Abstract
The effects of temperature on the expression patterns and enzyme activity of cathepsin B (HlCatB), cathepsin D (HlCatD) and acid phosphatase (HlACP) during the embryo development of Haemaphysalis longicornis (bisexual population) were investigated in this study. Eggs were exposed to 20 °C (low temperature), 26 °C (normal temperature), and 30 °C (high temperature) immediately after laying, and collected on odd days of embryo development to measure HlCatB, HlCatD and HlACP gene expression using quantitative real-time PCR, as well as three enzyme activities using spectrophotometry. Then the associations between mRNA expression levels of three enzymes and their enzyme activities were assessed. Compared with normal temperature, the mRNA expression peaks of HlCatB were higher and appeared later at low and high temperatures and the activity of HlCatB increased on most days of embryonic development at high temperature. As for HlCatD, the expression peak appeared later at low temperature, but earlier at high temperature. The activity peaks of HlCatD were lower and appeared earlier at low and high temperatures. As for HlACP, the expression peak was higher and appeared later at low temperature, whereas it formed no prominent peak at high temperature. The activity peak of HlACP was higher at low temperature, but lower at high temperature. The linear regression analysis showed that activities of three enzymes were associated with their mRNA expression levels (P < 0.05). Three enzymes are involved in the embryo adaptation to temperature stress. Moreover, the mRNA expression level may be another factor affecting its enzyme activity.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks (Acari: Ixodidae) are obligate hematophagous parasites that act as vector of a wide range of pathogens to humans and animals, including spotted fever group Rickettsia, Ehrlichia, Bartonella and Babesia, and thus cause significant economic losses (Alexander et al. 2018; Li et al. 2018; Palus et al. 2018). They are distributed globally (da Silva et al. 2004). Ticks are known for their reproductive capacity, laying up to several thousands of eggs at one time (Zhang et al. 2017). Like most other species, population growth of ticks is mainly depending on the environmental temperature (Dantas-Torres and Otranto 2011). It has been reported that, when exposed to temperature stresses, engorged female ticks of the species Rhipicephalus microplus, Rhipicephalus sanguineus, Dermacentor reticulatus, and Haemaphysalis longicornis can decrease egg numbers and production ratios, and prolong pre-oviposition (Dantas-Torres and Otranto 2011; Esteves et al. 2015; Jia et al. 2018; Yano et al. 1987; Zahler and Gothe 1995). Even though this temperature stress response is widely acknowledged in research, the mechanisms how temperature affects tick embryonic development are still poorly understood.
Embryogenesis success is mainly dependent on the occurrence of a precise sequence of hydrolytic enzymes (Wang et al. 2016). Cathepsin B, cathepsin D, and acid phosphatase are reported to be involved in insects’ embryo development, such as Culex quinquefasciatus, Rhodnius prolixus, and Periplaneta americana (Gomes et al. 2010; Moura et al. 2015; Oliveira et al. 2008). Cathepsin B has the ability to bind to the extracellular matrix where it takes part in various biological functions, such as major histocompatibility complex Class II (MHC-II)-mediated antigen presentation, inflammation, and apoptosis (Pezhman et al. 2017). Cathepsin D is a soluble aspartic endopeptidase – also localized to lysosomes – that usually presents as a glycoprotein. Studies have shown cathepsin D involved in several physiological processes, including vitellin production and degradation, fat body decomposition, and apoptosis process (Kang et al. 2017; Leyria et al. 2015; Rabossi et al. 2004). Acid phosphatase is a lysosomal enzyme responsible for hydrolyzing various phosphate-containing substrates. These lysosomal enzymes are interconnected and interworked to ensure embryo development. Acid phosphatase inhibitors could block cathepsin D activity during the embryo development of R. prolixus (Fialho et al. 2005). In Bombyx mori, when RNAi reduced either cathepsin B or cathepsin D, expression of the remaining cathepsin B or cathepsin D was augmented (Lee et al. 2009).
