Abstract
Homocysteine (Hcy) induced vascular endothelial injury leads to the progression of endothelial dysfunction in atherosclerosis. Epigallocatechin gallate (EGCG), a natural dietary antioxidant, has been applied to protect against atherosclerosis. However, the underlying protective mechanism of EGCG has not been clarified. The present study investigated the mechanism of EGCG protected against Hcy-induced human umbilical vein endothelial cells (HUVECs) apoptosis. Methyl thiazolyl tetrazolium assay (MTT), transmission electron microscope, fluorescent staining, flow cytometry, western blot were used in this study. The study has demonstrated that EGCG suppressed Hcy-induced endothelial cell morphological changes and reactive oxygen species (ROS) generation. Moreover, EGCG dose-dependently prevented Hcy-induced HUVECs cytotoxicity and apoptotic biochemical changes such as reducing mitochondrial membrane potential (MMP), decreasing Bcl-2/Bax protein ratio and activating caspase-9 and 3. In addition, EGCG enhanced the protein ratio of p-Akt/Akt, endothelial nitric oxide synthase (eNOS) activation and nitric oxide (NO) formation in injured cells. In conclusion, the present study shows that EGCG prevents Hcy-induced HUVECs apoptosis via modulating mitochondrial apoptotic and PI3K/Akt/eNOS signaling pathways. Furthermore, the results indicate that EGCG is likely to represent a potential therapeutic strategy for atherosclerosis associated with Hyperhomocysteinemia (HHcy).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Numerous risk factors involve in the process of atherosclerosis including Hyperhomocysteinemia (HHcy). Moreover, HHcy has been defined as an independent risk factor for cardiovascular disease [1, 2]. For instance, every 5 µM increase of homocysteine (Hcy) enhances the risk of chronic heart diseases, which is an important risk factor in plasma [3]. Studies have demonstrated that Hcy-induced vascular endothelial cells apoptosis is involved in several mechanisms including the accumulation of reactive oxygen species (ROS), activation of nuclear transcription factor kappa B, inhibition of endothelial nitric oxide production [4–7]. Although complement of vitamin B-Complex such as folic acid, vitamin B6, and vitamin B12 has been shown to reduce the level of plasma Hcy, but these vitamin B-Complex can not significantly inhibit Hcy-induced endothelial dysfunction [8–10]. So far, the associated mechanisms have not been elucidated. Thus, it is necessary to develop a new kind of medicine to prevent endothelial dysfunction and atherosclerosis diseases caused by HHcy.
Epigallocatechin-3-gallate (EGCG), a naturally occurring polyphenol antioxidant extracted and purified from green tea, is generally considered as the most anti-oxidative effect due to the presence of several phenolic hydroxyl groups in its chemical structure [11–14]. Previous study has shown that there is an inverse relationship between components of tea and cardiovascular events [15]. What is more, numerous studies have demonstrated that EGCG possesses the bioactivities such as anti-inflammatory activities and potent anti-atherosclerotic properties in vitro and in vivo [16–18]. However, there remains no direct evidence to demonstrate whether EGCG can protect human umbilical vein endothelial cells (HUVECs) from Hcy-induced apoptosis. Therefore, we hypothesized that EGCG may attenuate Hcy-induced endothelial apoptosis and attempted to study the molecular mechanisms underlying this potential protective effect.
To elucidate above hypothesis, the aim of the present study was to examine whether EGCG could suppress Hcy-induced vascular endothelial cells apoptosis and to clarify its possible effects on the various signaling pathways.
Materials and methods
Cell culture
HUVECs were purchased from Yu heng feng Technology Co., Ltd (Beijing, China). The culture medium was consisted of Dulbecco’s modified Eagle medium (DMEM) with high sugar, containing 10% fetal bovine serum, penicillin (100 units/mL) and streptomycin (100 mg/mL). Cells were incubated at 37 °C with 95% humidity and 5% CO2 and were used in the logarithmic growth phase.
