Abstract
Apoptotic cell death plays a pivotal role in the development and/or maintenance of several tissues including thymus. Deregulated thymic cell death is associated with autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), a prototype murine model for analysis of human multiple sclerosis. Because Thy28 expression is modulated during thymocyte development, we tested whether Thy28 affects induction of EAE as effectively as antigen-induced thymocyte deletion using Thy28 transgenic (TG) mice. Thy28 TG mice showed partial resistance to anti-CD3 monoclonal antibody (mAb)-induced thymic cell death in vivo, as assessed by annexin V-expression and loss of mitochondrial membrane potential. The resistance to anti-CD3 mAb-induced cell death in Thy28 TG mice appeared to correlate with a decreased c-Jun N-terminal kinase phosphorylation and reduced down-regulation of Bcl-xL. Moreover, thymic hyperplasia was detected in Thy28 TG mice, although thymocyte development was unaltered. Development of peripheral lymphoid tissues including spleen and lymph nodes was also unaltered. Thy28 TG spleen T cells showed an increased production of IFN-γ, but not IL-17, in response to both anti-CD3 and anti-CD28 mAbs. Finally, Thy28 TG mice displayed accelerated induction of EAE as assessed by disease incidence, clinical score, and pathology following immunization with myelin oligodendrocyte glycoprotein compared with control WT mice. These findings suggest that modulation of Thy28 expression plays a crucial role in the determination of thymic cell fate, which may contribute to the development of EAE through proinflammatory cytokine production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Apoptotic cell death plays a pivotal role in the development and maintenance of homeostasis in several tissues including thymus [1]. The majority of CD4+CD8+ double positive (DP) thymocytes, derived from CD4−CD8− double negative (DN) cells, die by neglect when they fail to receive survival signal(s) because of failure to recognize self major histocompatibility complex (MHC)/peptide complex molecules [2]. Only a minor portion of DP thymocytes that bind the self-MHC/peptide complex with intermediate affinity differentiate into CD4+CD8− or CD4−CD8+ single positive (SP) cells through positive selection. Moreover, the DP cells with high affinity for the self-MHC/peptide complex undergo apoptosis through negative selection, contributing to acquisition of self-tolerance. Although the positive–negative selection is affected by several factors including Bcl-2 family proteins [3], Nur 77, and mitogen-activated protein kinases (MAPKs) [4, 5], much remains unknown.
Apoptosis is accompanied by a loss of mitochondrial membrane potential (MMP), caspase activation, and annexin V-expression in multiple tissues including lymphocytes in response to a variety of stimuli [6–8]. Apoptotic cell death is regulated by several factors including Bcl-2 family members comprising proapoptotic (Bax and Bad) and anti-apoptotic (Bcl-2, Bcl-xL, and Mcl-1) components [3, 9]. MAPKs affect induction of apoptosis through modulation of Bcl-2 family members [10–12]. Inactivation of c-Jun N-terminal kinase (JNK)1 and JNK2 partially abrogates the apoptotic cell death of DP thymocytes on anti-CD3 administration in vivo [13]. Antigen-induced apoptosis in B cells involves JNK-mediated modulation of Bcl-2 family members [5].
Lymphocyte survival has some effect on the induction and/or enhancement of autoimmune diseases including experimental autoimmune encephalomyelitis (EAE), murine model for analysis of human multiple sclerosis (MS) [14, 15]. EAE is mediated mainly by CD4+ T cells specific for autoantigens such as myelin oligodendrocyte glycoprotein (MOG) [15], which migrate into the central nervous system resulting in demyelination. The severity of an inflammatory lesion is regulated by complex interactions among T cell subsets including helper T-cell (Th)1, Th2, Th17, and regulatory T cells [16, 17]. CD4+ T cell-derived IFN-γ plays a crucial role in directing the site of pathogenesis from the central nervous system [18, 19]: IFN-γ mediates the inflammation of classical EAE dominated by inflammatory lesions in the spinal cord, while preventing pathogenesis in the cerebellum and brain stem. IL-17 is considered to be a mediator of EAE [20, 21], which can be dampened by IL-4-producing Th2 cells [16].
We have recently cloned Thy28 cDNA, the sequence of which is highly conserved among bacteria, yeast, and vertebrates [22]. Thy28 protein (32 kDa) is substantially expressed in several tissues, including thymus, spleen, and testis, with low amounts in muscle, lung, and heart [23]. Interestingly, Thy28 protein expression is transiently down-regulated in the DP stage during thymocyte development [24]. Over-expression of Thy28 partially protects against antigen-mediated apoptosis in B cells [25]. To examine whether Thy28 expression levels are implicated in the fate of thymocytes in vivo, we established a line of Thy28 transgenic (TG) mice driven by a CAG promoter. DP thymocytes of Thy28 TG mice displayed partial resistance to anti-CD3-induced death in vivo, possibly resulting in thymic hyperplasia. Interestingly, Thy28 TG mice showed accelerated induction of EAE in response to MOG. A possible role for Thy28 in the physiological development of thymocytes and also in pathogenesis of EAE is discussed.