Cathepsin B and Cathepsin D also play important roles in the embryonic development of Rh. microplus (Nascimento-Silva et al. 2008; Oldiges et al. 2012). Previous research conducted by the authors also showcased that cathepsin B (HlCatB), cathepsin D (HlCatD), and acid phosphatase (HlACP) were involved in the embryonic development of H. longicornis (Qiu et al. 2020; Zhang et al. 2019). RNA interference of HlCatB, HlCatD, and HlACP in engorged female ticks significantly decreased the hatching rate of the eggs (Qiu et al. 2020). However, it remains to be determined how temperature stress – an important environmental factor – affects the three enzymes during the embryonic development of the tick. To further understand this uncertainty, the eggs of hard tick H. longicornis (bisexual population) were put under stress at varying temperatures. Following temperature stress induction, tests were designed to measure: mRNA expression and activity of HlCatB, HlCatD and HlACP during embryogenesis. This study would lay a scientific basis for further exploring the relationship between tick embryo development and temperature, and provide more information on the future forecast of the tick population dynamic under global climate changes.
Materials and methods
Tick reared and egg collection
Free-living H. longicornis ticks (bisexual population) were collected at the Xiaowutai National Natural Conservation Area (39°50’ − 40°07’ N, 114°47’ − 115°30’ E), Hebei Province, China. They were then reared inside small cloth bags and later glued onto ears of individual New Zealand white rabbit species, Oryctolagus cuniculus, during the parasitize stages. After engorgement, ticks were kept in an incubator for oviposition [26 ± 1 °C, 75% RH, 6L:18D]. To determine the effect of temperature on mRNA expression patterns and enzyme activity of HlCatB, HlCatD, and HlACP during embryonic development, eggs collected from 20 female ticks (a total of 0.5 g) were incubated at different temperatures immediately after laying: 26 °C as the normal-temperature group, 20 °C as the low-temperature group, and 30 °C as the high-temperature group. The eggs (0.05 g) were sampled on odd days (days 1, 3, 5, 7, 9, etc.) of embryonic development for analysis until the first tick hatched.
Expression analysis of three enzymes during embryo development under different temperatures
Egg samples were homogenized in micro-centrifuge flex tubes and AxyPrep™ Multisource Total RNA Miniprep Kit (Axygen, USA) was used to extract RNA in the instructed manner. RNA concentration and purity was evaluated using a NanoDrop device (Thermo Fisher Scientific, USA); complementary DNA synthesis was carried out immediately using a one-step complementary DNA (cDNA) synthesis kit (TransGen Biotech, China).
Quantitative real-time polymerase chain reactions (qPCRs) of HlCatB, HlCatD, and HlACP in sampled eggs were performed using a Mx3005P qPCR system (Agilent Technologies, USA). The primers of HlCatB, HlCatD, and HlACP were consistent with those presented in the previous Zhang et al. (2019) study. Measured PCR was initially denatured at 94 °C for 10 min, followed by 35 cycles of denaturation at 94 °C for 5 s, annealing at 60 °C for 30 s, as well as an extension period at 72 °C for 30 s. Melt curve analyses were traced for all three genes; the presence of a unique peak confirmed the PCR product specificity. Previous studies in our lab (Qiu et al. 2020; Zhang et al. 2019) showed that beta-actin (β-actin) expression level was stable at various developmental stages of egg, so it was chosen as the internal reference gene to normalize relative expression levels of the three genes. The Ct values were calculated and analyzed by using the comparative Ct (2−ΔΔCT) method (Qu et al. 2018).
Activity assays of three enzymes during embryo development under different temperatures
Eggs collected on odd days of embryonic development were individually homogenized with a cold phosphate-balanced solution containing protease inhibitors. Subsequently, samples were centrifuged at 4 °C for 40 min at 14,000× g. After this, the supernatant was transferred to a 1.5-mL tube for immediate testing. Total protein concentration was attained through the Bradford protein assay method, using a protein standard of bovine serum albumin.
The activity of cathepsin B and D was assessed using Activity Fluorometric Assay Kits (BioVision, USA) following the manufacturer’s instructions. Activity of HlCatB was detected using the substrate – labeled with amino-4-trifluoromethyl coumarin (AFC). Then AFC was cleaved from the synthetic substrate by HlCatB and quantified using a fluorescence plate reader (Thermo Fisher Scientific). The reaction product was detected with 400/505 nm excitation/emission filters. The HlCatD activity was detected using GKPILFFRLK (Dnp)-D-R-NH2-MCA peptides as the substrate, which could be cleaved by HlCatD to release fluorescence and then quickly quantified by using a fluorescence plate reader. The reaction product was detected using 328/460 nm excitation/emission filters. Acid phosphatase activity was detected using p-nitrophenyl phosphate (pNPP), a phosphatase substrate, which was transformed into top-nitrophenol (pNP) and turned the color yellow (BioVision). Enzymatic assays were conducted using 5 mM pNPP as the substrate and specific activity was detected at 405 nm.
Statistical analysis
All statistical analysis was performed by SPSS for Windows v.12.0 (SPSS, USA). Both expression amount and enzyme activity on the odd days of embryonic development was recorded, with the curves of expression amount and enzyme activity throughout the development then plotted. Test of normality was conducted using Shapiro–Wilk test. Two-way analysis of variance (ANOVA) was conducted to determine differences among the groups, followed by Tukey’s post hoc tests for multiple comparisons. The linear regression analysis was performed to assess associations with HlCatB, HlCatD and HlACP expression and their enzyme activities. The level of significance was all set at α = 0.05.
Results
The HlCatB dynamic changes during embryo development under different temperatures
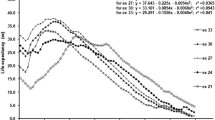
The effect of temperature on the HlCatB expression during the embryo development was assessed by comparing the normal-temperature-exposed eggs (26 °C) to the abnormal-temperature-exposed eggs (20 and 30 °C). The mRNA expression curves of HlCatB during embryonic development under the varying temperatures are depicted in Fig. 1. The results indicated that exposure to different temperatures significantly influenced HlCatB mRNA expression during embryonic development (2-way ANOVA: F8,54 = 113.5, P < 0.0001). Compared with the normal-temperature group, the expression level of HlCatB gene was significantly different at days 3, 7, and 15 in the low-temperature group and that was significantly different at days 1, 3, 5, 7, 9, 11, 13 and 15 in the high-temperature group (Fig. 1). The peaks of HlCatB expression in the low- and high-temperature groups both appeared 2 days later than that in the normal-temperature group, with the peak values of HlCatB expression being higher in the low- and high-temperature groups.
The effect of temperature on HlCatB activity during embryonic development was assessed. Activity curves of HlCatB throughout embryonic development under the varying temperatures are shown in Fig. 2. The results showed that exposure to different temperatures significantly influenced HlCatB activity during embryonic development (2-way ANOVA: F8,54 = 94.64, P < 0.0001). Compared with the normal-temperature group, the activity of HlCatB in the low-temperature group was significantly different at days 3, 7 and 17 and that in the high-temperature group was significantly different at days 1, 5, 7, 11, 13 and 15 (Fig. 2). On most days of embryonic development, the activity of HlCatB increased in the high-temperature group.
The HlCatD dynamic changes during egg development under different temperatures
The mRNA expression trends of HlCatD during embryonic development under different temperatures are illustrated in Fig. 3. The results indicated that exposure to different temperatures significantly influenced the mRNA expression of HlCatD during embryonic development (2-way ANOVA: F8,54 = 28.59, P < 0.0001). Compared with the normal-temperature group, the expression level of HlCatD gene in the low-temperature group was significantly different at days 3, 5, 11, 13, 15 and 17 and that in the high-temperature group was significantly different at days 3, 5, 7 and 9 (Fig. 3). The peak of HlCatD expression in the low-temperature group appeared 4 days later than that in the normal-temperature group, whereas it appeared 2 days earlier in the high-temperature group. Peak values in low- and high- temperature groups were both higher than that in normal temperature group.