Cell viability and morphological examination
Cell viability was determined by methyl thiazolyl tetrazolium (MTT, Sigma, USA) colorimetric assay. Briefly, the cells (2 × 105 cells/mL) were plated in 96-well plates, incubated at 37 °C for 24 h and pretreated with 10, 20 or 30 µM EGCG (Aladdin, China) for 1 h respectively, then stimulated with 3 mM Hcy (Sigma, USA) for 24 h. After that, MTT was added to each well (0.5 mg/mL final concentration) and incubated for 4 h. The absorbance was read using a Microtiter-plate reader (Thermo, USA) at a wavelength of 570 nm.
For examination of morphological appearance, the cells were plated in 6-well plates and pretreated with different concentrations of EGCG for 1 h, then stimulated with 3 mM Hcy for 24 h. The images were photographed by an inverted microscope (Nikon, Japan).
For transmission electron microscopy (TEM) assay, the cells were plated in 6-well plates and incubated overnight. After being pretreated as described above, the cells were harvested and fixed overnight at 4 °C in 2% glutaraldehyde. The samples were treated as previously described [19] and the ultramicrotomies were stained and imaged using a transmission electron microscope (JEM-2000EX, Japan).
AO/EB and DAPI staining
The cells (2 × 105 cells/mL) were plated in 6-well plates, incubated at 37 °C for 24 h and pretreated with 10, 20 or 30 µM EGCG for 1 h respectively, then stimulated with 3 mM Hcy for 24 h. After that, the cells were washed with cold phosphate buffered solution (PBS) for two times, and the mixture containing the same volume of acridine orange (AO, 100 µg/mL in PBS) and ethidium bromide (EB, 100 µg/mL in PBS) was placed onto the cells. Then the images were observed using a fluorescence microscope (Ex = 488 nm, Em = 515 nm), (Olympus, USA).
The cells were plated in 6-well plates and incubated overnight. After being pretreated as described above, the cells were washed with cold PBS for two times. Then the cells were fixed with 10% formaldehyde for 10 min. The fixed cells were washed with cold PBS for three times and stained with 6-diamidino-2-phenylindole (DAPI, 1 µg/mL) for 10 min at 37 °C. The images were observed using a fluorescence microscope (Ex = 358 nm, Em = 461 nm).
Cellular apoptosis determination
An Annexin V–fluorescein isothiocyanate (FITC) Apoptosis Detectable Kit (Nanjing KeyGen Biotech, China) was used for the determination of apoptosis. HUVECs (2 × 105 cells/mL) were plated in 6-well plates and incubated at 37 °C for 24 h, then treated the cells as the method described above. The treated cells were harvested and washed with cold PBS for two times, then gently resuspended in 500 µL binding buffer. Finally, 5 µL Annexin V-FITC and 5 µL propidium iodide (PI) solutions were incubated with the cells in the dark for 15 min. The percentages of apoptotic cells were analyzed by a FACCalibura flow cytometer (Ex = 488 nm, Em = 530 nm), (Becton Dickinson, Laguna Hills, CA, USA).
Biochemical assay
The release of nitric oxide (NO) and lactate dehydrogenase (LDH), the content of malondialdehyde (MDA), and the activities of superoxide dismutase (SOD), were determined with assay kits (Nanjing Jian cheng Bioengineering Institute Nanjing, China) by following the manufacturer’s instructions.
Intracellular ROS accumulation detection
ROS production in HUVECs was determined by 2,7-dichlorodihydrofluorescein diacetate (DCFH-DA) as a probe for the presence of ROS. Briefly, HUVECs (2 × 105 cells/mL) were plated in 6-well plates and pretreated with 10, 20 or 30 µM EGCG for 1 h respectively, then stimulated with 3 mM Hcy for 2 h. The cells were harvested and gently resuspended in 500 µL DCFH-DA diacetate (10 µM final concentration), then incubated at 37 °C for 30 min. After washing twice with PBS, cells were observed under a FACCalibura flow cytometer (Ex = 488 nm, Em = 530 nm).
