilization of fibrin clots [58]. TG3 (also known as a epidermal/hair follicle TG) and TG1 (also known as a Keratinocyte TG), which are also activated by proteolysis, are involved in the terminal differentiation of keratinocytes [10, 59, 60]. All members are of the papain-like superfamily of cysteine proteases and might be structurally similar.
Although the substrate specificity and regulation of their mechanisms of action vary significantly within this enzyme family, all members share high sequence identity and structural similarity. The sequence identities between TG2 and Factor XIII and TG3 are 38% and 39%, respectively. The crystal structure of human TG2 showed that the monomer has four distinct domains that are located similarly to those of Factor XIII and TG3 (Fig. 4a–c). A structural superposition analysis showed that the structure of TG2 superposed well with those of Factor XIII and TG3, which had root mean square deviation (RMSD) values of 2.3 and 2.0 Å, respectively (Fig. 4d). The catalytic triad on TG2 (C277, H335, and D358) is conserved in Factor XIII and TG3 (Fig. 5). The structure of the region containing the catalytic triad is also highly conserved, although the regulation and substrate specificity are distinctly different within the TG family (Fig. 5).
Structural comparison of transglutaminase 2 (TG2) family members. a 2.8 Å crystal structure of TG2 in the GDP bound form is shown in green (1KV3) [49], b 2.65 Å crystal structure of Factor XIII shown in magenta (1GGT) [57], c 2.1 Å crystal structure of TG3 shown in blue (1L9M) [56], d Three different structures of TG were superposed by using SuperPose Version 1.0 [61] (TG2 (green), Factor XIII (magenta), and TG3 (Blue)). Root mean square deviation (RMSD) and sequence identities based on the structure of TG2 are indicated. (Color figure online)
Conserved catalytic triad among the transglutaminase 2 family. The catalytic triads of three proteins are superposed by using SuperPose Version 1.0 [61]. Each triad is well conserved at the same structural position. Superimposed triads are boxed
Structural analysis of GTP specificity for TG2
The unique guanidine nucleotide-binding site on TG2 is located in a cleft between the catalytic core and β-barrel1 (Fig. 6a). Although other GTP binding proteins, such as many small G proteins, use Mg2+ to coordinate GTP on the GTP binding proteins, TG2 interacts with GTP in the absence of Mg2+ [52]. Several positively charged amino acids (K173, R476, and R580) on TG2 are involved in the interaction with the negatively charged phosphate moieties of GTP [52]. In addition to the charged interaction and hydrogen bonds, a hydrophobic interaction formed by F174, M483, Y583, and V479 from TG2 is important to stabilize the guanine moiety on GTP [52]. The majority of the residues contacting GTP come from the end of the first β-strand of the first β-barrel domain and the loop that connects it to the next β-strand. Only two residues, K173 and F174, from the catalytic core contribute to the interaction with GTP (Fig. 6b).
Comparison of GTP-binding site. a Each GTP-binding site of transglutaminase 2 (TG2), TG3, and Factor XIII were identified and compared by structural superposition performed by SuperPose Version 1.0 [61]. b GTP-binding sites are shown in close-up the black box. GDP bound at GTP-binding site of TG2 is shown by black sticks. Amino acids from TG2 that are involved in the interaction with GDP are shown and labeled. c Amino acid sequences of the GTP-binding regions from TG2, TG3, and Factor XIII are compared by alignment. Critical residues for the interaction with GTP on TG2 are shown in red, and those aligned residues on TG3 and Factor XIII are also shown in red. (Color figure online)
Although the TG family shares similar catalytic mechanisms, the activities of individual members are regulated differently. For example, GTP inhibits TG2, TG3, and TG4, but not TG1 and Factor XIII [62,63,64,65]. Sequence alignment showed that the amino acids involved in the GTP binding in TG2 are not conserved in Factor XIII (Fig. 6c). In the case of Factor XIII, K173 and F174 are replaced by asparagine and aspartic acid, which are not related at all. In addition, R476 and R478 are replaced by aspartic acid and glutamic acid, which are oppositely charged amino acids. This alignment clearly indicated that GTP cannot bind to or regulate Factor XIII. Further structural studies of other transglutaminase family members with various substrates should be conducted to understand how TG2 can possess multiple functions, can interact with various substrates, and can be regulated by many factors such as GTP, ATP, and Ca2+.
Regulatory diversity of TG2
To prevent random cross-linking and deamidation activity against the many proteins that contain glutamine and lysine residues on their surface, the enzymatic activities of TG2 should be tightly regulated by cofactors, spatial localization, post-translational modification, and endogenous protein regulators. The main TG2 activity regulators are Ca2+ and GTP. Transamidase activities, including cross-linking, which are the main activities of TG2, are controlled positively by Ca2+ and regulated negatively by nucleotides such as GTP, GDP, and ATP [7]. Although the Ca2+-mediated activation mechanism is not well characterized, it is proposed that Ca2+ and irreversible inhibitors activate TG2 by promoting transition to the extended, open conformational structure from the compact inactive form, which reveals the active site. By contrast, GTP, GDP, and ATP inactivate TG2 by maintaining the compact conformation. The binding sites of Ca2+ on TG2 are controversial, while the nucleotide-binding site is well-characterized. Upon GTP binding at the GTP-binding site of TG2 (located between catalytic core domain and the first β-barrel), the active site is blocked by two loops, and a hydrogen bond is formed between cysteine in the active site and a tyrosine residue, which blocks the extended conformational change.
Intracellular TG2 is mostly in the inactive state because of the relatively high concentration of GTP, GDP, and ATP, and the low concentration of Ca2+, inside the cell. Once the Ca2+ concentration is increased in the cell by releasing Ca2+ into the cytosol from intracellular compartments, or by bringing it in from outside the cell during extreme situations such as cellular injury and apoptosis, Ca2+ activates TG2 by promoting transition to the open conformational structure, which exposes the active site to the substrates. In the extracellular space, TG2 is expected to be in the active form because of the low concentration of GTP/GDP and the high levels of Ca2+. However, it was observed that most TG2 in the extracellular matrix was in the inactive form [66]; however, the reason is unknown.
Besides Ca2+ and nucleotides, endogenous amine compounds, including cystamine, cystamine, spermidine, and histamine, inhibit TG2 activity by competing with various protein substrates [67, 68]. In this case enzymatically active TG2 can crosslink glutamine(s) in the substrate with amine compound inhibitors. Among the amine compound inhibitors, cystamine might inhibit TG2 activity via multiple mechanisms. It has been reported that cystamine, the reduced form of cystamine, can inhibit TG2 activity by competing with its natural substrates [69]. However, it has been suggested that cystamine might inhibit TG2 activity by forming disulfide bonds with the active site cysteine (C277) or other cysteines that are critical for the activity of TG2 [70, 71]. Recently, an allosteric activity regulation mechanism for TG2 activity by oxidation/reduction of the disulfide bond was proposed, in which extracellular TG2 is inactivated by the formation of a disulfide bond and activated by reducing the disulfide bond by thioredoxin [66, 72]; therefore, cystamine-mediated cysteine modification on TG2 might be a critical regulation point in the control of TG2 activity. In addition, ribosomal proteins RPL7a and RPL13 were identified as endogenous protein inhibitors of TG2 [73]. In that study, the authors showed that RPL7a and RPL13 inhibit the transamidase activity of TG2 specifically by a direct interaction with the β-barrel2 domain at the C-terminus. Indirect activity control mechanisms have also been proposed. Cytokine (interferon-γ)-mediated TG2 activity control was suggested by showing that extracellular TG2 in the small intestine could be activated by interferon-γ via a phosphatidylinositol-3-kinase-dependent pathway [74]. Reactive oxygen species-mediated Ca2+ level control was also proposed as a TG2 activation mechanism [75, 76].
Future perspectives
The multiple activities of TG2 are involved in various cellular activities, including apoptosis, differentiation, angiogenesis, and bone development, and are linked to many human diseases, including celiac disease, neurodegeneration, inflammatory diseases, diabetes, tissue fibrosis, and the formation of certain types of cancer. Although knockout mice for this important enzyme are viable and develop normally, follow-up analysis of the TG2-knockout mice indicated that deficient wound healing and phagocytosis, and diabetes appeared in the absence of TG2 [77,78,79,80]. The importance of TG2 in human pathogenesis and its potential as a target for therapeutic intervention in human diseases including celiac sprue, neurodegenerative diseases, and several types of cancer, have led to the development and application of its inhibitors [67, 81]. Although TG2 inhibitors that target the active site of TG2 have yielded promising results in a number of different disease models, the effect and application of those inhibitors are limited by their low affinity, resulting from the absence of specific binding pockets at the active site of TG2 (flat-shaped active site). Therefore, high affinity inhibitors that can target either the active site or different positions on TG2 are desired.
In addition to the two distinct conformations of TG2, the open and closed states, it is possible that multiple conformations of TG2 exist [67, 82, 83]. Although no structural information for the TG2-Ca2+ complex is available, a putative Ca2+ binding site was proposed by comparing the structure of XIIIa [49] and TG3 [56]. Ca2+ binding at the specific site could weaken nucleotide binding and lead to subsequent conformational changes that expose the active site. The structure of TG3 also showed that the Ca2+-dependent conformational change would expose two critical Trp residues that control access of substrates to the active site [56]. Therefore, it is possible that TG2 shares a similar structural change upon calcium binding. The structure of the TG2-Ca2+ complex should be determined to confirm the Ca2+-dependent TG2 activation mechanism by fine structural changes of TG2. The extended, open conformation has not been detected for other members of the transglutaminase family. However, the active site and the structure are almost perfectly conserved; therefore, the mechanisms of activity control via large structural changes for the other transglutaminase family members must be investigated.
Besides the known TG2 activity regulators, including Ca2+, GTP, and amine compounds, allosteric regulation by oxidation/reduction of disulfide bonds and post-translational modification by glutathionylation were proposed recently [84, 85]. Moreover, inhibition of TG2 activity by the ribosomal proteins RPL7a and RPL13 was also proposed [73]. The authors showed that RPL7a and RPL13 inhibit the transamidase activity of TG2 specifically via direct interaction with the β-barrel2 domain of TG2. How this interaction inhibits the activity of TG2 should be determined in future studies.
Celiac disease, an autoimmune response to dietary gluten antigens, produces autoantibodies against TG2. Recent studies revealed that the N-terminal β-sandwich domain (R116 and H134) and the region between the β-sandwich and β-barrel2 domains (R19, E153, and M659) might be epitopes that bind the autoantibodies directly [53, 86] (Fig. 7a, b). Blocking autoantibody binding to the celiac epitopes of TG2 represents an ideal strategy for therapeutic intervention in celiac disease. Inhibiting the structural change from the closed to the open state could block the activity of TG2. To open up the β-barrel2 domain to gain active TG2, the β-barrel2 domain interacts loosely with the core domain (Fig. 7c). Thus, to design effective TG2 inhibitors, the surface pocket formed by the N-terminal β-sandwich and β-barrel2 domains might be a tentative target for small molecules that could inhibit both autoantibody binding for celiac disease and the structural changes (Fig. 7d).
Innovative inhibitory target site on transglutaminase 2 (TG2). Celiac autoantibody-binding site 1 (a) and site 2 (b). A black box indicates the amino acid residues on TG2 that are critical for the interaction with celiac autoantibodies. c Intermolecular binding interface formed between β-barrel2 domain and the core domain of TG2. A black box shows the amino acid residues on TG2 that are involved in the interaction. The β-barrel2 domain of TG2 is shown in red. d Surface representation of TG2. Blue arrows indicate the innovative inhibitory target site that might block both autoantibody interaction and conformational changes of TG2. The β-barrel2 domain is shown in red. (Color figure online)
References
Lee KN, Birckbichler PJ, Patterson MK Jr (1989) GTP hydrolysis by guinea pig liver transglutaminase. Biochem Biophys Res Commun 162(3):1370–1375
Griffin M, Casadio R, Bergamini CM (2002) Transglutaminases: nature’s biological glues. Biochem J 368(Pt 2):377–396
Hasegawa G, Suwa M, Ichikawa Y, Ohtsuka T, Kumagai S, Kikuchi M, Sato Y, Saito Y (2003) A novel function of tissue-type transglutaminase: protein disulphide isomerase. Biochem J 373(Pt 3):793–803
Mishra S, Saleh A, Espino PS, Davie JR, Murphy LJ (2006) Phosphorylation of histones by tissue transglutaminase. J Biol Chem 281(9):5532–5538
Mishra S, Murphy LJ (2006) The p53 oncoprotein is a substrate for tissue transglutaminase kinase activity. Biochem Biophys Res Commun 339(2):726–730
Akimov SS, Krylov D, Fleischman LF, Belkin AM (2000) Tissue transglutaminase is an integrin-binding adhesion coreceptor for fibronectin. J Cell Biol 148(4):825–838
Achyuthan KE, Greenberg CS (1987) Identification of a guanosine triphosphate-binding site on guinea pig liver transglutaminase. Role of GTP and calcium ions in modulating activity. J Biol Chem 262(4):1901–1906
Lesort M, Attanavanich K, Zhang J, Johnson GV (1998) Distinct nuclear localization and activity of tissue transglutaminase. J Biol Chem 273(20):11991–11994
Im MJ, Russell MA, Feng JF (1997) Transglutaminase II: a new class of GTP-binding protein with new biological functions. Cell Signal 9(7):477–482
Lorand L, Graham RM (2003) Transglutaminases: crosslinking enzymes with pleiotropic functions. Nat Rev Mol Cell Biol 4(2):140–156
Oliverio S, Amendola A, Di Sano F, Farrace MG, Fesus L, Nemes Z, Piredda L, Spinedi A, Piacentini M (1997) Tissue transglutaminase-dependent posttranslational modification of the retinoblastoma gene product in promonocytic cells undergoing apoptosis. Mol Cell Biol 17(10):6040–6048
Nemes Z Jr, Adany R, Balazs M, Boross P, Fesus L (1997) Identification of cytoplasmic actin as an abundant glutaminyl substrate for tissue transglutaminase in HL-60 and U937 cells undergoing apoptosis. J Biol Chem 272(33):20577–20583
Piacentini M, Fesus L, Farrace MG, Ghibelli L, Piredda L, Melino G (1991) The expression of “tissue” transglutaminase in two human cancer cell lines is related with the programmed cell death (apoptosis). Eur J Cell Biol 54(2):246–254
Jones RA, Kotsakis P, Johnson TS, Chau DY, Ali S, Melino G, Griffin M (2006) Matrix changes induced by transglutaminase 2 lead to inhibition of angiogenesis and tumor growth. Cell Death Differ 13(9):1442–1453
Haroon ZA, Hettasch JM, Lai TS, Dewhirst MW, Greenberg CS (1999) Tissue transglutaminase is expressed, active, and directly involved in rat dermal wound healing and angiogenesis. FASEB J 13(13):1787–1795
Upchurch HF, Conway E, Patterson MK Jr, Maxwell MD (1991) Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. J Cell Physiol 149(3):375–382
Matic I, Sacchi A, Rinaldi A, Melino G, Khosla C, Falasca L, Piacentini M (2010) Characterization of transglutaminase type II role in dendritic cell differentiation and function. J Leukoc Biol 88(1):181–188
Tee AE, Marshall GM, Liu PY, Xu N, Haber M, Norris MD, Iismaa SE, Liu T (2010) Opposing effects of two tissue transglutaminase protein isoforms in neuroblastoma cell differentiation. J Biol Chem 285(6):3561–3567
Eitan S, Solomon A, Lavie V, Yoles E, Hirschberg DL, Belkin M, Schwartz M (1994) Recovery of visual response of injured adult rat optic nerves treated with transglutaminase. Science 264(5166):1764–1768
Kaartinen MT, El-Maadawy S, Rasanen NH, McKee MD (2002) Tissue transglutaminase and its substrates in bone. J Bone Miner Res 17(12):2161–2173
Aeschlimann D, Mosher D, Paulsson M (1996) Tissue transglutaminase and factor XIII in cartilage and bone remodeling. Semin Thromb Hemost 22(5):437–443
Kim SY (2006) Transglutaminase 2 in inflammation. Front Biosci 11:3026–3035
Dieterich W, Ehnis T, Bauer M, Donner P, Volta U, Riecken EO, Schuppan D (1997) Identification of tissue transglutaminase as the autoantigen of celiac disease. Nat Med 3(7):797–801
Lesort M, Tucholski J, Miller ML, Johnson GV (2000) Tissue transglutaminase: a possible role in neurodegenerative diseases. Prog Neurobiol 61(5):439–463
Hoffner G, Djian P (2005) Transglutaminase and diseases of the central nervous system. Front Biosci 10:3078–3092
Porzio O, Massa O, Cunsolo V, Colombo C, Malaponti M, Bertuzzi F, Hansen T, Johansen A, Pedersen O, Meschi F, Terrinoni A, Melino G, Federici M, Decarlo N, Menicagli M, Campani D, Marchetti P, Ferdaoussi M, Froguel P, Federici G, Vaxillaire M, Barbetti F (2007) Missense mutations in the TGM2 gene encoding transglutaminase 2 are found in patients with early-onset type 2 diabetes. Hum Mutat 28(11):1150
Griffin M, Smith LL, Wynne J (1979) Changes in transglutaminase activity in an experimental model of pulmonary fibrosis induced by paraquat. Br J Exp Pathol 60(6):653–661
Birckbichler PJ, Orr GR, Conway E, Patterson MK Jr (1977) Transglutaminase activity in normal and transformed cells. Cancer Res 37(5):1340–1344
Barnes RN, Bungay PJ, Elliott BM, Walton PL, Griffin M (1985) Alterations in the distribution and activity of transglutaminase during tumour growth and metastasis. Carcinogenesis 6(3):459–463
Mangala LS, Mehta K (2005) Tissue transglutaminase (TG2) in cancer biology. Prog Exp Tumor Res 38:125–138
Birckbichler PJ, Bonner RB, Hurst RE, Bane BL, Pitha JV, Hemstreet GP 3rd (2000) Loss of tissue transglutaminase as a biomarker for prostate adenocarcinoma. Cancer 89(2):412–423
Johnson TS, Knight CR, el-Alaoui S, Mian S, Rees RC, Gentile V, Davies PJ, Griffin M (1994) Transfection of tissue transglutaminase into a highly malignant hamster fibrosarcoma leads to a reduced incidence of primary tumour growth. Oncogene 9(10):2935–2942
Mehta K, Kumar A, Kim HI (2010) Transglutaminase 2: a multi-tasking protein in the complex circuitry of inflammation and cancer. Biochem Pharmacol 80(12):1921–1929
Mangala LS, Fok JY, Zorrilla-Calancha IR, Verma A, Mehta K (2007) Tissue transglutaminase expression promotes cell attachment, invasion and survival in breast cancer cells. Oncogene 26(17):2459–2470
Ishizawa T, Mattila P, Davies P, Wang D, Dickson DW (2003) Colocalization of tau and alpha-synuclein epitopes in Lewy bodies. J Neuropathol Exp Neurol 62(4):389–397
Wang DS, Dickson DW, Malter JS (2008) Tissue transglutaminase, protein cross-linking and Alzheimer’s disease: review and views. Int J Clin Exp Pathol 1(1):5–18
Dudek SM, Johnson GV (1994) Transglutaminase facilitates the formation of polymers of the beta-amyloid peptide. Brain Res 651(1–2):129–133
Norlund MA, Lee JM, Zainelli GM, Muma NA (1999) Elevated transglutaminase-induced bonds in PHF tau in Alzheimer’s disease. Brain Res 851(1–2):154–163
Mian S, el Alaoui S, Lawry J, Gentile V, Davies PJ, Griffin M (1995) The importance of the GTP-binding protein tissue transglutaminase in the regulation of cell cycle progression. FEBS Lett 370(1–2):27–31
Nakaoka H, Perez DM, Baek KJ, Das T, Husain A, Misono K, Im MJ, Graham RM (1994) Gh: a GTP-binding protein with transglutaminase activity and receptor signaling function. Science 264(5165):1593–1596
Malorni W, Farrace MG, Matarrese P, Tinari A, Ciarlo L, Mousavi-Shafaei P, D’Eletto M, Di Giacomo G, Melino G, Palmieri L, Rodolfo C, Piacentini M (2009) The adenine nucleotide translocator 1 acts as a type 2 transglutaminase substrate: implications for mitochondrial-dependent apoptosis. Cell Death Differ 16(11):1480–1492
Dorner A, Schultheiss HP (2007) Adenine nucleotide translocase in the focus of cardiovascular diseases. Trends Cardiovasc Med 17(8):284–290
Tsujimoto Y, Shimizu S (2007) Role of the mitochondrial membrane permeability transition in cell death. Apoptosis 12(5):835–840
Mishra S, Murphy LJ (2004) Tissue transglutaminase has intrinsic kinase activity: identification of transglutaminase 2 as an insulin-like growth factor-binding protein-3 kinase. J Biol Chem 279(23):23863–23868
Mishra S, Melino G, Murphy LJ (2007) Transglutaminase 2 kinase activity facilitates protein kinase A-induced phosphorylation of retinoblastoma protein. J Biol Chem 282(25):18108–18115
Jung SH, Ji SH, Han ET, Park WS, Hong SH, Kim YM, Ha KS (2016) Real-time monitoring of glucose-6-phosphate dehydrogenase activity using liquid droplet arrays and its application to human plasma samples. Biosensors Bioelectronics 79:930–937
Yoo JO, Lim YC, Kim YM, Ha KS (2012) Transglutaminase 2 promotes both caspase-dependent and caspase-independent apoptotic cell death via the calpain/Bax protein signaling pathway. J Biol Chem 287(18):14377–14388
Wang Z, Collighan RJ, Gross SR, Danen EH, Orend G, Telci D, Griffin M (2010) RGD-independent cell adhesion via a tissue transglutaminase-fibronectin matrix promotes fibronectin fibril deposition and requires syndecan-4/2 alpha5beta1 integrin co-signaling. J Biol Chem 285(51):40212–40229
Liu S, Cerione RA, Clardy J (2002) Structural basis for the guanine nucleotide-binding activity of tissue transglutaminase and its regulation of transamidation activity. Proc Natl Acad Sci USA 99(5):2743–2747
Iismaa SE, Chung L, Wu MJ, Teller DC, Yee VC, Graham RM (1997) The core domain of the tissue transglutaminase Gh hydrolyzes GTP and ATP. Biochemistry 36(39):11655–11664
Han BG, Cho JW, Cho YD, Jeong KC, Kim SY, Lee BI (2010) Crystal structure of human transglutaminase 2 in complex with adenosine triphosphate. Int J Biol Macromol 47(2):190–195
Jang TH, Lee DS, Choi K, Jeong EM, Kim IG, Kim YW, Chun JN, Jeon JH, Park HH (2014) Crystal structure of transglutaminase 2 with GTP complex and amino acid sequence evidence of evolution of GTP binding site. PloS ONE 9(9):e107005
Chen X, Hnida K, Graewert MA, Andersen JT, Iversen R, Tuukkanen A, Svergun D, Sollid LM (2015) Structural basis for antigen recognition by transglutaminase 2-specific autoantibodies in celiac disease. J Biol Chem 290(35):21365–21375
Di Niro R, Mesin L, Zheng NY, Stamnaes J, Morrissey M, Lee JH, Huang M, Iversen R, du Pre MF, Qiao SW, Lundin KE, Wilson PC, Sollid LM (2012) High abundance of plasma cells secreting transglutaminase 2-specific IgA autoantibodies with limited somatic hypermutation in celiac disease intestinal lesions. Nat Med 18(3):441–445
Pinkas DM, Strop P, Brunger AT, Khosla C (2007) Transglutaminase 2 undergoes a large conformational change upon activation. PLoS Biol 5(12):e327
Ahvazi B, Kim HC, Kee SH, Nemes Z, Steinert PM (2002) Three-dimensional structure of the human transglutaminase 3 enzyme: binding of calcium ions changes structure for activation. EMBO J 21(9):2055–2067
Yee VC, Pedersen LC, Le Trong I, Bishop PD, Stenkamp RE, Teller DC (1994) Three-dimensional structure of a transglutaminase: human blood coagulation factor XIII. Proc Natl Acad Sci USA 91(15):7296–7300
Sicker T, Hilgenfeld R (2002) Blood coagulation factor XIII: activation, substrates and structure of a transglutaminase. Hamostaseologie 22(1):20–27
Thacher SM, Rice RH (1985) Keratinocyte-specific transglutaminase of cultured human epidermal cells: relation to cross-linked envelope formation and terminal differentiation. Cell 40(3):685–695
Hitomi K, Presland RB, Nakayama T, Fleckman P, Dale BA, Maki M (2003) Analysis of epidermal-type transglutaminase (transglutaminase 3) in human stratified epithelia and cultured keratinocytes using monoclonal antibodies. J Dermatol Sci 32(2):95–103
Maiti R, Van Domselaar GH, Zhang H, Wishart DS (2004) SuperPose: a simple server for sophisticated structural superposition. Nucl Acids Res 32:W590-W594
Hitomi K, Kanehiro S, Ikura K, Maki M (1999) Characterization of recombinant mouse epidermal-type transglutaminase (TGase 3): regulation of its activity by proteolysis and guanine nucleotides. J Biochem 125(6):1048–1054
Spina AM, Esposito C, Pagano M, Chiosi E, Mariniello L, Cozzolino A, Porta R, Illiano G (1999) GTPase and transglutaminase are associated in the secretion of the rat anterior prostate. Biochem Biophys Res Commun 260(2):351–356
Hitomi K, Yamagiwa Y, Ikura K, Yamanishi K, Maki M (2000) Characterization of human recombinant transglutaminase 1 purified from baculovirus-infected insect cells. Biosci Biotechnol Biochem 64(10):2128–2137
Hitomi K, Ikura K, Maki M (2000) GTP, an inhibitor of transglutaminases, is hydrolyzed by tissue-type transglutaminase (TGase 2) but not by epidermal-type transglutaminase (TGase 3). Biosci Biotechnol Biochem 64(3):657–659
Jin X, Stamnaes J, Klock C, DiRaimondo TR, Sollid LM, Khosla C (2011) Activation of extracellular transglutaminase 2 by thioredoxin. J Biol Chem 286(43):37866–37873
Siegel M, Khosla C (2007) Transglutaminase 2 inhibitors and their therapeutic role in disease states. Pharmacol Ther 115(2):232–245
Karpuj MV, Becher MW, Springer JE, Chabas D, Youssef S, Pedotti R, Mitchell D, Steinman L (2002) Prolonged survival and decreased abnormal movements in transgenic model of Huntington disease, with administration of the transglutaminase inhibitor cystamine. Nat Med 8(2):143–149
Jeitner TM, Delikatny EJ, Ahlqvist J, Capper H, Cooper AJ (2005) Mechanism for the inhibition of transglutaminase 2 by cystamine. Biochem Pharmacol 69(6):961–970
Lorand L (1998) DRPLA aggregation and transglutaminase, revisited. Nat Genet 20(3):231
Lorand L, Conrad SM (1984) Transglutaminases. Mol Cell Biochem 58(1–2):9–35
Plugis NM, Palanski BA, Weng CH, Albertelli M, Khosla C (2017) Thioredoxin-1 selectively activates transglutaminase 2 in the extracellular matrix of the small intestine: implications for celiac disease. J Biol Chem 292(5):2000–2008
Hitomi K, Kojima S, Fesus L (2015) Transglutaminase, multiple functional modifiers and targets for new drug discovery, 1st edn. Springer, New York
Diraimondo TR, Klock C, Khosla C (2012) Interferon-gamma activates transglutaminase 2 via a phosphatidylinositol-3-kinase-dependent pathway: implications for celiac sprue therapy. J Pharmacol Exp Ther 341(1):104–114
Jeong EM, Kim CW, Cho SY, Jang GY, Shin DM, Jeon JH, Kim IG (2009) Degradation of transglutaminase 2 by calcium-mediated ubiquitination responding to high oxidative stress. FEBS Lett 583(4):648–654
Shin DM, Jeon JH, Kim CW, Cho SY, Lee HJ, Jang GY, Jeong EM, Lee DS, Kang JH, Melino G, Park SC, Kim IG (2008) TGFbeta mediates activation of transglutaminase 2 in response to oxidative stress that leads to protein aggregation. FASEB J 22(7):2498–2507
Bernassola F, Federici M, Corazzari M, Terrinoni A, Hribal ML, De Laurenzi V, Ranalli M, Massa O, Sesti G, McLean WH, Citro G, Barbetti F, Melino G (2002) Role of transglutaminase 2 in glucose tolerance: knockout mice studies and a putative mutation in a MODY patient. FASEB J 16(11):1371–1378
Mastroberardino PG, Iannicola C, Nardacci R, Bernassola F, De Laurenzi V, Melino G, Moreno S, Pavone F, Oliverio S, Fesus L, Piacentini M (2002) ‘Tissue’ transglutaminase ablation reduces neuronal death and prolongs survival in a mouse model of Huntington’s disease. Cell Death Differ 9(9):873–880
Telci D, Griffin M (2006) Tissue transglutaminase (TG2)–a wound response enzyme. Front Biosci 11:867–882
Sarang Z, Toth B, Balajthy Z, Koroskenyi K, Garabuczi E, Fesus L, Szondy Z (2009) Some lessons from the tissue transglutaminase knockout mouse. Amino Acids 36(4):625–631
Song M, Hwang H, Im CY, Kim SY (2017) Recent Progress in the development of transglutaminase 2 (TGase2) Inhibitors. J Med Chem 60(2):554–567
Begg GE, Holman SR, Stokes PH, Matthews JM, Graham RM, Iismaa SE (2006) Mutation of a critical arginine in the GTP-binding site of transglutaminase 2 disinhibits intracellular cross-linking activity. J Biol Chem 281(18):12603–12609
Begg GE, Carrington L, Stokes PH, Matthews JM, Wouters MA, Husain A, Lorand L, Iismaa SE, Graham RM (2006) Mechanism of allosteric regulation of transglutaminase 2 by GTP. Proc Natl Acad Sci USA 103(52):19683–19688
Stamnaes J, Pinkas DM, Fleckenstein B, Khosla C, Sollid LM (2010) Redox regulation of transglutaminase 2 activity. J Biol Chem 285(33):25402–25409
Chiang BY, Chou CC, Hsieh FT, Gao S, Lin JC, Lin SH, Chen TC, Khoo KH, Lin CH (2012) In vivo tagging and characterization of S-glutathionylated proteins by a chemoenzymatic method. Angew Chem Int Ed Engl 51(24):5871–5875
Simon-Vecsei Z, Kiraly R, Bagossi P, Toth B, Dahlbom I, Caja S, Csosz E, Lindfors K, Sblattero D, Nemes E, Maki M, Fesus L, Korponay-Szabo IR (2012) A single conformational transglutaminase 2 epitope contributed by three domains is critical for celiac antibody binding and effects. Proc Natl Acad Sci USA 109(2):431–436
Acknowledgements
This study was supported in part by the Yeungnam University research grant at 2015.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Rights and permissions
About this article
Cite this article
Lee, C.S., Park, H.H. Structural aspects of transglutaminase 2: functional, structural, and regulatory diversity. Apoptosis 22, 1057–1068 (2017). https://doi.org/10.1007/s10495-017-1396-9
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1396-9