Materials and methods
Mice
Thy28 TG mice (C57BL/6 background) and control WT mice 8–12 weeks old were bred and maintained at the animal facility of Tokyo Medical University. TG mice were established by microinjecting a transgene construct (Thy28-V5-His6/pCAGGS) into a fertilized egg from a C57BL/6 mouse. The murine cDNA for Thy28 tagged with V5-His6 was amplified by a standard polymerase chain reaction (PCR) method, which was inserted into a TOPO vector (Invitrogen, Carlsbad, CA, USA). The amplified Thy28-V5-His6 was inserted into a pCAGGS vector (a gift from K. Miyake, Nippon Medical School, Tokyo, Japan) that contains a CMV enhancer, a chicken β-actin promoter. Three independent lines (Thy28-19, Thy28-121, and Thy28-123) were established. All experiments were approved by the Ethical Committee of Animal Experiments of Tokyo Medical University.
Western blotting
Protein samples from various tissues resolved on a 12.5 % SDS-PAGE were transferred to PVDF membranes, as previously described [26]. The blots were incubated with anti-V5 monoclonal antibody (mAb) (Invitrogen), rabbit anti-Thy28 Abs [24], and anti-actin (Sigma, St. Louis, MO, USA), followed by several washes. The blots were developed with ECL Plus Western Blotting Detection System (GE Healthcare, Buckinghamshire, UK) according to the manufacturer’s recommendations.
RT-PCR
Reverse transcription-PCR (RT-PCR) was done as previously described [24]. Briefly, total RNA was isolated from each tissue with Tri Reagent (Molecular Research Center, Inc, Cincinnati, OH, USA), according to the manufacturer’s recommendation. For preparation of cDNA, RNA was reverse transcribed using an RNA PCR kit version 2.1 (Takara, Kyoto, Japan). The sequences of the primer pairs used, 5′ and 3′ were the following: mThy28, GAATTCCACCATGCCGAGACCCCGCAAGAG and GCG GCCGCTTATTTCTCTAGCCGGCTTTCTG (fragment of 200 bp); mThy28-V5, GAATTCCGGTATAGACATGAAGTTCAGCATCGAGG and ATGACCGGTACGCGTAGAATGG (fragment of 550 bp); GAPDH, ACCACAGTCCATGCCATCAC and TCCACCACCCTGTTGCTGTA (fragment of 551 bp).
Flow cytometry of thymic, spleen, lymph node, and bone marrow cells
Cells from thymus, spleen, lymph node, and bone marrow were stained with APC-labeled anti-CD3 (BD Bioscience, Franklin Lakes, NJ, USA), FITC-anti-CD4, PE-anti-CD8, PE-anti-CD21, FITC-anti-IgD (BioLegend, San Diego, CA, USA), APC-anti-CD11c, PE/Cy7-anti-CD93, PE-, APC-, APC/Cy7-anti-B220 and APC-anti-IgM (eBioscience, San Diego, CA, USA) mAbs, followed by analysis using a flow cytometer (FACSCanto II, BD Bioscience).
Thymic cell death in vivo
Thy28 TG and WT control mice were injected intraperitoneally with 10 μg anti-CD3 mAbs (145-2C11) (BioLegend) per mouse. Two days later, thymocytes were stained with APC-anti-CD4, and PerCP-Cy5.5-anti-CD8 mAbs (BioLegend), followed by analysis using a flow cytometer. Numbers of thymocytes were determined by trypan blue dye exclusion. For determination of MMP, cells were stained with 2 μM JC-1 for 30 min at 37 °C using a MitoProbe JC-1 Assay Kit (Molecular Probes, Eugene, OR, USA), as previously described [27].
Alternatively, the percentage of cells undergoing apoptosis was evaluated by staining 1 × 106 cells with FITC-annexin V in combination with 7-amino-actinomycin-D (7-AAD) using Annexin V-FITC/7-AAD Kit (Beckman coulter, Marseille, France), according to the manufacturer’s instruction. The annexin V/7-AAD assay was used to distinguish viable (annexin V/7-AAD DN), apoptotic (annexin V+/7-AAD−), and secondary necrotic (annexin V/7-AAD DP) cells [28]. In some experiments, the cells were permeabilized with cold 50 % MeOH/PBS and stained with Abs specific for PE-anti-phospho-JNKs and FITC-anti-Bcl-xL (Cell Signaling Technology, Danvers, MA, USA), followed by analysis on a flow cytometer.