Activity curves of HlCatD throughout embryonic development exposure to varying temperatures are shown in Fig. 4. The results showed that exposure to different temperatures significantly influenced HlCatD activity during embryonic development (2-way ANOVA: F8,54 = 143.4, P < 0.0001). Compared with the normal-temperature group, the activity of HlCatD in the low-temperature group was significantly different at days 1, 3, 5, 7 and 13 and that in the high-temperature group was significantly different at days 3, 5, 7, 11 and 13 (Fig. 4). The trends of HlCatD activity during embryonic development in the low- and high-temperature groups were similar, with peak values in the low- and high-temperature groups being lower and appearing earlier than that in the normal-temperature group.
The HlACP dynamic changes during egg development under different temperatures
The mRNA expression curves of HlACP during embryonic development under varying temperatures are illustrated in Fig. 5. The result showed that exposure to different temperatures significantly influenced HlACP mRNA expression during embryonic development (2-way ANOVA: F8,54 = 81.95, P < 0.0001). Compared with the normal-temperature group, the expression level of HLACP gene in the low-temperature group was significantly different at days 1, 3, 5, 11, 15 and 17 and that in the high-temperature group was significantly different at days 3, 5 and 11 (Fig. 5). The expression peak of HlACP was higher and appeared later in the low-temperature group, whereas it was lower and formed no prominent peak in the high-temperature group.
Curves of HlACP activity throughout embryonic development when placed under different temperatures are shown in Fig. 6. The results showed that exposure to different temperatures significantly influenced HlACP activity during embryonic development (2-way ANOVA: F8,54 = 115.2, P < 0.0001). Compared with the normal-temperature group, the activity of HlCatD in the low-temperature group was significantly different at days 1, 3, 5, 7, 9, 13, 15 and 17 and that in the high-temperature group was significantly different at days 1, 5, 9 and 11 (Fig. 6). When compared with the normal-temperature group, the activity level of HlACP was higher and formed no prominent peak in low-temperature group, whereas the peak was lower in the high-temperature group.
Regression analysis
Linear regression analysis revealed a statistically significant relationship between HlCatB, HlCatD and HlACP expression with their enzyme activities. Regression coefficient of HlCatB, HlCatD and HlACP were 32.63 (F1,77 = 77.66), 3.30 (F1,77 = 99.35) and 3.79 (F1,77 = 185.4, all P < 0.001), respectively. The results indicated gene expression level affected its enzyme activity.
Discussion
A variety of proteolytic enzymes act precisely and orderly to ensure the embryo successfully develop (Giorgi et al. 1999). Cathepsin B, cathepsin D, and acid phosphatase were all involved in the process of tick embryo development. But it was poorly understood that how these three enzymes work together in an orderly way. In the normal-temperature group, the mRNA expression of HlCatB was at a high level from the 3rd day to the 7th day of embryo development, the mRNA expression level of HlCatD was high from the 9th day to the 15th day of embryo development, and the mRNA expression level of HlACP was high from the 9th day to the 13th day of embryo development. The results suggested that HlCatB may mainly participate in the early stage of embryo development; HlCatD and HlACP in the middle and late stages of embryo development. Additionally, activity peaks of HlCatB and HlCatD occurred 2 days after their gene expression peak, respectively.
As for HlCatB, the results showed that the expression peaks of HlCatB in response to low and high temperatures were enhanced during embryo development. The previous study demonstrated that the higher cathepsin B mRNA level might be predictive of deficient oocytes and embryos quality, since it was related to activation of the apoptotic pathway (Bettegowda et al. 2008). Then the enhancement expression of HlCatB that temperature stress-induced in this study may result in excessive apoptosis in H. longicornis, which could inhibit the development of embryo and decrease the hatching rate. Therefore, up-regulated expression of HlCatB may play an important role in reducing the hatching rate of tick eggs. Our result showed that the high temperature could increase the activity of HlCatB, which was consistent with a previous study in mammals (Yamanaka et al. 2018).
It also analyzed whether HlCatD expression affected by temperature stress, and the results showed that the temperature stress enhanced the HlCatD expression peaks during embryo development. In B. mori, the high temperature could increase the expression level of cathepsin D, which our findings in eggs of H. longicornis were consistent with (Kim et al. 2011). Kang et al. (2017) showed that the expression of cathepsin D was negatively correlated with the hatching rate in the beet armyworm (Spodoptera exigua). We also found a similar phenomenon in eggs of H. longicornis. Inappropriate temperature could reduce egg hatchability in H. longicornis (Yano et al. 1987). Meanwhile, low temperature also increased expression level of HlCatD in our study. Therefore, hatchability induced by inappropriate temperature may be related to the increased expression level of HlCatD in H. longicornis. It was notable that the activity of HlCatD was much lower than the activity of HlCatB during embryo development in this study, suggesting that HlCatD may play a less important role than HlCatB during embryo development.
As for HlACP, the result showed that low temperature enhanced the expression peak of HlACP, and the high temperature made it lose its expression peak during embryo development. Meanwhile, the enzyme activity of HlACP was decreased with the temperature in the range of 20 to 30 °C, and the highest activity was at 20 °C. The effect of temperature on acid phosphatase expression and its enzyme activity in insects has not been studied. A similar phenomenon was also observed in the yeast Yarrowia lipolytica that the level of the acid phosphatase activity decreased as temperature increased from 15 to 36 °C (Vasileva-Tonkova et al. 1996). The evidence above suggested that acid phosphatase activity may be decreased with temperature and participated in the temperature adaptation.
Conclusion
This study is the first to show that temperature affects gene expression and enzyme activity of HlCatB, HlCatD, and HlACP during embryo development of ticks. The mRNA expression peaks of HlCatB were higher and appeared later at low and high temperatures and the activity of HlCatB increased at high temperature on most days of embryonic development. As for HlCatD, the expression peak appeared later at low temperature, but earlier at high temperature. The activity peaks of HlCatD were lower and appeared earlier at low and high temperatures than that at normal temperature. Regarding for HlACP, the expression peak was higher and appeared later at low temperature, whereas it was lower and formed no prominent peak at high temperature. The activity level of HlACP was higher at low temperature, but was lower at high temperature. In addition, regression analysis showed that gene expression levels of three enzymes affected their activities. This study provides more information about the relationship between tick embryo development and temperature, and advanced our knowledge about the underlying mechanism that the tick embryo responds to temperature stress.
References
Alexander GW, Santiago S, Claire J, Patrick L, Lisa W, Marie-Josée F (2018) Host functional connectivity and the spread potential of Lyme disease. Landsc Ecol 33:1925–1938. https://doi.org/10.1007/s10980-018-0715-z
Bettegowda A, Patel OV, Lee KB, Park KE, Salem M, Yao J, Ireland JJ, Smith GW (2008) Identification of novel bovine cumulus cell molecular markers predictive of oocyte competence: functional and diagnostic implications. Biol Reprod 79(2):301–309. https://doi.org/10.1095/biolreprod.107.067223
da Silva Vaz I Jr, Imamura S, Ohashi K, Onuma M (2004) Cloning, expression and partial characterization of a Haemaphysalis longicornis and a Rhipicephalus appendiculatus glutathione S-transferase. Insect Mol Biol 13(3):329–335. https://doi.org/10.1111/j.0962
Dantas-Torres F, Otranto D (2011) Cold-stress response of engorged females of Rhipicephalus sanguineus. Exp Appl Acarol 54(3):313–318. https://doi.org/10.1007/s10493-011-9439-3
Esteves E, Pohl PC, Klafke GM, Reck J, Fogaça AC, Martins JR, Daffre S (2015) Low temperature affects cattle tick reproduction but does not lead to transovarial transmission of Anaplasma marginale. Vet Parasitol 214(3–4):322–326. https://doi.org/10.1016/j.vetpar
Fialho E, Nakamura A, Juliano L, Masuda H, Silva-Neto MA (2005) Cathepsin D-mediated yolk protein degradation is blocked by acid phosphatase inhibitors. Arch Biochem Biophys 436(2):246–253. https://doi.org/10.1016/j.abb.2005.01.005
Giorgi F, Bradley JT, Nordin JH (1999) Differential vitellin polypeptide processing in insect embryos. Micron 30:579–596
Gomes FM, Oliveira DM, Motta LS, Ramos IB, Miranda KM, Machado EA (2010) Inorganic polyphosphate inhibits an aspartic protease-like activity in the eggs of Rhodnius prolixus (Stahl) and impairs yolk mobilization in vitro. J Cell Physiol 222(3):606–611. https://doi.org/10.1002/jcp.21975
Jia Q, Wang H, Wang T, Dong N, Ren S, Yang X, Liu J, Yu Z (2018) Effect of temperature on reproductive fitness of the engorged tick, Haemaphysalis longicornis (Acari: Ixodidae). Pakistan J Zool 50(1):369–372. https://doi.org/10.17582/journal.pjz/2018. 50.1.sc2
Kang T, Jin R, Zhang Y, Wan H, Lee KS, Jin BR, Li J (2017) Functional characterization of the aspartic proteinase cathepsin D in the beet armyworm (Spodoptera exigua). Gene 617:1–7. https://doi.org/10.1016/j.gene.2017.03.035
Kim BY, Lee KS, Sohn MR, Kim KY, Choi KH, Kang PD, Jin BR (2011) Bombyx mori cathepsin D expression is induced by high temperature and H2O2 exposure. J Asia-Pac Entomol 14:285–288. https://doi.org/10.1016/j.aspen.2011.04.002
Lee KS, Kim BY, Choo YM, Yoon HJ, Kang PD, Woo SD, Sohn HD, Roh JY, Gui ZZ, Je YH, Jin BR (2009) Expression profile of cathepsin B in the fat body of Bombyx mori during metamorphosis. Comp Biochem Physiol B Biochem Mol Biol 154(2):188–194. https://doi.org/10.1016/j.cbpb.2009.06.002
Leyria J, Fruttero LL, Nazar M, Canavoso LE (2015) The role of DmCatD, a cathepsin D-Like peptidase, and acid phosphatase in the process of follicular atresia in Dipetalogaster maxima (Hemiptera: Reduviidae), a vector of Chagas’ disease. PLoS ONE 10(6):e0130144. https://doi.org/10.1371/journal.pone.0130144
Li S, Li Y, Wang Q, Yu X, Liu M, Xie H, Qian L, Ye L, Yang Z, Zhang J, Zhu H, Zhang W (2018) Multiple organ involvement in severe fever with thrombocytopenia syndrome: an immunohistochemical finding in a fatal case. Virol J 15(1):97. https://doi.org/10.1186/s12985-018-1006-7
Moura AS, Cardoso AF, Costa-da-Silva AL, Winter CE, Bijovsky AT (2015) Two cathepsins B are responsible for the yolk protein hydrolysis in Culex quinquefasciatus. PLoS ONE 10(2):e0118736. https://doi.org/10.1371/journal.pone.0118736
Nascimento-Silva MC, Leal AT, Daffre S, Juliano L, da Silva Vaz I Jr, Paiva-Silva Gde O, Oliveira PL, Sorgine MH (2008) BYC, an atypical aspartic endopeptidase from Rhipicephalus (Boophilus) microplus eggs. Comp Biochem Physiol B Biochem Mol Biol 149(4):599–607. https://doi.org/10.1016/j.cbpb.2007.12.007
Oldiges DP, Parizi LF, Zimmer KR, Lorenzini DM, Seixas A, Masuda A, da Silva Vaz I Jr, Termignoni C (2012) A Rhipicephalus (Boophilus) microplus cathepsin with dual peptidase and antimicrobial activity. Int J Parasitol 42(7):635–645. https://doi.org/10.1016/j.ijpara
Oliveira DM, Ramos IB, Reis FC, Lima AP, Machado EA (2008) Interplay between acid phosphatase and cysteine proteases in mediating vitellin degradation during early embryogenesis of Periplaneta americana. J Insect Physiol 54(5):883–891. https://doi.org/10.1016/j.jinsphys.2008.04.005
Palus M, Sohrabi Y, Broman KW, Strnad H, Šíma M, Růžek D, Volkova V, Slapničková M, Vojtíšková J, Mrázková L, Salát J, Lipoldová M (2018) A novel locus on mouse chromosome that influences survival after infection with tick-borne encephalitis virus. BMC Neurosci 19(1):39. https://doi.org/10.1186/s12868-018-0438-8
Pezhman M, Hosseini SM, Ostadhosseini S, Rouhollahi Varnosfaderani S, Sefid F, Nasr-Esfahani MH (2017) Cathepsin B inhibitor improves developmental competency and cryo-tolerance of in vitro ovine embryos. BMC Dev Biol 17(1):10. https://doi.org/10.1186/s12861-017-0152-2
Qiu ZX, Li Y, Li MM, Wang WY, Zhang TT, Liu JZ (2020) Investigation of three enzymes and their roles in the embryonic development of parthenogenetic Haemaphysalis longicornis. Parasite Vector 13(1):46. https://doi.org/10.1186/s13071-020-3916-7
Qu C, Wang R, Che W, Zhu X, Li F, Luo C (2018) Selection and evaluation of reference genes for expression analysis using quantitative real-time PCR in the asian Ladybird Harmonia axyridis (Coleoptera: Coccinellidae). PLoS ONE 13(6):e0192521. https://doi.org/10.1371/journal.pone.0192521
Rabossi A, Stoka V, Puizdar V, Turk V, Quesada-Allué LA (2004) Novel aspartyl proteinase associated to fat body histolysis during Ceratitis capitata early metamorphosis. Arch Insect Biochem Physiol 57(2):51–67. https://doi.org/10.1002/arch.20011
Vasileva-Tonkova E, Balasheva DM, Galabova D (1996) Influence of growth temperature on the acid phosphatase activity in the yeast Yarrowia lipolytica. FEMS Microbiol Lett 145(2):267–271. https://doi.org/10.1111/j.1574-6968.1996.tb08588.x
Wang D, Zhang Y, Dong Z, Guo P, Ma S, Guo K, Xia Q, Zhao P (2016) Serine protease P-IIc is responsible for the digestion of yolk proteins at the late stage of silkworm embryogenesis. Insect Biochem Mol Biol 74:42–49. https://doi.org/10.1016/j.ibmb.2016.03.003
Yamanaka KI, Khatun H, Egashira J, Balboula AZ, Tatemoto H, Sakatani M, Takenouchi N, Wada Y, Takahashi M (2018) Heat-shock-induced cathepsin B activity during IVF and culture compromises the developmental competence of bovine embryos. Theriogenology 114:293–300. https://doi.org/10.1016/j.theriogenology.2018.04.005
Yano Y, Shiraishi S, Uchida TA (1987) Effects of temperature on development and growth in the tick, Haemaphysalis longicornis. Exp Appl Acarol 3(1):73–78. https://doi.org/10.1007/BF01200415
Zahler M, Gothe R (1995) Effect of temperature and humidity on egg hatch, moulting and longevity of larvae and nymphs of Dermacentor reticulatus (Ixodidae). Appl Parasitol 36(1):53–65
Zhang CM, Li NX, Zhang TT, Qiu ZX, Li Y, Li LW, Liu JZ (2017) Endosymbiont CLS-HI plays a role in reproduction and development of Haemaphysalis longicornis. Exp Appl Acarol 73(3–4):429–438. https://doi.org/10.1007/s10493-017-0194-y
Zhang TT, Qiu ZX, Li Y, Wang WY, Li MM, Guo P, Liu JZ (2019) The mRNA expression and enzymatic activity of three enzymes during embryo development of the hard tick Haemaphysalis longicornis. Parasite Vector 12(1):96. https://doi.org/10.1186/s13071-019-3360-3368
Acknowledgements
This work was supported by the National Natural Science Foundation of China (Grant No. 31672365). The funder had no role in the study design, data collection, and analysis, decision to publish or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
D.W. and J.L. conceived the study and designed the experiments. D.W., M.L., X.W. and J.M performed the experiments. M.L. and J.M. performed the data analysis. D.W. wrote the original draft. All authors reviewed, edited and contributed to the final version of the manuscript and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Wang, D., Li, M., Ma, J. et al. Effects of temperature on cathepsin B, cathepsin D and acid phosphatase during embryo development of the hard tick Haemaphysalis longicornis. Exp Appl Acarol 89, 105–115 (2023). https://doi.org/10.1007/s10493-022-00774-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-022-00774-z