Measurement of mitochondrial membrane potential
Mitochondrial membrane potential (MMP) was identified with 5,5′,6,6′-tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine iodide (JC-1, Fanbo Biochemical, China) staining. Briefly, HUVECs (2 × 105 cells/mL) were seeded overnight in 6-well plates, and pre-treated with and without 10, 20 or 30 µM EGCG for 1 h respectively, then stimulated with 3 mM Hcy for 24 h. After that cells were rinsed with the medium and incubated with JC-1 (10 µg/mL final concentration) for 15 min at 37 °C in the dark. Subsequently, cells were washed with DMEM for two times. After treatment, cells were observed under a FACCalibura flow cytometer (Ex = 530 nm, Em = 590 nm).
Western blot assay
The cells (2 × 105 cells/mL) were plated in 6-well plates and pretreated with 10, 20 and 30 µM EGCG for 1 h respectively, then stimulated with 3 mM Hcy for 24 h. Total cytosolic proteins from different groups were extracted with Radio immunoprecipitation assay (RIPA, 150 mM NaCl, 1% Triton X-100, sodium pyrophosphate, β-glycerophosphate, ethylene diamine tetraacetic acid, Na3VO4 and leupeptin, 20 mM Tris, pH = 7.5) and Phenylmethanesulfonyl fluoride (PMSF, 100 µM), then the lysates were centrifuged at 12,000×g for 10 min at 4 °C. The protein concentration was determined with the BCA Protein Assay Kit (bicinchonininc acid, Thermo Fisher Scientific Inc.). Total proteins were loaded onto sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS–PAGE) gel (10–15%), separated by gel electrophoresis, and transferred to polyvinylidene fluoride (PVDF) membranes (Millipore, Bedford, MA, USA). After putting the PVDF membranes into 5% skimmed milk for 2 h at room temperature, the membranes were incubated overnight at 4 °C with primary antibody (1:1000 dilution) against Akt, caspase-9, caspase-3, Bcl-2, Bax (Proteintech Group, USA), Akt (Thr450), Akt (Thr308), Akt (Ser473) and eNOS (Bio world Consulting Laboratories, USA), then incubated with HRP-labeled Goat Anti-Rabbit IgG (H + L) (Beyotime Institute of Biotechnology, Jiangsu, China) at a 1:1000 dilution for 2 h at room temperature. After the final washing, the blots were detected by enhanced chemiluminescence (ECL) system and photographed by Bio-Spectrum Gel Imaging System (UVP, USA).
Statistical analysis
The data were performed as mean ± SD. The significance of difference between two groups was evaluated by Student’s t test. Multiple group comparisons were performed using one-way analysis of variance (ANOVA). ##p < 0.01 versus Control, *p < 0.05, **p < 0.01 versus 3 mM Hcy treatment only and △p < 0.05, △△p < 0.01 versus 20 µM EGCG treatment were deemed to be statistically significant respectively.
Results
EGCG attenuates the viability of Hcy-injured HUVECs and the morphological changes of cells
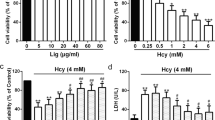
We firstly examined the effect of EGCG on the viability of Hcy-injured HUVECs by MTT assay. As shown in (Fig. 1a), incubation with EGCG did not induce cytotoxicity, but 3 mM Hcy induced a reduction of cell viability. However, pre-treatment with 10–30 µM EGCG in dose dependently prevented the inhibition of cell proliferation induced by Hcy.
Effects of EGCG on HUVECs viability in response to Hcy. a Cell viability was determined by MTT method. Data are presented as mean ± SD, n = 3. ##p < 0.01 versus control and *p < 0.05, **p < 0.01 versus 3 mM Hcy treatment only. b Representative photomicrographs of Hcy-treated cells with and without EGCG. The bright field images for morphology changes of HUVECs (100×, final magnification) under different concentrations of EGCG. The fluorescence images for apoptosis changes of HUVECs stained by AO/EB (200×, final magnification) and DAPI (800×, final magnification) respectively. c TEM micrographs of HUVECs without and with EGCG (8000×, final magnification)
The morphological changes, including bright field images, AO/EB and DAPI fluorescent staining of HUVECs were examined as shown in (Fig. 1b). AO/EB staining revealed that the viable cells exhibited bright green, while the apoptotic cells triggered by Hcy shown red color. Through DAPI staining, changes in apoptotic cells, including karyorrhexis and karyopyknosis were observed. The treatment of cells with Hcy for 24 h significantly increased the number of apoptotic cells relative to untreated control. However, pre-treatment with EGCG for 1 h in dose dependently suppressed apoptosis in HUVECs induced by Hcy.
The ultrastructure of HUVECs was observed as shown in (Fig. 1c). The typical, normal cellular structures, including abundant microvilli on the cell surface, intact cell membranes and good nuclei were displayed in the control group. Hcy-treated cells displayed fewer microvilli on the cell surface, nucleus chromatin condensation and marginalization. However, EGCG could alleviate morphological changes in cells induced by Hcy.
EGCG suppress of Hcy-induced apoptosis
To examine whether Hcy-induced cell death is involved in apoptosis, flow cytometry analysis using Annexin V-FITC and PI double staining was used. As shown in (Fig. 2a, b), treatment of cells with Hcy for 24 h markedly increased cell apoptosis about ~20% relative to untreated control. Enhanced apoptosis was significantly suppressed by EGCG in dose dependent manner.
Effects of EGCG on Hcy-induced apoptosis in HUVECs. a Representative cytograms of Annexin V-FITC binding and PI staining of Hcy-stimulated HUVECs. The horizontal axis shows the AnnexinV intensity, and the vertical axis represents the PI binding. a control, b Hcy, c Hcy + EGCG (10 µM), d Hcy + EGCG (20 µM), e Hcy + EGCG (30 µM). b The mean percentage of cells in early apoptosis as analyzed by flow cytometry. Data are presented as mean ± SD, n = 3. ##p < 0.01 versus control and *p < 0.05, **p < 0.01 versus 3 mM Hcy treatment only
EGCG inhibits Hcy-induced oxidative stress
Treatment of HUVECs with Hcy for 24 h caused a significant increase of LDH, MDA levels and a marked decreased SOD activity in the culture medium as shown in (Fig. 3). However, pre-treatment with various concentrations of EGCG for 1 h prior to incubation with Hcy significantly prevented the LDH, MDA release and increased the SOD level in dose dependent manner.
To investigate whether increased oxidative stress is associated with Hcy-induced apoptosis in HUVECs, flow cytometry analysis using DCFH-DA staining was performed. As shown in (Fig. 3d), treatment of cells with Hcy for 24 h markedly caused ROS generation. ROS production was considerably reduced under treatment with EGCG in dose dependently manner.
EGCG attenuates Hcy-induced HUVECs apoptosis through the mitochondrial-dependent apoptotic signaling pathway
We used the fluorescent probe JC-1 to verify whether the suppression of mitochondrial function disruption was involved in the anti-apoptotic effects of EGCG (Fig. 4a, b). The MMP was depolarized in Hcy-treated cells as shown by the increase in green fluorescence and the inhibition of red fluorescence (Fig. 4a, b). However, pretreatment with EGCG prevented the loss in MMP as indicated by the repression of green fluorescence and restoration of red fluorescence (Fig. 4a, c–e).
Effects of EGCG on Hcy-induced mitochondrial membrane permeability in HUVECs. a Representative cytograms of JC-1 staining of Hcy-stimulated HUVECs. a control, b Hcy, c Hcy + EGCG (10 µM), d Hcy + EGCG (20 µM), e Hcy + EGCG (30 µM). b Relative level of MMP was quantified by the ratio of red and green fluorescence intensity per cell. Data are presented as mean ± SD, n = 3. ##p < 0.01 versus control and **p < 0.01 versus 3 mM Hcy treatment only
It has been reported that a decrease of Bcl-2/ Bax ratio is sufficient to promote apoptosis in cells by activating the mitochondrial apoptotic pathway [20]. As shown in (Fig. 5a), the stimulation of cells with Hcy caused a significant reduction in the Bcl-2/Bax ratio. Though pre-treatment with EGCG in dose dependently attenuated the decrease in the Bcl-2/Bax ratio. We also detected the activity of cleaved caspase-9, procaspase-9, cleaved caspase-3 and procaspase-3, which is a hallmark of apoptotic execution enzymes, by Western blot. Hcy treatment of HUVECs increased the cleaved caspase-9/procaspase-9 ratio and cleaved caspase-3/procaspase-3 ratio, but pre-incubation with EGCG inhibited the ratio of both caspases compared with untreated cells (Fig. 5b, c).
Effects of EGCG on Bax and Bcl-2 expression and caspase activation in Hcy-treated HUVECs. a Representative Western blot of Bcl-2 and Bax proteins. b Representative Western blot of cleaved caspase-9 and caspase-9 proteins. c Representative Western blot of cleaved caspase-3 and caspase-3 proteins. β-actin was used as an internal control. a control, b Hcy, c Hcy + EGCG (10 µM), d Hcy + EGCG (20 µM), e Hcy + EGCG (30 µM). Data are presented as mean ± SD, n = 3. ##p < 0.01 versus control and *p < 0.05, **p < 0.01 versus 3 mM Hcy treatment only
EGCG attenuates Hcy-induced HUVECs apoptosis through the PI3K/Akt/eNOS signaling pathway
It has been reported that PI3K/Akt/eNOS/NO signaling pathway is an important survival pathway in endothelial cells [21]. To investigate whether PI3K/Akt/eNOS signaling was involved in the action of EGCG, we pretreated cells with LY-294002, 1 h prior to EGCG treatment. As shown in (Fig. 6a), we observed that EGCG could effect on different phosphorylation sites of Akt in the damaged cells. The activity of Akt was partially inhibited by Hcy, which induces cell death and apoptosis. But pre-treatment with different concentrations of EGCG increased the Akt of different phosphorylation sites against Hcy-induced cell apoptosis. However, when the Akt pathways were inhibited by LY-294002, EGCG failed to protect cells against Hcy-induced cell apoptosis as shown in (Fig. 6a). eNOS is an important downstream target of Akt. Thus, we examined eNOS protein expression. We found that Hcy reduced eNOS protein expression, but this tendency was reversed by EGCG (Fig. 6b). Furthermore, total nitrite was likewise measured. Hcy treatment significantly decreased the nitrite content in the culture medium, while EGCG significantly reversed this tendency in dose dependent manner as shown in (Fig. 6c).
Effects of EGCG on Akt signaling pathways in Hcy-treated HUVECs. a Representative Western blot of Akt, Akt (Thr450), Akt (Thr308) and Akt (Ser473) proteins. a control, b Hcy, c Hcy + EGCG (20 µM), d Hcy + EGCG (20 µM) + LY294002 (20 µM). b Representative Western blot of eNOS protein expression. β-actin was used as an internal control. a control, b Hcy, c Hcy + EGCG (10 µM), d Hcy + EGCG (20 µM), e Hcy + EGCG (30 µM). c Effects of EGCG on nitric oxide (NO) production. Data are presented as mean ± SD, n = 3. ##p < 0.01 versus control, *p < 0.05, **p < 0.01 versus 3 mM Hcy treatment only and △p < 0.05, △△p < 0.01 versus 20 µM EGCG treatment
Discussion
Since vascular endothelial cells have a crucial function in regulating and maintaining the health of vascular system, oxidative stress-induced endothelial apoptosis is a driving force in the development of atherosclerosis [22]. The major findings of the present study are that EGCG protected HUVECs against Hcy-induced endothelial apoptosis by suppressing overloading of intracellular ROS, dephosphorylation of Akt, depolarization of MMP, down-regulation of Bcl-2 and the subsequent activation of cleaved caspase-9 and 3. Based on these results, a schematic presentation was proposed to lay out the possible mechanisms for the protective effect of EGCG. Both mitochondrial-dependent apoptosis and PI3K/Akt/eNOS signaling pathways were involved in the apoptotic response in HUVECs cells (Fig. 7).
It has been reported that the autoxidation of Hcy generates ROS and causes lipid peroxidation in endothelial cells, which is one of the main reasons leading to endothelial functional impairment [23]. LDH is a stable enzyme in the cytosol, which is quickly released into the medium upon damage of the plasma membrane. Hence it is a biomarker for cell membrane damage. MDA is one of the most regularly used indicators of lipid peroxidation. Intensification of endogenous antioxidants SOD has been accepted as an important pharmacological property presenting in nature as well as many synthetic compounds. Meanwhile, it has been proved that the intervention of Hcy increases the level of LDH, MDA and decreases the SOD activity in endothelial cells [24]. The experimental results of our present study revealed that oxidative stress was a crucial factor in Hcy-induced cells apoptosis. Moreover, our results shown that EGCG played an important protective role in Hcy-induced endothelial cells apoptosis by reducing the release of LDH and MDA and increasing the activity of SOD activity. Hence, the anti-apoptotic features of EGCG might be attributed to their antioxidative capacity.
Mitochondrion regulates the production of cellular energy, which plays a key role in triggering apoptotic events [25]. During the process of apoptosis, the opening of the permeability transition pore (PTP) results in the reduction of MMP, which causes the release of pro-apoptotic factors [26]. Pro-apoptotic factors activate the caspase cascades, cause nuclear condensation, and generate secondary ROS. The increased ROS also induces mitochondrial membranal depolarization [27]. The Bcl-2 family of pro-apoptotic and anti-apoptotic proteins can regulate outer mitochondrial membranal permeability for initiating apoptosis [20]. In this study, our results are consistent with previous studies that Hcy can induce intracellular ROS production that leads to the loss of MMP [28, 29]. Since the activation of mitochondrial-dependent apoptotic signaling pathway is the most important toxic mechanism, it is significant to study whether EGCG could inhibit the activation of this signaling pathway. Results indicated that EGCG prevented the loss of MMP, decreased the ratio of cleaved caspase-9/procaspase-9 and cleaved caspase-3/procaspase-3, and attenuated the decrease in the ratio of Bcl-2/Bax protein. Taken together, we proposed that EGCG could protect HUVECs from Hcy-induced cell apoptosis by regulating mitochondrial-dependent apoptotic signaling pathway.
Endothelial NO synthase (eNOS) plays a critical role in endothelial cell function and survival, which is triggered by the activation of protein kinase Akt and phosphoinositide-3-kinase [30]. Nitric oxide (NO) is one of the types of endothelium-derived factors, which are triggered by eNOS in endothelial cells [7, 31]. Decreased bioavailability of NO is a key factor in vascular pathobiology that is associated with elevated Hcy level, which results in endothelial dysfunction [32]. In this study, we found that EGCG promoted the Akt of different phosphorylation sites phosphorylation, eNOS expression and NO production in HUVECs injured by Hcy. LY294002 is a PI3K inhibitor, which can significantly inhibit the activity of PI3K and reduce the phosphorylation of Akt. Pretreatment with LY294002 partially antagonized the anti-cytotoxic and anti-apoptotic effects of EGCG, suggesting that the protective effect of EGCG is at least partially due to its ability to activate the PI3K/Akt/eNOS signaling pathway.
Conclusions
The present study demonstrated that EGCG protected HUVECs against Hcy-induced apoptosis by modulating PI3K/Akt/eNOS pathway, suppressing mitochondria-dependent apoptotic signaling and subsequent stimulation of cleaved caspase-9 and 3. This novel anti-apoptotic effect of EGCG demonstrates that it has promising applications in the therapy of HHcy-related atherosclerosis or other oxidative stress-related cardiovascular diseases.
Abbreviations
- Hcy:
-
Homocysteine
- EGCG:
-
Epigallocatechin gallate
- HHcy:
-
Hyperhomocysteinemia
- HUVEC:
-
Human umbilical vein endothelial cell
- LDH:
-
Lactate dehydrogenase
- MDA:
-
Malondialdehyde
- SOD:
-
Superoxide dismutase
- ROS:
-
Reactive oxygen species
- MMP:
-
Mitochondrial membrane potential
- Akt:
-
Protein kinase B
- p-Akt:
-
Phosphorylated protein kinase B
- eNOS:
-
Endothelial nitric oxide synthase
- NO:
-
Nitric oxide
References
Bautista LE, Arenas IA, Penuela A, Martinez LX (2002) Total plasma homocysteine level and risk of cardiovascular disease: a meta-analysis of prospective cohort studies. J Clin Epidemiol 55:882–887
Refsum H, Ueland PM, Nygard O, Vollset SE (1998) Homocysteine and cardiovascular disease. Annu Rev Med 49:31–62
Clarke R, Daly L, Robinson K et al (1991) Hyperhomocysteinemia: an independent risk factor for vascular disease. N Engl J Med 324:1149–1155
Dong F, Zhang X, Li SY et al (2005) Possible involvement of NADPH oxidase and JNK in homocysteine-induced oxidative stress and apoptosis in human umbilical vein endothelial cells. Cardiovasc Toxicol 5:9–20
Moselhy SS, Demerdash SH (2003) Plasma homocysteine and oxidative stress in cardiovascular disease. Dis Markers 19:27–31
Postea O, Krotz F, Henger A, Keller C, Weiss N (2006) Stereospecific and redox-sensitive increase in monocyte adhesion to endothelial cells by homocysteine. Arterioscler Thromb Vasc Biol 26:508–513
Upchurch GR Jr, Welch GN, Fabian AJ et al (1997) Homocyst(e)ine decreases bioavailable nitric oxide by a mechanism involving glutathione peroxidase. J Biol Chem 272:17012–17017
Lonn E, Yusuf S, Arnold MJ et al (2006) Homocysteine lowering with folic acid and B vitamins in vascular disease. N Engl J Med 354:1567–1577
Toole JF, Malinow MR, Chambless LE et al (2004) Lowering homocysteine in patients with ischemic stroke to prevent recurrent stroke, myocardial infarction, and death: the Vitamin Intervention for Stroke Prevention (VISP) randomized controlled trial. Jama 291:565–575
Woodman RJ, Celermajer DE, Thompson PL, Hung J. (2004) Folic acid does not improve endothelial function in healthy hyperhomocysteinaemic subjects. Clin Sci 106:353–358
Choi YJ, Jeong YJ, Lee YJ, Kwon HM, Kang YH (2005) (-)Epigallocatechin gallate and quercetin enhance survival signaling in response to oxidant-induced human endothelial apoptosis. J Nutr 135:707–713
Guo Q, Zhao B, Li M, Shen S, Xin W (1996) Studies on protective mechanisms of four components of green tea polyphenols against lipid peroxidation in synaptosomes. Biochim Biophys Acta 1304:210–222
Guo Q, Zhao B, Shen S, Hou J, Hu J, Xin W (1999) ESR study on the structure-antioxidant activity relationship of tea catechins and their epimers. Biochim Biophys Acta 1427:13–23
Ou HC, Song TY, Yeh YC, et al. (2010) EGCG protects against oxidized LDL-induced endothelial dysfunction by inhibiting LOX-1-mediated signaling. J Appl Physiol 108:1745–1756
Riemersma RA, Rice-Evans CA, Tyrrell RM, Clifford MN, Lean ME (2001) Tea flavonoids and cardiovascular health. Mon J Assoc Phys 94:277–282
Ludwig A, Lorenz M, Grimbo N et al (2004) The tea flavonoid epigallocatechin-3-gallate reduces cytokine-induced VCAM-1 expression and monocyte adhesion to endothelial cells. Biochem Biophys Res Commun 316:659–665
Tedeschi E, Suzuki H, Menegazzi M (2002) Antiinflammatory action of EGCG, the main component of green tea, through STAT-1 inhibition. Ann N Y Acad Sci 973:435–437
Xu H, Lui WT, Chu CY, Ng PS, Wang CC, Rogers MS (2009) Anti-angiogenic effects of green tea catechin on an experimental endometriosis mouse model. Human Reprod 24:608–618
Wen YD, Wang H, Kho SH et al (2013) Hydrogen sulfide protects HUVECs against hydrogen peroxide induced mitochondrial dysfunction and oxidative stress. PloS One 8:e53147
Brunelle JK, Letai A (2009) Control of mitochondrial apoptosis by the Bcl-2 family. J Cell Sci 122:437–441
Ho FM, Lin WW, Chen BC et al (2006) High glucose-induced apoptosis in human vascular endothelial cells is mediated through NF-kappaB and c-Jun NH2-terminal kinase pathway and prevented by PI3K/Akt/eNOS pathway. Cell Signal 18:391–399
Choy JC, Granville DJ, Hunt DW, McManus BM (2001) Endothelial cell apoptosis: biochemical characteristics and potential implications for atherosclerosis. J Mol Cell Cardiol 33:1673–1690
Mujumdar VS, Aru GM, Tyagi SC (2001) Induction of oxidative stress by homocyst(e)ine impairs endothelial function. J Cell Biochem 82:491–500
Lin R, Liu J, Gan W, Ding C (2007) Protective effect of quercetin on the homocysteine-injured human umbilical vein vascular endothelial cell line (ECV304). Basic Clin Pharmacol Toxicol 101:197–202
Ishikawa K, Takenaga K, Akimoto M et al (2008) ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320:661–664
Ly JD, Grubb DR, Lawen A. (2003) The mitochondrial membrane potential (deltapsi(m)) in apoptosis; an update. Apoptosis 8:115–128
Petit PX, Susin SA, Zamzami N, Mignotte B, Kroemer G. (1996) Mitochondria and programmed cell death: back to the future. FEBS Lett 396:7–13
Sipkens JA, Hahn N, van den Brand CS et al (2013) Homocysteine-induced apoptosis in endothelial cells coincides with nuclear NOX2 and peri-nuclear NOX4 activity. Cell Biochem Biophys 67:341–352
Tyagi N, Ovechkin AV, Lominadze D, Moshal KS, Tyagi SC (2006) Mitochondrial mechanism of microvascular endothelial cells apoptosis in hyperhomocysteinemia. J Cell Biochem 98:1150–1162
Vanhaesebroeck B, Leevers SJ, Panayotou G, Waterfield MD (1997) Phosphoinositide 3-kinases: a conserved family of signal transducers. Trends Biochem Sci 22:267–272
Dimmeler S, Zeiher AM (1999) Nitric oxide–an endothelial cell survival factor. Cell Death Differ 6:964–968
Weiss N (2005) Mechanisms of increased vascular oxidant stress in hyperhomocys-teinemia and its impact on endothelial function. Curr Drug Metab 6:27–36
Acknowledgements
This study was financially supported by the Natural Science Foundation of China (No. 30772601) and the University Innovation Team Project Foundation of Education Department of Liaoning Province (No. LT2013019).
Author information
Authors and Affiliations
Corresponding author
Additional information
Shumin Liu, Zhengwu Sun and Peng Chu equally contributed to the work.
Rights and permissions
About this article
Cite this article
Liu, S., Sun, Z., Chu, P. et al. EGCG protects against homocysteine-induced human umbilical vein endothelial cells apoptosis by modulating mitochondrial-dependent apoptotic signaling and PI3K/Akt/eNOS signaling pathways. Apoptosis 22, 672–680 (2017). https://doi.org/10.1007/s10495-017-1360-8
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-017-1360-8