Proliferation assay
Thymocytes and spleen cells at various cell density were cultured in RPMI-1640 medium supplemented with 10 % v/v fetal bovine serum, 50 μM 2-mercaptoethanol, and 100 μg/ml kanamycin at 37 °C in a humidified atmosphere of 5 % CO2 for 3 days and pulsed with 0.5 μCi/well (1 mCi = 37 MBq) tritiated thymidine for the final 6 h, as previously described [29].
Measurement of cytokine production
Spleen naïve CD4+ T cells were isolated on an autoMACS ProSeparator (Miltenyi Biotech, Auburn, CA, USA) with a CD4+CD62L+ T cell isolation Kit II (Miltenyi), respectively. T cells were stimulated with microplates coated with anti-CD3 mAb (5 μg/ml) in the presence of anti-CD28 mAb (1 μg/ml) for 5 days and incubated with GolgiStop (monensin) for 5 h before fixation and permeabilization with BD Cytofix/Cytoperm Plus Fixation/Permeabilization Kit (BD Biosciences). The cells were stained with PE/Cy7-anti-IFN-γ, APC-anti-IL-4 (BioLegend), PE-anti-IL-17 (BD Biosciences), PE-anti-PD-1 (eBioscience), and APC-anti-CXCR5 (BioLegends) mAbs together with surface staining for FITC-anti-CD4 (BioLegend).
Induction of EAE
Thy28 TG and control WT mice were immunized by subcutaneous injection with 150 μg of MOG35-55 peptide (MEVGWYRSOFSRVVHLYRNGK) (Life Technology Corporation, Carlsbad, CA, USA) emulsified in a complete Freund’s adjuvant containing 5 mg/ml H37RA (Difco Laboratories, Inc., Detroit, MI, USA). On day 0 and 2 after immunization, 200 ng pertussis toxin (List Biological Laboratories, Inc., Campbell, CA, USA) was given intravenously. Animals were scored every 2–3 day for clinical signs: 0, no clinical signs; 1, limp tail; 2, hind limb weakness; 3, hind limb paralysis; 4, hind and forelimb paralysis; 5, severe morbidity; 6, death.
Histological staining
Animals were perfused with 20 % formalin in buffered saline under deep anesthesia. Spinal cords were taken, post fixed in the same fixative, and embedded in paraffin. They were cut into 6–8 μm sections on a vibrating microtome, followed by staining with H & E or Luxol fast blue to observe cellular infiltration or demyelination, respectively.
Statistical analysis
Data are expressed as means ± SE for each group. Statistical significance was determined by Student’s t test and was set at 0.01 or 0.05.
Results
Establishment of transgenic mice that carry Thy28 cDNA
To examine the role of Thy28 in lymphoid development, we generated three strains of TG mice (Thy28-19, Thy28-121, and Thy28-123) that harbor Thy28 cDNA under the control of a CAG promoter. The Thy28 transgene product in Thy28 TG mice (Thy28-123) was detected in several tissues including kidney, testis, and parts of the immune system such as thymus, bone marrow, spleen, and lymph node (Fig. 1). Thy28 expression in lymphoid organs including thymus, bone marrow, and spleen was also confirmed in another line Thy28-121 (Supplemental data 1). Thus, the Thy28-123 line was used in this study.
Establishment of Thy28 TG mice. Levels of Thy28 expression in various tissues from individual Thy28 TG and control WT mice were determined by Western blotting using anti-Thy28 Abs and anti-V5 mAb. Actin expression levels were also determined as control. Experiments were done twice, with essentially similar results
Development of thymus, bone marrow, spleen, and lymph node cells in Thy28 TG mice
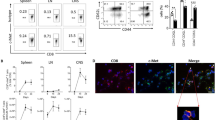
To examine whether Thy28 modulates lymphocyte development in vivo, total cell numbers and surface markers of thymus, bone marrow, spleen, and lymph node cells from Thy28 TG and control wild type (WT) mice were analyzed by flow cytometry. The number of total thymocytes was substantially increased in Thy28-TG mice, although the numbers in bone marrow, spleen, and lymph node were comparable to those in control mice (Fig. 2a). The proportions of thymus subpopulations (CD4−CD8−, CD4+CD8+, CD4+CD8−, CD4−CD8+) from Thy28 TG mice were comparable to those from control mice (Fig. 2b). Similarly, the development of bone marrow (precursor B, immature B, transitional B, mature recirculation B cells), spleen (T, B, marginal zone B, follicular B, DCs), and lymph node (T, B cells) from Thy28 TG mice was unaltered compared with control WT mice (Fig. 2b). These results suggest that Thy28-TG mice display thymic hyperplasia, but that the proportion of cells in lymphoid tissues is comparable between Thy28 TG and control mice.
Thymic hyperplasia with unaltered development of T cells and B cells in Thy28 TG mice relative to control WT mice. (a) Bone marrow, thymus, spleen, and lymph nodes from Thy28 TG and WT mice were removed. The total cell number of tissues from individual mice (n = 8 for thymus; n = 3 for other tissues) was enumerated. The results are represented as the mean ± SE. *p < 0.05. (b) Development of immune cells from each tissue (two mice pooled) is evaluated as follows: thymus, CD4−CD8−, CD4+CD8+, CD4+CD8−, and CD4−CD8+; bone marrow, precursor-B (B220+IgM−IgD−), immature B (B220+IgM+IgD−), transitional B (IgMhiIgDint/lo), mature recirculation B (IgM+IgD+) gating on B220+ cells, as reported [42]; spleen, B (B220+CD3−), marginal zone B (IgM+CD21hi) and follicular (IgM+CD21int/lo) B gating on B220+CD93− cells, T (B220−CD3+), CD4+CD8− T, CD4−CD8+ T, DCs (CD11c+) cells; lymph node, T (CD3+) cells and B (B220+) cells. The data are representative of three independent experiments
Administration of anti-CD3 mAb results in cell death, which is substantially reduced in Thy28 TG mice
Programmed cell death of DP cells in response to anti-CD3 mAb in vivo is reported to mimic negative selection [30], which is inhibited by some agents including cyclosporin A and IL-2 deficiency [31, 32], resulting in autoimmunity. Thy28-TG and control mice were injected with 10 μg anti-CD3 mAb, followed by a flow cytometry assay. Forty eight hours later, thymus from anti-CD3 mAb-administered WT mice was substantially decreased in size, whereas Thy28 TG mice showed a partial resistance to such treatment (Fig. 3a). The partial resistance to anti-CD3 mAb treatment in Thy28 TG mice was also demonstrated by percent reduction of cell number after anti-CD3 mAb administration (44.6 % in TG vs. 88.6 % in WT) (Fig. 3b). It is of note that thymus size of Thy28 TG mice was moderately bigger that that of WT mice (Fig. 3a). The decline in the proportion of DP cells relative to controls in Thy28 TG mice was partially prevented (from 84.7 to 53.1 % vs. 84.6 to 14.0 %) (Fig. 3c). Essentially similar results were obtained from other independent line Thy28-121 (Supplemental data 2).
Partial resistance to anti-CD3 mAb-induced reduction of CD4+CD8+ cells in Thy28 TG mice relative to control. Thy28 and control WT mice were injected intraperitoneally without or with 10 μg/ml anti-CD3 mAb. (a) Two days after injection, thymuses from Thy28 TG and WT mice injected without or with anti-CD3 mAb were shown. (b) Number of total viable cells without or with anti-CD3 mAb was enumerated. c Number and percentage of DP cells without or with anti-CD3 mAb administration were shown. The results are represented as mean ± SE (3 mice/group). **p < 0.01, *p < 0.05. Experiments were done six times, with essentially similar results
Number of apoptotic cells was determined by flow cytometric analysis of loss of MMP and annnexin V/7-AAD assay, as reported [7, 8]. Following anti-CD3 mAb administration, loss of MMP in Thy28 TG mice was moderately reduced compared with the control (Fig. 4a). Moreover, number of dead cells with annexin+/7-AAD– and annexin V+/7-AAD+ was also diminished in anti-CD3 mAb-treated Thy28 TG mice relative to control (Fig. 4b), suggesting that Thy28 TG mice are relatively resistant to anti-CD3 mAb-mediated apoptotic cell death in vivo.
Partial resistance to anti-CD3 mAb-induced cell death in Thy28 TG mice. Thymocytes from anti-CD3 mAb-injected TG (n = 3) and control (n = 3) individual mice were stained with JC-1 (a) or annexin-V/7-AAD (b), followed by flow cytometric analysis. The data are represented as the mean ± SE from one (b) and two (a) independent experiments. **p < 0.01, *p < 0.05
Anti-CD3 mAb-mediated JNK activation and modulation of Bcl-xL are partially prevented in Thy28 TG mice
To examine whether the anti-CD3 mAb-induced loss of MMP is associated with modulation of Bcl-2 family members, Bcl-xL expression levels in DP thymocytes from Thy28 TG and control mice were determined by flow cytometry after anti-CD3 mAb administration. Thy28 DP thymocytes showed resistance to anti-CD3 mAb-mediated down-regulation of Bcl-xL relative to WT controls (% Bcl-xL expression (median channel): 79 in TG vs. 63) (Fig. 5a).
