Abstract
The chilli thrips, Scirtothrips dorsalis Hood, is a recently established pest in the USA and poses a serious risk to many economically important ornamental and food crops. In this study the biological control potential of the phytoseiid mites Amblydromalus limonicus (Garman and McGregor) and Amblyseius swirskii (Athias-Henriot) was compared by examining their predation and oviposition rates when fed different developmental stages of S. dorsalis. Gravid females were offered 10 individuals of either first instar, second instar, or adult S. dorsalis using a no-choice leaf disc bioassay and oviposition and predation rates were assessed daily for 2 and 3 days, respectively. There was no significant difference in predation and oviposition rates between mite species fed specific S. dorsalis life stages. There was, however, a significant effect of S. dorsalis life stage on the oviposition and predation rates observed for each mite species. The larval stage was the most preferred stage for both mite species, with A. swirskii consuming 4.6–6.3 and A. limonicus 4.8–6.4 individuals/day compared to only 1.6–1.7 adults/day consumed by both species. Female A. swirskii and A. limonicus laid 0.55–0.75 and 0.73 eggs/day on the two larval stages, respectively, compared to only 0.25–0.30 eggs/day observed for individuals feeding on adults. Although the results showed that the biological control potential of both mite species was similar, having an additional predator available that may be as effective as A. swirskii, a proven control agent against S. dorsalis in the field, warrants additional research into its potential utility.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), is an invasive pest represented by a cryptic species complex (Dickey et al. 2015) native to East and Southeast Asia that has become established in areas representing a broad global distribution (Seal et al. 2006; Kumar et al. 2013). By 2010 in the USA, S. dorsalis had become well-established in Florida and Texas (Silagyi and Dixon 2006) and recent interceptions suggest there is a risk of S. dorsalis gaining a foothold in Alabama, California, Georgia, Louisiana, and New York (Diffie and Srinivasan 2010; Kumar et al. 2011; Dickey et al. 2015). In Florida where S. dorsalis has been most studied, it has been shown to be highly polyphagous, feeding on at least 50 plant species (Seal and Kumar 2010; Kumar et al. 2012) including many economically important ornamental, vegetable, and fruit crops. Scirtothrips dorsalis larvae and adults feed primarily on new leaf foliage, which can lead to stunted growth, unmarketable fruit, and even death of the plant during severe infestations. In addition to physical damage to the plant via feeding, S. dorsalis is also a vector for at least nine tospoviruses including melon yellow spot virus and tobacco streak virus (Rao et al. 2003; Chiemsombat et al. 2008) further increasing its ability to damage impacted crops.
Traditional control of S. dorsalis around the world has relied heavily on chemical options (Chu et al. 2006; Seal et al. 2006); however, recent studies have also demonstrated the utility of biorational insecticides and entomopathogenic fungi for controlling S. dorsalis (Seal et al. 2007; Seal and Kumar 2010; Aristizábal et al. 2017; Kumar et al. 2017; Dale and Borden 2018). Due to the rapid generational turnover of S. dorsalis, which can reproduce both sexually and parthenogenically (Dev 1964), there is elevated risk of populations developing resistance to insecticides. This has already been demonstrated for some Indian populations of S. dorsalis, which have exhibited resistance to organochlorine, organophosphate, and carbamate insecticides (Reddy et al. 1992; Sridhar and Rani 2003). Recent S. dorsalis control tactics have focused more on the use of natural enemies as a means of biological control in order to help prevent the development of insecticidal resistance, which can lead to decreased economic input by growers.
The mite Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) is a generalist predator and has been used in the biological control of many mites (Messelink et al. 2006, 2010; van Maanen et al. 2010) and insects including whiteflies (Nomikou et al. 2002; Hoogerbrugge et al. 2011) and thrips (Wimmer et al. 2008; Chow et al. 2010; Kakkar et al. 2016). Use of A. swirskii against S. dorsalis is currently the primary means of its biological control and has shown potential for managing S. dorsalis populations (Arthurs et al. 2009; Doğramaci et al. 2011). The direct release of A. swirskii onto S. dorsalis infested crops (Kumar et al. 2015) or preventative release using banker plants (Arthurs et al. 2009; Xiao et al. 2012; Avery et al. 2014) have both been shown to be effective control tactics. Feedback from growers suggests that A. swirskii may have difficulty establishing on some host plants of S. dorsalis however, such as roses, which may be due to having few leaf trichomes which promote oviposition. Developing alternative biological control tactics for use with these hosts is essential to provide growers the necessary tools to prevent damage by S. dorsalis, and there is increased need to assess the utility of A. swirskii for S. dorsalis control beyond a select few vegetable crops.
In late 2011 the phytoseiid predatory mite Amblydromalus limonicus (Garman and McGregor) became commercially available. Like A. swirskii, A. limonicus is a generalist predator and an effective biological control agent against whiteflies (Hoogerbrugge et al. 2011; Knapp et al. 2013; Lee and Zhang 2018), psyllids (Davidson et al. 2016; Patel and Zhang 2017), thrips (van Houten et al. 1995; Knapp et al. 2013; Vervoort et al. 2017), broad mites (McMurtry et al. 1984), and to a lesser extent spider mites due to its reduced performance on eggs (McMurtry and Scriven 1965) and poor ability to deal with webbing (van Houten et al. 2008; Vangansbeke et al. 2014). When compared to other phytoseiid species, A. limonicus has exhibited superior predation and oviposition rates feeding on thrips (van Houten et al. 1995; Houten et al. 2016). In studies directly comparing overall levels of thrips suppression between A. limonicus and A. swirskii, overall levels of thrips control were shown to be higher for A. limonicus against western flower thrips, Frankliniella occidentalis (Pergande) (Messelink et al. 2006).
Previous studies have shown that A. swirskii prefers feeding on the first instar of many thrips species and may have difficulty preying on later stages, as seen for poinsettia thrips, Echinothrips americanus Morgan (Ghasemzadeh et al. 2017), melon thrips, Thrips palmi Lindeman (Cuthbertson et al. 2012), and F. occidentalis (Wimmer et al. 2008). Due to the relatively small size of S. dorsalis compared to other species, however, A. swirskii can feed on both the larval and adult stages, albeit at a reduced rate for the latter (Arthurs et al. 2009). There is evidence that A. limonicus may have the capacity to feed on a broader range of thrips stages (Ghasemzadeh et al. 2017; Lam et al. 2019) than many phytoseiid species and it has been marketed for this behavior (Limonica®, Koppert Biological Systems). The aggressiveness of A. limonicus against the second instar and adult thrips may allow it to achieve better control of S. dorsalis than A. swirskii. Given evidence in the literature that suggests A. limonicus may be a superior natural enemy, the overall goal of this study was to compare the baseline effectiveness of A. swirskii and A. limonicus in controlling S. dorsalis in order to enhance ongoing biological control programs.
Materials and methods
Arthropod colonies
Chilli thrips were obtained from wild populations collected from ornamental roses (Rosa L.) in Apopka, FL, USA. Greenhouse colonies were maintained on bell peppers (Capsicum annum L.) at the University of Florida’s Mid-Florida Research and Education Center (MREC) in Apopka. Colonies were held in screened cages (59 × 59 × 59 cm) within greenhouses (27.8 ± 0.13 °C, 85 ± 0.43% RH, under a natural light regime) and provided with clean host plants weekly.
Commercially reared A. swirskii and A. limonicus used in experiments were obtained from Koppert Biological Systems (Romulus, MI, USA). Mite species were confined separately within the bottom halves of 15-cm-diameter Petri dishes containing a black 8 × 8 cm cardboard square (which had been dipped in paraffin and etched with 1 mm2 screening) placed on top of stacks of wet cotton pads (Kumar et al. 2015). Petri dishes were filled partially with water to keep the cotton moist and provide mites with a source of water. A glass microscope slide was placed over 5–10 pieces of 1-cm-long fibers (75% jute, 25% polyester) to provide mites an oviposition substrate and microspace to hide. Mites were provided cattail pollen (Typha sp.) twice a week as food. Mature adult females were removed from the laboratory colonies at their initiation, so that newly mature and gravid females (11–12 days old) in the colonies could be identified to ensure that even-aged individuals were used for laboratory trials. Mite populations were maintained up to 2 weeks in the laboratory before being restarted.
Plants
The commercial hybrid bell pepper (‘RPP24272’ Rogers/Syngenta Seeds, Boise, ID, USA) was selected for insect colony maintenance and to supply leaf discs for experiments. Clean pepper plants were housed in screened cages in greenhouses at the conditions described above at MREC. Plants were initiated in seed trays containing Jolly Gardener Pro-Line C/20 Growing Mix (Old Castle Lawn & Garden, Atlanta, GA, USA) and transplanted into 15-cm-diameter pots 2 weeks post-germination and fertilized with 5 g of Osmocote Plus 15-9-12 (N-P-K) (Scotts-Sierra Horticultural Products, Marysville, OH, USA). Pepper plants were watered as needed (2–3 × per week) and fertilized with Peter’s Professional 20-10-20 (N-P-K, 325 ppm) (Scotts-Sierra Horticultural Products) once a week. Pepper plants were utilized for experiments 45 days after germination (6–8 true leaves) and were free of pesticides.
Laboratory assays
Predation and oviposition rates of A. swirskii and A. limonicus fed on different stages of S. dorsalis were assessed under laboratory conditions. Oviposition rates were assessed as a meaningful surrogate metric for population growth rates of phytoseiid mites (Janssen and Sabelis 1992). The experiment had a total of nine treatments consisting of each of the predatory mite species provided either the L1, L2, or adult stage of S. dorsalis. Controls consisted of each S. dorsalis stage in the absence of predators to assess baseline mortality. Bioassay arenas consisted of small Petri dishes (4.7 cm diameter) arranged in a 3 × 3 array within a larger Petri dish filled partially with water to isolate dishes. Within each small Petri dish, a 2.5-cm-diameter pepper leaf disc was placed onto a cotton round saturated with water to confine the mites and S. dorsalis to the leaf disc. A piece of rice husk was placed into the center of the leaf discs as a refuge to help encourage mites to stay on the leaf disc and a 1 cm fiber was added as an oviposition substrate.
A total of 10 thrips of the desired stage were added to each leaf disc using a fine tip brush under a dissecting microscope. A single female of A. swirskii or A. limonicus at the start of their oviposition period (11–12 days old) were also placed into the arenas as described above and arenas were covered with lids containing holes covered in fine mesh screening for ventilation. The arenas were placed into environmental chambers (25 ± 2 °C, 75 ± 10% RH, and L16:D8 h), with the large Petri dishes containing the nine smaller Petri dishes serving as blocks to control for potential positional effects within the chambers. The number of dead/alive thrips and total number of mite eggs were assessed once every 24 h for 3 days. Predation rate data from all 3 days was utilized; however, oviposition rate data from the first day were omitted to reduce the potential effects of mites’ prior food source (Sabelis 1990). Leaf discs were replaced with fresh ones daily containing new thrips. The experiment was repeated with four blocks in time, with five treatment replicates performed during each experimental block in time for a total of 20 replicates per treatment.
Statistical analysis
All data were analyzed using SAS v.9.4 statistical software (SAS Institute 2013). Predation rate and oviposition rate data were log(x + 1)-transformed to meet the assumptions of homogeneity of variance and normality. Data were analyzed using a repeated measurements two-way ANOVA, with predator treatment and thrips stage as fixed effects and experimental subject and observation day as random effects in Proc MIXED. Any significant differences observed among treatments were analyzed by Tukey’s honestly significant difference (HSD) posthoc tests (α = 0.05).
Results
The analyses indicated that experimental blocks were not statistically different for predation rates (F3,168 = 1.27, p = 0.29) and oviposition rates (F3,111 = 1.64, p = 0.18), so the data were averaged by experimental blocks for each mite species and thrips stage (n = 4 for each species-stage combination).
Predation rates
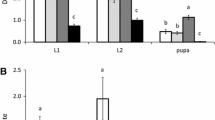
All three S. dorsalis life stages examined were consumed by A. limonicus and A. swirskii and there was no evidence of a pattern in daily predation rates exhibited by either mite species. There was a significant interaction between mite species and S. dorsalis life stage on observed mortality (F4,171 = 47.22, p < 0.0001). This interaction, however, was due to the mortality patterns of the control differing from the mite treatments and had no influence on the interpretation of the results. There was no significant difference in overall S. dorsalis mortality rates between mite species observed, however overall S. dorsalis mortality was significantly higher in both the A. limonicus and A. swirskii treatments compared to the control (Fig. 1). There were no significant differences in the number of S. dorsalis consumed between mite species for either first or second stage larvae, as well as adults.
Mean (± SEM) daily predation rates (days 1–3) of the phytoseiid mites Amblydromalus limonicus and Amblyseius swirskii provided with 10 of either first or second stage larvae or adults of Scirtothrips dorsalis using a leaf disc bioassay. The control represents baseline thrips mortality. Letter differences indicate significant differences in predation/mortality rates between S. dorsalis stages within each predator treatment (Tukey’s HSD test: p < 0.05)
Oviposition rates
There was no significant interaction effect between mite species and S. dorsalis life stage (F2,114 = 0.44, p = 0.65), or mite species treatment effect observed on mean daily oviposition rates (F1,114 = 0.94, p = 0.33) (Fig. 2). There was, however, a significant effect of S. dorsalis life stage observed on mean ovipositional rates of mites during days 2 and 3 (F2,114 = 11.35, p < 0.0001) (Fig. 2). Mean daily oviposition rates of mites fed on adult S. dorsalis were significantly lower than of individuals fed first and second stage larvae (Fig. 2). There was no difference in oviposition rates of mites fed first or second stage larvae (Fig. 2).
Mean (± SEM) daily oviposition rates (days 2–3) of the phytoseiid mites Amblydromalus limonicus and Amblyseius swirskii provided with 10 of either first or second stage larvae or adults of Scirtothrips dorsalis using a leaf disc bioassay. Letter differences indicate differences in oviposition rates between mite treatments for each S. dorsalis life stage (Tukey’s HSD test: p < 0.05)
Discussion
Chilli thrips predation rates observed for A. swirskii observed in this study were slightly higher those observed by Arthurs et al. (2009) who found that A. swirskii consumed an average of 2.73 and 1.09 individuals/day of second stage larvae and adults respectively when provided 15 prey. Oviposition rates of A. swirskii observed in this study, however, were lower than the 1.33 and 0.52 eggs/day they observed for females fed second stage larvae and adults respectively. As in this study, a similar pattern of lower predation rates and fewer eggs laid by A. swirskii fed adult S. dorsalis was observed by Arthurs et al. (2009), suggesting adult S. dorsalis are a suboptimal prey. Our observations of A. swirskii predatory behavior in both the lab and field suggest that A. swirskii has difficulty subduing adult chili thrips to feed. In most encounters adult S. dorsalis drag mites across the leaf or kick them until the mites give up. However, in the field predation of adult thrips appears to be facilitated by leaf structures such as large veins and domatia that mites can use to pin the adult thrips against and successfully kill them. On rare occasion we have also observed additional A. swirskii adults, that were nearby S. dorsalis adults dragging pursuing adult mites, assist in successful predation attempts by jointly pinning down fleeing thrips. Both these observations suggest that adult S. dorsalis predation rates in the lab may underestimate predation rates of adults in the field.
As this was the first study to examine A. limonicus feeding on S. dorsalis, no direct comparisons of our findings with the literature can be made for this specific predator-prey combination; however, similar types of data exist for A. limonicus feeding on other thrips species. Ghasemzadeh et al. (2017) examined predation and oviposition rates on E. americanus by A. limonicus and A. swirskii and observed similar results, with no difference in predation and oviposition rates of A. limonicus provided 10 first or second stage larvae. Unlike in this study, however, predation and oviposition rates of A. limonicus feeding on larvae were higher than those observed for A. swirskii. A study by van Houten et al. (1995) found that A. limonicus provided with 12 first instar F. occidentalis in a leaf disc bioassay consumed an average of 6.9 individuals/day and laid 3.2 eggs/day. Both studies saw considerably higher A. limonicus oviposition rates than what were observed in this study. Possible explanations for observed differences in these metrics may be attributed to population genetics of source populations of A. limonicus used as well as differences in the suitability of thrips species. It has been shown that A. limonicus performs well on plant material such as pollen and may also feed directly on plant tissue (Messelink et al. 2006). Studies examining A. limonicus oviposition rates on pollen saw higher oviposition rates than those observed in this study (van Houten et al. 1995; Vangansbeke et al. 2014; Leman and Messelink 2015; Nguyen et al. 2015; Samaras et al. 2015), and it would have been useful to include a pollen treatment as a reference to assess whether S. dorsalis is a poor food source.
In conclusion, results from this study show for the first time that A. limonicus is a promising candidate for use as a biological control agent against S. dorsalis. Although the hypothesis that A. limonicus would exhibit higher predation and oviposition rates than A. swirskii (especially on later developmental stages) was not supported by the data, the results still suggest that A. limonicus may be an equally effective predator. As A. swirskii has already been shown to provide effective control of S. dorsalis on some hosts such as pepper, there is the possibility that A. limonicus may possess other traits which could allow it to perform better in systems where A. swirskii is providing insufficient control. Documentation in the literature of greater thrips control achieved by A. limonicus compared to A. swirskii (e.g. Messelink et al. 2006; Hoogerbrugge et al. 2014) gives further credence to the hypothesis that behavior or biological traits other than predation and oviposition rates may better explain the effectiveness of A. limonicus as a biological control agent and needs to be examined in future studies.
References
Aristizábal LF, Chen Y, Cherry RH, Cave RD, Arthurs SP (2017) Efficacy of biorational insecticides against chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae), infesting roses under nursery conditions. J Appl Entomol 141:274–284
Arthurs S, McKenzie CL, Chen J, Dogramaci M, Brennan M, Houben K, Osborne L (2009) Evaluation of Neoseiulus cucumeris and Amblyseius swirskii (Acari: Phytoseiidae) as biological control agents of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) on pepper. Biol Control 49:91–96
Avery PB, Kumar V, Xiao Y, Powell CA, McKenzie CL, Osborne LS (2014) Selecting an ornamental pepper banker plant for Amblyseius swirskii in floriculture crops. Arthropod Plant Interact 8:49–56
Chiemsombat P, Gajanandana O, Warin N, Hongprayoon R, Bhunchoth A, Pongsapich P (2008) Biological and molecular characterization of tospoviruses in Thailand. Arch Virol 153:571–577
Chow A, Chau A, Heinz KM (2010) Compatibility of Amblyseius (Typhlodromips) swirskii (Athias-Henriot) (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae) for biological control of Frankliniella occidentalis (Thysanoptera: Thripidae) on roses. Biol Control 53:188–196
Chu CC, Ciomperlik MA, Gibbs I, Bell P, Taylor B, Henneberry TJ (2006) Insecticide evaluations to reduce Scirtothrips dorsalis (Thysanoptera: Thripidae) on mature sea island cotton (Gossypium barbadense L.) in Barbados. USDA, ARS. Western Cotton Research Laboratory, Phoenix, p 20
Cuthbertson AG, Mathers JJ, Croft P, Nattriss N, Blackburn LF, Luo W, Northing P, Murai T, Jacobson RJ, Walters KF (2012) Prey consumption rates and compatibility with pesticides of four predatory mites from the family Phytoseiidae attacking Thrips palmi Karny (Thysanoptera: Thripidae). Pest Manage Sci 68:1289–1295
Dale AG, Borden MA (2018) Evaluation of reduced-risk insecticides to control chilli thrips (Thysanoptera: Thripidae) and conserve natural enemies on ornamental plants. Fla Entomol 101:237–243
Davidson MM, Nielsen MC, Butler RC, Silberbauer RB (2016) Prey consumption and survival of the predatory mite, Amblydromalus limonicus on different prey and host plants. Biocontrol Sci Technol 26:722–726
Dev HN (1964) Preliminary studies on the biology of Assam thrips, Scirtothrips dorsalis Hood on tea. Indian J Entomol 26:184–194
Dickey AM, Kumar V, Hoddle MS, Funderburk JE, Morgan JK, Jara-Cavieres A et al (2015) The Scirtothrips dorsalis species complex: endemism and invasion in a global pest. PLoS ONE 10:e0123747
Diffie S, Srinivasan R (2010) Occurrence of Leucothrips furcatus, Scirtothrips dorsalis, and Tenothrips frici (Thysanoptera: Thripidae) previously unreported from Georgia. J Entomol Sci 45:394–396
Doğramaci M, Arthurs SP, Chen J, McKenzie CL, Irrizary F, Osborne LS (2011) Management of chilli thrips Scirtothrips dorsalis (Thysanoptera: Thripidae) on peppers by Amblyseius swirskii (Acari: Phytoseiidae) and Orius insidiosus (Hemiptera: Anthocoridae). Biol Control 59:340–347
Ghasemzadeh S, Leman A, Messelink GJ (2017) Biological control of Echinothrips americanus by phytoseiid predatory mites and the effect of pollen as supplemental food. Exp Appl Acarol 73:209–221
Hoogerbrugge H, van Houten Y, Knapp M, Bolckmans K (2011) Biological control of greenhouse whitefly on roses with phytoseiid mites. Integrated control in protected crops, temperate climate. IOBC-WPRS Bull 68:59–63
Hoogerbrugge H, Oude Lenferink K, van Houten Y, Bolckmans K (2014) Screening of three phytoseiid mite species as biocontrol agents of Echinothrips americanus. IOBC-WPRS Bull 102:97–101
Janssen A, Sabelis MW (1992) Phytoseiid life-histories, local predator–prey dynamics, and strategies for control of tetranychid mites. Exp Appl Acarol 14:233–250
Kakkar G, Kumar V, Seal DR, Liburd OE, Stansly P (2016) Predation by Neoseiulus cucumeris and Amblyseius swirskii on Thrips palmi and Frankliniella schultzei on cucumber. Biol Control 92:85–91
Knapp M, van Houten Y, Hoogerbrugge H, Bolckmans K (2013) Amblydromalus limonicus (Acari: Phytoseiidae) as a biocontrol agent: literature review and new findings. Acarologia 53:191–202
Kumar V, Seal DR, Schuster DJ, McKenzie CL, Osborne LS, Maruniak J, Zhang S (2011) Scirtothrips dorsalis (Thysanoptera: Thripidae): scanning electron micrographs of key taxonomic traits and a preliminary morphometric analysis of the general morphology of populations of different continents. Fla Entomol 94:941–955
Kumar V, Seal DR, Kakkar G, McKenzie CL, Osborne LS (2012) New tropical fruit hosts of Scirtothrips dorsalis (Thysanoptera: Thripidae) and its relative abundance on them in South Florida. Fla Entomol 95:205–207
Kumar V, Kakkar G, McKenzie CL, Seal DR, Osborne LS (2013) An overview of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae) biology, distribution and management. In: Soloneski S, Larramendy M (eds) Weed and pest control: conventional and new challenges. InTech, London
Kumar V, Xiao Y, McKenzie CL, Osborne LS (2015) Early establishment of the phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a Predator-In-First approach. Exp Appl Acarol 65:465–481
Kumar V, Kakkar G, Seal DR, McKenzie CL, Osborne LS (2017) Evaluation of insecticides for curative, preventative, and rotational use on Scirtothrips dorsalis South Asia 1 (Thysanoptera: Thripidae). Fla Entomol 100:634–646
Lam W, Paynter Q, Zhang ZQ (2019) Predation, prey preference and reproduction of predatory mites Amblydromalus limonicus (Garman), Amblyseius herbicolus (Chant) and Neoseiulus cucumeris (Oudemans) (Mesostigmata: Phytoseiidae) on immature Sericothrips staphylinus Haliday (Thysanoptera: Thripidae), a biocontrol agent of gorse. Syst Appl Acarol 24:508–519
Lee MH, Zhang ZQ (2018) Assessing the augmentation of Amblydromalus limonicus with the supplementation of pollen, thread, and substrates to combat greenhouse whitefly populations. Sci Rep 8:12189
Leman A, Messelink GJ (2015) Supplemental food that supports both predator and pest: a risk for biological control? Exp Appl Acarol 65:511–524
McMurtry JA, Scriven GT (1965) Life-history studies of Amblyseius limonicus, with comparative observations on Amblyseius hibisci (Acarina: Phytoseiidae). Ann Entomol Soc Am 58:106–111
McMurtry JA, Badii MH, Johnson HG (1984) The broad mite, Polyphagotarsonemus latus as a potential prey for phytoseiid mites in California. Entomophaga 29:83–86
Messelink GJ, van Steenpaal SEF, Ramakers PMJ (2006) Evaluation of phytoseiid predators for control of western flower thrips on greenhouse cucumber. Biocontrol 51:753–768
Messelink GJ, van Maanen R, van Holstein-Saj R, Sabelis MW, Janssen A (2010) Pest species diversity enhances control of spider mites and whiteflies by a generalist phytoseiid predator. Biocontrol 55:387–398
Nguyen DT, Vangansbeke D, De Clercq P (2015) Performance of four species of phytoseiid mites on artificial and natural diets. Biol Control 80:56–62
Nomikou M, Janssen A, Schraag R, Sabelis MW (2002) Phytoseiid predators suppress populations of Bemisia tabaci on cucumber plants with alternative food. Exp Appl Acarol 27:57–68
Patel K, Zhang ZQ (2017) Prey preference and reproduction of predatory mites, Amblydromalus limonicus and Neoseiulus cucumeris, on eggs of and 1st instar nymphs of the tomato/potato psyllid. Int J Acarol 43:468–474
Rao P, Reddy AS, