Abstract
Epithelial-mesenchymal transition (EMT) is implicated in the metastasis of human prostate cancer (PCa). Notch signaling has been established as a regulator of EMT. Notch-4 has emerged as a mammary proto-oncogene and a target in several cancers. However, the role and the mechanism of action of Notch-4 in PCa are still unclear. In the present study, we first observed a marked increase in Notch-4 expression in the PCa cell lines DU145, PC3 and LnCAP compared with the non-malignant prostate epithelial cell line RWPE1. Knocking down the expression of Notch-4 suppressed the viability and proliferation in the PCa cell lines DU145 and PC3. Also, further study showed that a decline in Notch-4 significantly promoted apoptosis in PC3 cells. Notch-4 silencing also resulted in decreased cell migration and invasion and affected the expression of EMT markers. We hypothesized that Notch-4 ablation suppresses the activity of NF-κB, so we used PMA to stimulate NF-κB p50 and p65 activation in PC3 cells. The results indicate that PMA treatment impaired the action of Notch-4 ablation in the biology of PC3 cells including cell growth, apoptosis, migration, invasion and EMT. The results of the present study show that RNAi targeting against Notch-4 expression suppresses PCa progression.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Prostate cancer (PCa), one of the prevalent malignancies in men, is a main cause of cancer-related mortality [1]. Evidence indicates that PCa is apt to invasion, and clinically localized cancer can be treated with surgery, hormone therapy and radiotherapy [2]. The development of PCa is always asymptomatic; thus, this disease is often diagnosed at an advanced stage accompanied by tumor metastasis [1]. Recently, conventional therapy of PCa rarely works, and the lethality of this disease is a result of PCa metastasis. Although clinical therapy has improved, the survival of prostate cancer patients is still low. Thus, it is necessary to look for reliable biomarkers that can distinguish tumors in a latent stage and play roles in cancer grow and metastasize [3].
Emerging studies have revealed that metastasis contributes to the mortality of multiple types of cancer, including PCa [4]. In PCa, the developmental program epithelial-mesenchymal transition (EMT) is a key step in cancer initiation and progression via regulating the mesenchymal and epithelial phenotypes [5]. A considerable body of work has addressed the link between EMT in tumor cells and tumor aggressiveness [6]. EMT participates in the process of tumor cell metastasis including migration, cell detachment, dispersion and invasion [7]. EMT markers including vimentin, fibronectin, E-cadherin, N-cadherin and ZEB1 have been implicated in controlling mesenchymal and epithelial cellular states [8, 9].
Byles et al. showed that SIRT1 regulates cell migration and metastasis via increasing the expression of the EMT transcription factor ZEB1 in prostate cancer cells [10]. Wellner et al. showed that the EMT-activator zinc finger E-box binding homeobox 1 can regulate tumorigenicity via inhibiting microRNA-200 expression [9]. Also, accumulating evidence indicates that Notch signaling is implicated in tumor cell migration, invasion and EMT [11]. Moreover, Bui et al. have shown that Notch-4 plays an essential role in the EMT process in tamoxifen-resistant MCF-7 breast cancer cells [12]. Emerging studies have indicated an important role for Notch-4 in cancer [13]. However, the function and mechanism of Notch-4 in PCa biology including cell growth, metastasis and EMT is still unclear.
According to published estimates, there are four members of the Notch family in mammals, Notch1 to Notch4 [14]. Notch1 and Notch2 have similar epidermal growth factor (EGF)-like repeats; Notch3 has 34 EGF-like repeats whereas Notch4 has 29 EGF-like repeats. These repeats affect Notch1–Notch4 affinity for ligands [14–16]. It is accepted that Notch signaling acts as a tumor suppressor or oncogene and is involved in the development and progression of a wide range of cancers [17, 18]. Shambhavi et al. have shown that Notch4 activity is enhanced in basal breast cancer cells and has been associated with tumor progression [19]. Thus, we speculated that Notch-4 may play a role in PCa cell growth and EMT. In agreement with the action of Notch-4 in other tumor types, the present study shows that Notch-4 expression is increased in PCa cells and Notch-4 expression inhibits PCa cell growth and metastasis and impacts on the EMT phenotype.
Methods
Cell culture and transfection
The prostate cancer cell lines DU145, PC3 and LnCAP and the non-malignant prostate epithelial cell line RWPE1 were all obtained from American Type Culture Collection (ATCC). All cancer cells were maintained in RPMI-1640 medium containing 10% FBS, and supplemented with 1% penicillin/streptomycin at 37 °C. RWPE1 cells were cultured in keratinocyte serum-free media in an incubator with 5% CO2 at 37 °C. DU145 and PC3 cells was transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for indicated time by using Lipofectamine 2000 (Invitrogen, Carlsbad, CA, USA) following the manufacturer’s protocol. After transfection, cells were treated with the potent NF-κB activator phorbol 12-myristate 13-acetate (PMA) (Sigma, St. Louis, MO, USA; 100 ng/mL) for 6 h.
Cell viability assay
DU145 and PC3 cells were maintained in RPMI-1640 medium and plated in 96-well plate at a density of 2 × 104 cells /well with five replicates for each group. Cells were transfected with Notch-4 siRNA or control siRNA for 48 h. Cell viability was measured by the Cell Titer-Glo (CTG) Luminescent Cell Viability Assay (Promega; Madison, Wisconsin, USA) following the manufacturer’s instructions.
Cell proliferation assay
DU145 and PC3 cells were cultured and treated as above. Proliferation of transfected cells and controls was assayed by the MTS assay using the CellTiter AQueous assay system (Promega) following the manufacturer’s instructions [20]. In brief, MTS reagent (10 µL) was added to the cells and incubated for 1 h. The same volume of DMSO was added to cells as a control. The OD value was measured at 490 nm.
Apoptosis assay
For the apoptosis assay, PC3 cells were plated in 96-well plates at a density of 2 × 104 cells/well with five replicates for each group. Cells were transfected with Notch-4 siRNA or control siRNA for 48 h. Apoptosis was determined by a cell death detection ELISA according to the manufacturer’s instructions. The apoptosis assay was performed by quantitating DNA fragmentation generated by apoptotic cells.
Cell migration and invasion assay
The Transwell assay was used to investigate the migration and invasion ability of PC3 cells as described previously [21]. The cells were transfected as described above. For the migration assay, PC3 cells were directly seeded in the upper chamber, whereas lower chamber contained medium containing 20% FBS which acted as a chemoattractant. After 24 h of incubation, cells were washed with PBS three times and the non-migrated cells in the upper chamber were removed. The cells that migrated to the lower chamber were fixed and stained with 0.1% crystal violet. Cell invasion studies were performed according to a similar Transwell method described above, except the upper chamber was pre-coated with Matrigel (BD Biosciences). The migrating or invading cell counts were recorded using a microscope.
RNA isolation and qRT-PCR
Total RNA was extracted from PC3 cells using the RNeasy mini kit (Qiagen, Valencia, CA) following the manufacturer’s protocol. Then, 2 μg of RNA was used to synthesize cDNA using the Omniscript RT kit (Qiagen, Courtaboeuf, France) according to the manufacturer’s instructions. qRT-PCR was performed on an ABI Prism 7500 Fast RT-PCR System (Applied Biosystems, Branchburg, NJ, USA). The primers for Notch-4 used in this study were as follows [22]: forward 5′-TCAACACTCCTGGCTCCTTCAACT-3′ and reverse 5′-AGAGGCACTCATTGTGATCA- GCCT-3′.
Western blot assay
PC3 cells were washed with ice cold PBS and lysed using RIPA buffer containing protease and phosphatase inhibitors [23]. The same quantity of total protein was electrophoresed by 10% SDS–PAGE. A rabbit monoclonal antibody to Notch-4 and a rabbit polyclonal antibody to NF-κB p65 were used as the primary antibodies for Western blotting. An HRP-conjugated goat anti-rabbit IgG antibody was used as the secondary antibody for Western blotting.
Statistical analysis
All quantified data are depicted as mean ± SD. Statistical analyses were performed using SPSS 16.0 software. One-way ANOVA followed by a two-tailed Student’s t test was used for comparisons. The results were considered significant at P < 0.05. GraphPad Prism 5 software was used to illustrate the data in this study.
Results
Notch-4 is increased in PCa cell lines
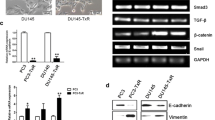
Previous studies have shown that Notch-4 play essential roles in cancer progression; however, the function and mechanism of Notch-4 in PCa remains unclear. In order to determine the expression of Notch-4 in the PCa cell lines DU145, PC3 and LnCAP compared to the normal prostate epithelial cell line RWPE1, we performed RT-PCR and western blot to show the mRNA and protein levels of Notch-4 in PCa cells and RWPE1 cells. Compared to prostate epithelial cell line RWPE1, increased Notch-4 levels were particularly obvious in DU145, PC3 and LnCAP cells (Fig. 1a). Also, the western blot showed similar results in that the protein expression of Notch-4 was significantly up-regulated in PCa cells (Fig. 1b).
Notch-4 expression is increased in the PCa cell lines DU145, PC3 and LnCAP. a RT-PCR was used to assess the mRNA level of Notch-4 in the PCa cell lines DU145, PC3 and LnCAP and the normal prostate epithelial cell line RWPE1. Relative Notch-4 mRNA levels were normalized to β-actin; b Western blot showed that Notch-4 protein expression was increased in DU145, PC3 and LnCAP cells compared with RWPE1 cells. Notch-4 protein expression was normalized to β-actin. The RT-PCR and Western blot results are representative of three independent experiments in performed triplicate. Results are presented as mean ± SEM, *P < 0.05 versus control
Silencing Notch-4 expressions inhibits PCa cell growth and promotes apoptosis
To further determine the effects of Notch-4 on PCa cell biology, Notch-4 deletion cells were constructed. RT-PCR and western blot showed that Notch-4 levels were remarkably decreased in Notch-4 deletion cells (Fig. 2a, b). We next determined the effects of Notch-4 on PCa cell growth; the results indicate that Notch-4 depletion decreased DU145 and PC3 viability and proliferation (P < 0.05, Fig. 2c, d). Finally, to determine whether Notch-4 deletion affects DU145 and PC3 apoptosis, a cell death detection ELISA was used; the results show that, compared to the control group, Notch-4 silencing markedly promoted DU145 and PC3 apoptosis (P < 0.05, Fig. 2e), whereas there was no obvious change between the control and the siCon group (P > 0.05, Fig. 2e).
Notch-4 ablation in PCa cells suppresses cell growth and promotes apoptosis. a–b Notch-4 mRNA levels (a) and protein expression levels (b) were determined by qRT-PCR and western blot in Du145 and PC3. c Notch-4 ablation inhibits in vitro cytotoxicity in DU145 and PC3 determined using the Cell Titer-Glo (CTG) Luminescent Cell Viability Assay. d DU145 and PC3 cell proliferation was determined by the MTS assay. e Effects of Notch-4 ablation on Du145 and PC3 apoptosis were determined by a cell death detection ELISA. Cells were transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for 48 h. Results are presented as mean ± SEM from three independent experiments performed in triplicate. * P < 0.05 versus control
Depleted Notch-4 expression suppresses PCa cell migration and invasion
We next determined whether Notch-4 silencing has an effect on cell migration and invasion. The results of the Transwell assay show that Notch-4 inhibition impaired PC3 cell migration by ~40% when compared with the control group and the siCon group (P < 0.05, Fig. 3a). Similarly, compared with the control group and the siCon group, we observed a significant decline in the number of invaded PC3 cells in the Notch-4 inhibition group (P < 0.05, Fig. 3b). Also, as shown in Fig. 3c, d, the cell migration and invasion of DU145 cells was both decreased in the Notch-4 inhibition group (P < 0.05, Fig. 3c, d). These data suggest that Notch-4 depletion inhibits cell migration and invasion in PCa prostate cells.
Notch-4 ablation in Du145 and PC3 cells suppresses cell migration and invasion. a–b Cells migration and invasion were measured by the Transwell assay in PC3 cells. c–d Cells migration and invasion were measured by the Transwell assay in DU145 cells. Cells were transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for 48 h. Results are presented as mean ± SEM from three independent experiments performed in triplicate. *P < 0.05 versus control
Depleted Notch-4 expression reverses PCa cell EMT
It is now well-established that cell metastasis is associated with EMT [24]. Also, Notch-4 has been implicated in this process [12]. EMT is characterized particular mesenchymal and epithelial markers such as E-cadherin, Vimentin and N-cadherin. In the present study, western blot analysis showed a significant increase in the expression of E-cadherin in PC3 cells transfected with siNotch-4. Meanwhile, an obvious decrease in Vimentin was observed in the Notch-4 silenced prostate cancer PC3 cells. The expression of another mesenchymal marker, N-cadherin was also measured; it displayed a similar trend with Vimentin in Notch-4 silenced PC3 cells (Fig. 4a). Also, as shown in Fig. 4b, the increased E-cadherin expression and decreased Vimentin and N-cadherin was observed in SiNotch-4 treated group (P < 0.05, Fig. 4b). These results suggest that Notch-4 knockdown in PCa cells reverses EMT and promotes a shift towards an epithelial phenotype.
Notch-4 ablation reverses EMT in Du145 and PC3 cells. EMT markers E-cadherin, N-cadherin and Vimentin affected by Notch-4 ablation in PC3 (a) and Du145 (b) cells were detected by western blot. Cells were transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for 48 h. Results are presented as mean ± SEM from three independent experiments performed in triplicate. *P < 0.05 versus control
Silencing Notch-4 expression suppresses NF-κB signaling
Previous studies have found that Notch-1 signaling can increase NF-κB transcriptional activity; moreover, Notch-4 is similar to Notch-1, which may also activate the NF-κB pathway [25]. This signaling pathway plays roles in cancer cell growth and metastasis. This study shows that, compared with the control group, Notch-4 silencing significantly inhibited NF-κB subunit p50 and p65 expression in PC3 cells (Fig. 5a, b). The results indicate that Notch-4 depletion suppressed the activity of NF-κB. Next, we used PMA to stimulate NF-κB signal pathway activation in PC3 cells (Fig. 5a, b), and the Notch-4 siRNA transfected cell biology was assessed (Fig. 6). A significant increase in cell viability and a decrease in apoptosis were observed in PMA-stimulated Notch-4 siRNA transfected PC3 cells (Fig. 6a, b). Cell invasion was increased in Notch-4 siRNA transfected PC3 cells after PMA treatment (Fig. 6c). The effects of Notch-4 depletion on the levels of E-cadherin, vimentin and N-cadherin were also attenuated. Compared with the siNotch-4 group, the level of E-cadherin was reduced, whereas the expression of vimentin and N-cadherin were increased after PMA treatment and Notch-4 depletion cells (Fig. 6d). These results suggest that Notch-4 silencing inhibits PCa cell growth, metastasis and EMT, at least partly dependent on the NF-κB pathway.
Notch-4 ablation inhibits NF-κB pathway activation in PC3 cells. NF-κB p50 (a) and p65 (b) protein expression was detected by western blot. Cells were transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for 48 h and then treated with the potent NF-κB activator PMA (100 ng/mL) for 6 h. Results are presented as mean ± SEM from three independent experiments performed in triplicate. *P < 0.05 versus control, #P < 0.05 versus siNotch-4 group
Notch-4 ablation inhibits PC3 cell growth and metastasis dependent on NF-κB signaling. a Notch-4 ablation inhibits in vitro cytotoxicity in DU145 and PC3 cells, determined using the Cell Titer-Glo (CTG) Luminescent Cell Viability Assay. b The effects of Notch-4 ablation on PC3 apoptosis were determined by a cell death detection ELISA. c Cell invasion was measured by the Transwell assay. d E-cadherin, N-cadherin and Vimentin levels were detected by western blot. Cells were transfected with Notch-4 siRNA (siNotch-4) or control siRNA (siCon) for 48 h and then treated with the potent NF-κB activator PMA (100 ng/mL) for 6 h. Results are presented as mean ± SEM from three independent experiments performed in triplicate. *P < 0.05 versus control, #P < 0.05 versus siNotch-4 group
Discussion
The evolutionarily conserved Notch family of proteins has been shown to play fundamental roles in the progression of multiple types of cancer, such as breast cancer, hepatocellular carcinoma and prostate cancer [26–28]. There are four Notch receptors expressed in the mammalian system, i.e. Notch-1, Notch-2, Notch-3 and Notch-4. A considerable body of work suggests that Notch signaling, including Notch-4, is a mammary proto-oncogene and a target for cancer treatment, such for as human breast cancer [29]. However, the role and the mechanism of action of Notch-4 in prostate cancer are still unknown. Elevated expression of Notch has been found in human breast cancers and the increased Notch is harmful in the overall survival of breast cancer patients [30]. Also, a high level of Notch-4 protein has been observed in adenoid cystic carcinoma [31]. Notably, Li et al. showed that Notch-4 is overexpressed in LNCaP-AI cells and aggressive prostate cancer samples by proteomics pathway array analysis (PPAA) [32]. Consistent with these results, our study shows that Notch-4 is increased in the PCa cell lines DU145, PC3 and LnCAP compared to the normal prostate epithelial cell line RWPE1 at both the mRNA and protein levels.
As proto-oncogenes, Notch proteins have been implicated in tumor progression and initiation [33]. Notch signaling can determine cellular fate and induce changes in cell activity, including cell proliferation, survival, apoptosis and differentiation [34, 35]. Wang et al. indicated that silencing Notch-1 and its ligand Jagged-1 markedly induces PCa apoptosis and suppresses cell growth, migration and invasion [25]. Studies have revealed that Notch-4 knockdown inhibits estrogen-independent growth of T47D cells transfected with PKCα, whereas increased Notch-4 expression promotes cell growth [36]. Consistent with these findings, the present study showed that Notch-4 silencing markedly suppressed DU145 and PC3 cell viability and proliferation. Moreover, we observed that Notch-4 knockdown promoted PC3 apoptosis. Another study has also implicated Notch-4 signaling in the metastasis of salivary adenoid cystic carcinoma [37].
EMT is associated with the invasion and metastasis of malignant cancer and is characterized by an increase in mesenchymal gene expression and a decrease in epithelial gene expression [38]. In the present study, we observed that Notch-4 silencing increase the level of E-cadherin, whereas it significantly inhibited the expression of vimentin and N-cadherin. Thus, we believe that Notch-4 silencing could affect the invasion and metastasis of PCa cells by regulating the expression of E-cadherin, vimentin and N-cadherin.
Accumulating evidence strongly indicates that down-regulated Notch-1 expression could inactivate the Akt, mTOR and NF-κB signaling pathways [25]. Notably, NF-κB signaling has also been reported to play an important role in the pathobiology of PCa. We have confirmed that Notch-4 silencing suppresses NF-κB signaling. To achieve this, we used PMA to stimulate NF-κB p50 and p65 activation in PC3 cells. The results indicate that Notch-4 silencing induced PCa cell migration and invasion; this inhibition was dependent on the NF-κB signaling pathway.
In conclusion, this study shows that the knockdown of Notch-4 expression successfully inhibits PCa cell growth and metastasis in vitro. Also, we confirmed that Notch-4 silencing can regulate EMT marker expression and inhibit NF-κB pathway activity. These results suggest that Notch-4 may be a new target gene for PCa and knockdown of Notch-4 may inhibit the malignant behavior of PCa.
References
Zhao W, Guo W, Zhou Q, Ma SN, Wang R, Qiu Y, Jin M, Duan HQ, Kong D (2013) In vitro antimetastatic effect of phosphatidylinositol 3-kinase inhibitor ZSTK474 on prostate cancer PC3 cells. Int J Mol Sci 14(7):13577–13591
Andreoiu M, Cheng L (2010) Multifocal prostate cancer: biologic, prognostic, and therapeutic implications. Hum Pathol 41(6):781–793
Crawford ED, Higano CS, Shore ND, Hussain M, Petrylak DP (2015) Treating patients with metastatic castration resistant prostate cancer: a comprehensive review of available therapies. J Urol 194(6):1537–1547
Shi J, Xia Y, Song Q, Zhou X, Mizokami A, Keller E, Zhang J, Lu Y (2014) Interaction of prostate cancer cells with tumor microenvironment promotes EMT and DTCs activation. Cancer Res 74(19 Supplement):1–1
Liu YN, Yin JJ, Abou-Kheir W, Hynes PG, Casey OM, Fang L, Yi M, Stephens RM, Seng V, Sheppard-Tillman H, Martin P, Kelly K (2013) MiR-1 and miR-200 inhibit EMT via Slug-dependent and tumorigenesis via Slug-independent mechanisms. Oncogene 32(3):296–306
Prasad CP, Rath G, Mathur S, Bhatnagar D, Parshad R, Ralhan R (2009) Expression analysis of E-cadherin, Slug and GSK3beta in invasive ductal carcinoma of breast. BMC Cancer 9(325):1471–2407
Voulgari A, Pintzas A (2009) Epithelial-mesenchymal transition in cancer metastasis: mechanisms, markers and strategies to overcome drug resistance in the clinic. Biochim Biophys Acta 2:75–90
Onder TT, Gupta PB, Mani SA, Yang J, Lander ES, Weinberg RA (2008) Loss of E-cadherin promotes metastasis via multiple downstream transcriptional pathways. Cancer Res 68(10):3645–3654
Wellner U, Schubert J, Burk UC, Schmalhofer O, Zhu F, Sonntag A, Waldvogel B, Vannier C, Darling D, zur Hausen A, Brunton VG, Morton J, Sansom O, Schuler J, Stemmler MP, Herzberger C, Hopt U, Keck T, Brabletz S, Brabletz T (2009) The EMT-activator ZEB1 promotes tumorigenicity by repressing stemness-inhibiting microRNAs. Nat Cell Biol 11(12):1487–1495
Byles V, Zhu L, Lovaas JD, Chmilewski LK, Wang J, Faller DV, Dai Y (2012) SIRT1 induces EMT by cooperating with EMT transcription factors and enhances prostate cancer cell migration and metastasis. Oncogene 31(43):4619–4629
Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U (2008) Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA 105(17):6392–6397
Bui Q, Kang K (2016) Abstract P1-05-06: Essential role of notch-4/STAT3 signaling in epithelial-mesenchymal transition of tamoxifen-resistant human breast cancer. Cancer Res 76(4 Supplement):P1-05-06–P01-05-06
Speiser J, Foreman K, Drinka E, Godellas C, Perez C, Salhadar A, Ersahin C, Rajan P (2012) Notch-1 and Notch-4 biomarker expression in triple-negative breast cancer. Int J Surg Pathol 20(2):139–145
Ntziachristos P, Lim JS, Sage J, Aifantis I (2014) From fly wings to targeted cancer therapies: a centennial for notch signaling. Cancer Cell 25(3):318–334
Haines N, Irvine KD (2003) Glycosylation regulates Notch signalling. Nat Rev Mol Cell Biol 4(10):786–797
Okajima T, Irvine KD (2002) Regulation of notch signaling by o-linked fucose. Cell 111(6):893–904
Delury C, Hart C, Brown M, Clarke N, Parkin E (2016) Stroma-induced Jagged1 expression drives PC3 prostate cancer cell migration; disparate effects of RIP-generated proteolytic fragments on cell behaviour and Notch signaling. Biochem Biophys Res Commun 472(1):255–261
Yin L, Velazquez OC, Liu ZJ (2010) Notch signaling: emerging molecular targets for cancer therapy. Biochem Pharmacol 80(5):690–701
Naik S, MacFarlane M, Sarin A (2015) Notch4 signaling confers susceptibility to TRAIL-induced apoptosis in breast cancer cells. J Cell Biochem 116(7):1371–1380
Chan GK, Kleinheinz TL, Peterson D, Moffat JG (2013) A simple high-content cell cycle assay reveals frequent discrepancies between cell number and ATP and MTS proliferation assays. PLoS ONE 8(5):e63583
Fan X, Chen X, Deng W, Zhong G, Cai Q, Lin T (2013) Up-regulated microRNA-143 in cancer stem cells differentiation promotes prostate cancer cells metastasis by modulating FNDC3B expression. BMC Cancer 13(61):1471–2407
Chen W, Zhang H, Wang J, Cao G, Dong Z, Su H, Zhou X, Zhang S (2013) Lentiviral-mediated gene silencing of Notch-4 inhibits in vitro proliferation and perineural invasion of ACC-M cells. Oncol Rep 29(5):1797–1804
Saini S, Majid S, Shahryari V, Tabatabai ZL, Arora S, Yamamura S, Tanaka Y, Dahiya R, Deng G (2014) Regulation of SRC kinases by microRNA-3607 located in a frequently deleted locus in prostate cancer. Mol Cancer Ther 13(7):1952–1963
Kudo-Saito C, Shirako H, Takeuchi T, Kawakami Y (2009) Cancer metastasis is accelerated through immunosuppression during Snail-induced EMT of cancer cells. Cancer Cell 15(3):195–206
Wang Z, Li Y, Banerjee S, Kong D, Ahmad A, Nogueira V, Hay N, Sarkar FH (2010) Down-regulation of Notch-1 and Jagged-1 inhibits prostate cancer cell growth, migration and invasion, and induces apoptosis via inactivation of Akt, mTOR, and NF-kappaB signaling pathways. J Cell Biochem 109(4):726–736
Bolos V, Mira E, Martinez-Poveda B, Luxan G, Canamero M, Martinez AC, Manes S, de la Pompa JL (2013) Notch activation stimulates migration of breast cancer cells and promotes tumor growth. Breast Cancer Res 15(4):R54 doi:10.1186/bcr3447
Wan X, Cheng C, Shao Q, Lin Z, Lu S, Chen Y (2016) CD24 promotes HCC progression via triggering Notch-related EMT and modulation of tumor microenvironment. Tumour Biol 37(5):6073–6084. doi:10.1007/s13277-015-4442-7
Carvalho FL, Simons BW, Eberhart CG, Berman DM (2014) Notch signaling in prostate cancer: a moving target. Prostate 74(9):933–945. doi:10.1002/pros.22811
Clementz AG, Rogowski A, Pandya K, Miele L, Osipo C (2011) NOTCH-1 and NOTCH-4 are novel gene targets of PEA3 in breast cancer: novel therapeutic implications. Breast Cancer Res 13(3):R63
Reedijk M, Odorcic S, Chang L, Zhang H, Miller N, McCready DR, Lockwood G, Egan SE (2005) High-level coexpression of JAG1 and NOTCH1 is observed in human breast cancer and is associated with poor overall survival. Cancer Res 65(18):8530–8537
Bell D, Hanna EY, Miele L, Roberts D, Weber RS, El-Naggar AK (2014) Expression and significance of notch signaling pathway in salivary adenoid cystic carcinoma. Ann Diagn Pathol 18(1):10–13
Li Y, Chen L, Yang Y et al (2014) MP31-17 NOTCH 4 REGULATED, LEF1 MEDIATED PROSTATE CANCER METASTASIS AND ANDROGEN INDEPENDENCE. J Urol 191(4):e328–e329
Yabuuchi S, Pai SG, Campbell NR, de Wilde RF, De Oliveira E, Korangath P, Streppel MM, Rasheed ZA, Hidalgo M, Maitra A, Rajeshkumar NV (2013) Notch signaling pathway targeted therapy suppresses tumor progression and metastatic spread in pancreatic cancer. Cancer Lett 335(1):41–51
Kopan R, Ilagan MX (2009) The canonical Notch signaling pathway: unfolding the activation mechanism. Cell 137(2):216–233
Somnay YR, Yu XM, Lloyd RV, Leverson G, Aburjania Z, Jang S, Jaskula-Sztul R, Chen H (2016) Notch3 expression correlates with thyroid cancer differentiation, induces apoptosis, and predicts disease prognosis. Cancer 2(10):30403
Yun J, Pannuti A, Espinoza I, Zhu H, Hicks C, Zhu X, Caskey M, Rizzo P, D’Souza G, Backus K, Denning MF, Coon J, Sun M, Bresnick EH, Osipo C, Wu J, Strack PR, Tonetti DA, Miele L (2013) Crosstalk between PKCalpha and Notch-4 in endocrine-resistant breast cancer cells. Oncogenesis 5(2):26
Ding LC, She L, Zheng DL, Huang QL, Wang JF, Zheng FF, Lu YG (2010) Notch-4 contributes to the metastasis of salivary adenoid cystic carcinoma. Oncol Rep 24(2):363–368
Sekhon K, Bucay N, Majid S, Dahiya R, Saini S (2016) MicroRNAs and epithelial-mesenchymal transition in prostate cancer. Oncotarget 30(10):11708
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Zhang, J., Kuang, Y., Wang, Y. et al. Notch-4 silencing inhibits prostate cancer growth and EMT via the NF-κB pathway. Apoptosis 22, 877–884 (2017). https://doi.org/10.1007/s10495-017-1368-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1368-0