Partial resistance to anti-CD3 mAb-induced Bcl-xL down-regulation and JNK phosphorylation in Thy28 TG DP cells relative to control. Thymocytes (n = 3) from each animal injected with anti-CD3 mAb (solid line) or control PBS (shaded) were stained with anti-CD4 and anti-CD8 mAbs, and then intracellularly stained with anti-Bcl-xL (a) and anti-phospho JNK Abs (b). Median channel from each group was shown. The data are representative of two independent experiments, with essentially similar results
Receptor-mediated modulation of Bcl-xL is preceded by JNK activation in a variety of cell types following apoptotic stimuli [4]. Compared with the control, levels of anti-CD3 mAb-mediated JNK phosphorylation were partially reduced in Thy28 TG DP thymocytes relative to the WT (% pJNK expression (median channel): 175 in TG vs. 248) (Fig. 5b), probably contributing to apoptosis resistance to anti-CD3 mAbs in vivo. Thus, Thy28 protects against the antigen-induced apoptosis through the JNK-Bcl-xL signaling pathway.
Cytokine production of Thy28 TG spleen T cells in response to anti-CD3 plus anti-CD28 mAbs
To examine whether Thy28 influences cytokine production, naïve spleen T cells were stimulated with both anti-CD3 and anti-CD28 mAbs for 5 days, followed by incubation with GolgiStop for 5 h. The cells were then stained intracellularly with anti-IFN-γ, anti-IL-4, and anti-IL-17 mAbs. The frequency of IFN-γ-positive T cells on anti-CD3/anti-CD28 mAbs stimulation was increased in Thy28 TG mice compared with WT controls (72.7 vs. 27.0 %) (Fig. 6). In contrast, the proportion of high IL-4+ cells was decreased in Thy28 TG mice (1.0 vs. 3.3 % in WT), although T cells producing total IL-4 and IL-17 were almost comparable in frequency between Thy28 TG and control mice. The proportion of follicular helper T cells expressing PD-1 and CXCR5 in Thy28 TG mice in response to anti-CD3/anti-CD28 mAbs was also similar to that of WT mice. These findings indicate that Thy28 TG mice have an increased proportion of IFN-γ+ cells with a decreased proportion of high IL-4+ cells in response to anti-CD3 and anti-CD28 mAbs.
Increased IFN-γ production by Thy28 TG spleen naïve T cells following stimulation with anti-CD3 and anti-CD28 mAbs in vitro. Spleen naïve T cells (three mice pooled) were stimulated with anti-CD3 together with anti-CD28 mAb for 5 days. The cells were stained with anti-CD4, anti-PD-1, and anti-CXCR5 mAb, and then permeabilized for intracellular staining with anti-IFN-γ, anti-IL-4, and anti-IL-17 mAbs. The data are representative of four independent experiments. IFN-γ-positive cells (%) are represented as the mean ± SE from four independent experiments. *p < 0.05
Thy28 TG mice showed accelerated induction of EAE after immunization with MOG in vivo
Because the proinflammatory cytokine IFN-γ plays a crucial role in the induction of autoimmunity including EAE [18, 33, 34], Thy28 TG and control WT mice were immunized with MOG, followed by assays for disease onset, clinical score, and pathological criteria. Thy28 TG mice showed earlier onset of MOG-induced EAE that was more severe compared with WT mice, as assessed by disease incidence and clinical score (Fig. 7a). Accumulation of inflammatory cells in the spinal cords was substantially increased in Thy28 TG mice relative to controls on day 10 and more severe on day 30, accompanied by demyelination as revealed by H&E and Luxol fast blue staining (Fig. 7b). Essentially similar findings were observed in several other independent experiments (Supplemental data 3). Thus, Thy28 affects EAE induction after MOG stimulation.
Accelerated induction of EAE by immunization with MOG in Thy28 TG mice relative to control. (a) Thy28 TG (b, d, and f) (n = 10) and control WT (a, c, and e) (n = 10) mice were immunized with MOG and assessed by clinical scores for 30 days after immunization. (b) Spinal sections were stained with H & E (a and b, day 10; c and d, day 30) and Luxol fast blue (e and f, day 30) after immunization. The location of cellular infiltration (b, d) and myelin sheath (e, f) was shown by arrows. The results are the mean ± SE. *p < 0.05. Experiments were done six times, with essentially similar results
Discussion
Thy28 is expressed in several tissues including the immune system [22], and Thy28 expression is transiently down-regulated in DP stage [24], where positive–negative selection is proposed to take place [2]. We recently demonstrated that antigen-mediated apoptotic cell death was partially prevented by Thy28 in vitro [23, 25], suggesting an anti-apoptotic role for Thy28. Cell death, including apoptosis and necrosis, has been demonstrated to be associated with inflammation such as that caused by autoimmune diseases [35, 36]. To elucidate the possible action of Thy28 in vivo, we generated Thy28 TG mice driven by a CAG promoter. Thy28 TG and control WT mice were evaluated by anti-CD3 mAb-mediated deletion of thymocytes and MOG-induced EAE [14, 15], a useful model to dissect the cellular and molecular mechanisms underlying autoimmunity.
Anti-CD3-mediated thymic apoptotic cell death is supposed to mimic negative selection in vivo [30, 37]. Apoptotic cell death in various cell types including lymphocytes is accompanied by loss of MMP and annexin V-expression [6–8]. Thymocytes from Thy28 mice were resistant to anti-CD3-mediated apoptotic cell death, as assessed by total viable cell number, annexin V-expression, and MMP (Figs. 3b, 4a, and 4b). Apoptotic cell death is regulated by several components including the Bcl-2 family consisting of pro-apoptotic (Bax, Bad) and anti-apoptotic Bcl-2 (Bcl-2, Bcl-xL, Mcl-1) members [3]. Down-regulation of anti-apoptotic protein Bcl-xL was found in DP thymocytes after anti-CD3 mAb administration to WT control mice, and it was substantially impaired in TG mice (Fig. 5a), favoring survival.
Anti-CD3-mediated death of thymocytes was impaired in JNK-deficient mice [4, 13]. Consistent with these findings, a diminished anti-CD3 mAb-mediated JNK activation in TG DP thymocytes was found relative to the control (Fig. 5b). The decreased JNK activation in Thy28 TG DP thymocytes appeared to correlate with reduced down-regulation of Bcl-xL on anti-CD3 mAb administration (Fig. 5a and b). Because JNK activation modulates functions of Bcl-2 family members such as Bcl-xL [4, 5], resulting in cell death, it may be concluded that Thy28 regulates the JNK-Bcl-xL signaling pathway leading to apoptotic cell death. Although the precise molecular mechanisms underlying Thy28-mediated JNK inactivation remain unclear, it is possible that the nuclear protein Thy28 somehow affects molecule(s) responsible for activator protein 1 (AP-1) activation because JNK phosphorylation results in AP-1 activation, which regulates cell behavior including proliferation, cell death, and inflammatory responses [4].
A partial resistance to anti-CD3-mediated cell death in Thy28 TG mice may lead to thymic hyperplasia (Fig. 2), as reported [38]. Moreover, the augmented proliferation of thymocytes in response to a low concentration of anti-CD3 mAbs in Thy28 TG mice might also contribute to spontaneous thymic hyperplasia (Toyota et al. unpublished observation). However, lymphocyte development in thymus, bone marrow, lymph node, and spleen was unaltered in Thy28 TG and control WT mice. Thymic hyperplasia has been reported to be associated with autoimmune diseases including myasthenia gravis [39]. Consistent with this notion, Thy28 TG mice displayed a more severe EAE in response to MOG relative to control (Fig. 7a and b), suggesting that threshold for positive–negative selection in thymus plays a crucial role in the induction of autoimmune diseases including EAE, as proposed by Gatzka and Walsh [40]. Although the causal relationship between the Thy28-mediated thymic hyperplasia and accelerated induction of EAE remains unclear in the present study, it is possible that auto-reactive proinflammatory T cells escaping the positive–negative selection somehow play a critical role in the induction of EAE. However, the participation of Thy28 for human MS has not yet been reported. Further studies will reveal these points.
Effector T cells and their derived cytokines, such as IFN-γ and IL-17, play crucial roles in the development of autoimmune central nervous diseases including EAE [17, 41]. The proportion of IFN-γ-positive CD4+ T cells from Thy28 TG spleen was increased on stimulation with anti-CD3/anti-CD28 mAbs relative to control WT mice (Fig. 6), whereas that of IL-4+ T cells was decreased, favoring a balance toward Th1 cytokine production. However, the frequencies of IL-17+ and follicular helper T cells were unaltered in Thy28 TG and control WT mice. The higher production of Th1 cytokine IFN-γ may contribute to the development and/or enhancement of the classical spinal form of EAE, as reported previously [18].
Together, the present studies clearly demonstrated that Thy28 partially prevents deletion of DP thymocytes in response to anti-CD3 mAbs in vivo. The partial resistance to antigen-induced cell death may contribute to T cell repertoire shifts as well as spontaneous thymic hyperplasia, which can result in organ-specific autoimmune disease such as murine model of MS, EAE. Murine EAE was induced by immunization with the auto-antigen MOG in the presence of complete Freund’s adjuvant and pertussis toxin, which mimic environmental insults including microbial pathogens. These findings have implications for understanding auto-antigen-mediated self-tolerance and also auto-immunity in vivo.
Abbreviations
- DP:
-
Double positive
- JNK:
-
c-Jun N-terminal kinase
- EAE:
-
Experimental autoimmune encephalomyelitis
- MS:
-
Multiple sclerosis
- MOG:
-
Myelin oligodendrocyte glycoprotein
- Th :
-
Helper T cell
- TG:
-
Transgenic
- WT:
-
Wild type
- MAb:
-
Monoclonal antibody
- MMP:
-
Mitochondrial membrane potential
- AP-1:
-
Activator protein 1
- CFA:
-
Complete Freund’s adjuvant
References
Sohn SJ, Rajpal A, Winoto A (2003) Apoptosis during lymphoid development. Curr Opin Immunol 15:209–216
von Boehmer H (1997) Aspects of lymphocyte developmental biology. Immunol Today 18:260–262
Reed JC (1998) Bcl-2 family proteins. Oncogene 17:3225–3236
Weston CR, Davis RJ (2002) The JNK signal transduction pathway. Curr Opin Genet Dev 12:14–21
Takada E, Hata K, Mizuguchi J (2006) Requirement for JNK-dependent upregulation of BimL in anti-IgM-induced apoptosis in murine B lymphoma cell lines WEHI-231 and CH31. Exp Cell Res 312:3728–3738
Susin SA, Zamzami N, Kroemer G (1998) Mitochondria as regulators of apoptosis: doubt no more. Biochim Biophys Acta 1366:151–165
Hildeman DA, Mitchell T, Teague TK et al (1999) Reactive oxygen species regulate activation-induced T cell apoptosis. Immunity 10:735–744
Roger J, Chalifour A, Lemieux S, Duplay P (2001) Cutting edge: Ly49A inhibits TCR/CD3-induced apoptosis and IL-2 secretion. J Immunol 167:6–10
Zinkel S, Gross A, Yang E (2006) BCL2 family in DNA damage and cell cycle control. Cell Death Differ 13:1351–1359
Dong C, Davis RJ, Flavell RA (2002) MAP kinases in the immune response. Annu Rev Immunol 20:55–72
Minden A, Karin M (1997) Regulation and function of the JNK subgroup of MAP kinases. Biochim Biophys Acta 1333:F85–F104
Xia Z, Dickens M, Raingeaud J, Davis RJ, Greenberg ME (1995) Opposing effects of ERK and JNK-p38 MAP kinases on apoptosis. Science 270:1326–1331
Sabapathy K, Kallunki T, David JP, Graef I, Karin M, Wagner EF (2001) c-Jun NH2-terminal kinase (JNK)1 and JNK2 have similar and stage-dependent roles in regulating T cell apoptosis and proliferation. J Exp Med 193:317–328
Baxter AG (2007) The origin and application of experimental autoimmune encephalomyelitis. Nat Rev Immunol 7:904–912
Gold R, Linington C, Lassmann H (2006) Understanding pathogenesis and therapy of multiple sclerosis via animal models: 70 years of merits and culprits in experimental autoimmune encephalomyelitis research. Brain 129:1953–1971
Steinman L (2007) A brief history of T(H)17, the first major revision in the T(H)1/T(H)2 hypothesis of T cell-mediated tissue damage. Nat Med 13:139–145
Petermann F, Korn T (2011) Cytokines and effector T cell subsets causing autoimmune CNS disease. FEBS Lett 585:3747–3757
Lees JR, Golumbek PT, Sim J, Dorsey D, Russell JH (2008) Regional CNS responses to IFN-gamma determine lesion localization patterns during EAE pathogenesis. J Exp Med 205:2633–2642
Chu CQ, Wittmer S, Dalton DK (2000) Failure to suppress the expansion of the activated CD4 T cell population in interferon gamma-deficient mice leads to exacerbation of experimental autoimmune encephalomyelitis. J Exp Med 192:123–128
Sutton C, Brereton C, Keogh B, Mills KH, Lavelle EC (2006) A crucial role for interleukin (IL)-1 in the induction of IL-17-producing T cells that mediate autoimmune encephalomyelitis. J Exp Med 203:1685–1691
Park H, Li Z, Yang XO et al (2005) A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat Immunol 6:1133–1141
Miyaji H, Yoshimoto T, Asakura H et al (2002) Molecular cloning and characterization of the mouse thymocyte protein gene. Gene 297:189–196
Jiang XZ, Toyota H, Yoshimoto T, Takada E, Asakura H, Mizuguchi J (2003) Anti-IgM-induced down-regulation of nuclear Thy28 protein expression in Ramos B lymphoma cells. Apoptosis 8:509–519
Jiang X, Toyota H, Takada E et al (2003) Modulation of mThy28 nuclear protein expression during thymocyte development. Tissue Cell 35:471–478
Toyota H, Jiang XZ, Asakura H, Mizuguchi J (2012) Thy28 partially prevents apoptosis induction following engagement of membrane immunoglobulin in WEHI-231 B lymphoma cells. Cell Mol Biol Lett 17:36–48
Toyota H, Yanase N, Yoshimoto T, Moriyama M, Sudo T, Mizuguchi J (2003) Calpain-induced Bax-cleavage product is a more potent inducer of apoptotic cell death than wild-type Bax. Cancer Lett 189:221–230
Furuhata M, Takada E, Noguchi T, Ichijo H, Mizuguchi J (2009) Apoptosis signal-regulating kinase (ASK)-1 mediates apoptosis through activation of JNK1 following engagement of membrane immunoglobulin. Exp Cell Res 315:3467–3476
Vermes I, Haanen C, Reutelingsperger C (2000) Flow cytometry of apoptotic cell death. J Immunol Methods 243:167–190
Cao Y, Takada E, Hata K, Sudo K, Furuhata M, Mizuguchi J (2010) Enhanced T cell-independent antibody responses in c-Jun N-terminal kinase 2 (JNK2)-deficient B cells following stimulation with CpG-1826 and anti-IgM. Immunol Lett 132:38–44
Smith CA, Williams GT, Kingston R, Jenkinson EJ, Owen JJ (1989) Antibodies to CD3/T-cell receptor complex induce death by apoptosis in immature T cells in thymic cultures. Nature 337:181–184
Shi YF, Sahai BM, Green DR (1989) Cyclosporin A inhibits activation-induced cell death in T-cell hybridomas and thymocytes. Nature 339:625–626
Bassiri H, Carding SR (2001) A requirement for IL-2/IL-2 receptor signaling in intrathymic negative selection. J Immunol 166:5945–5954
Baccala R, Kono DH, Theofilopoulos AN (2005) Interferons as pathogenic effectors in autoimmunity. Immunol Rev 204:9–26
Cope A, Le Friec G, Cardone J, Kemper C (2011) The Th1 life cycle: molecular control of IFN-gamma to IL-10 switching. Trends Immunol 32:278–286
Patel VA, Lee DJ, Longacre-Antoni A et al (2009) Apoptotic and necrotic cells as sentinels of local tissue stress and inflammation: response pathways initiated in nearby viable cells. Autoimmunity 42:317–321
Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME (2008) The consequences of apoptosis in autoimmunity. J Autoimmun 31:257–262
McConkey DJ, Hartzell P, Amador-Perez JF, Orrenius S, Jondal M (1989) Calcium-dependent killing of immature thymocytes by stimulation via the CD3/T cell receptor complex. J Immunol 143:1801–1806
Volkmann A, Doffinger R, Ruther U, Kyewski BA (1996) Insertional mutagenesis affecting programmed cell death leads to thymic hyperplasia and altered thymopoiesis. J Immunol 156:136–145
Sherer Y, Bardayan Y, Shoenfeld Y (1997) Thymoma, thymic hyperplasia, thymectomy and autoimmune diseases (Review). Int J Oncol 10:939–943
Gatzka M, Walsh CM (2007) Apoptotic signal transduction and T cell tolerance. Autoimmunity 40:442–452
Ercolini AM, Miller SD (2006) Mechanisms of immunopathology in murine models of central nervous system demyelinating disease. J Immunol 176:3293–3298
Acknowledgments
We thank Dr. K. Miyake (Department of Biochemistry and Molecular Biology, Nippon Medical School, Tokyo, Japan) for pCAGGS vector. This work was supported by a Grant from the Intractable Immune System Disease Center of Tokyo Medical University, which is supported by the Ministry of Education, Culture, Sports, Science, and Technology of Japan. We also thank Zenji Sakamoto for his support.
Conflict of interest
We declare no conflicts of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
S1
Thy28 expression in Thy28-121 TG mice. Thy28 mRNA expression levels from Thy28 TG and control individual mice were determined by RT-PCR. Expression levels of GAPDH were also determined as control. The data are representative of three independent experiments. (PPTX 12 kb)
S2
Anti-CD3 mAb-induced reduction in proportion of DP thymocytes is abrogated in Thy28-121 TG mice. The percentage of DP cells from Thy28-121 or control mice after administration of 10 μg anti-CD3 mAb or control PBS was enumerated. The data are representative of three independent experiments. (PPTX 109 kb)
S3
Thy28-121 TG mice demonstrate an augmented clinical score, accompanied by increased cell infiltration and demyelination relative to control mice following MOG administration. (A) Thy28-121 TG (n=10) and control WT mice (n=10) were immunized with MOG and assessed by clinical score every 1–3 day up to day 25. (B) In a separate experiment, Thy28 TG (b and d, n=5) and WT mice (a and c, n=5) were assessed by disease incidence, clinical score, and pathology on day 25 after MOG immunization. Spinal sections stained with H & E (a and b) and Luxol fast blue (c and d). The location of cellular infiltration (b) and myelin sheath (d) was shown by arrows. (PPTX 128 kb)
Rights and permissions
About this article
Cite this article
Toyota, H., Sudo, K., Kojima, K. et al. Thy28 protects against anti-CD3-mediated thymic cell death in vivo. Apoptosis 20, 444–454 (2015). https://doi.org/10.1007/s10495-014-1082-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10495-014-1082-0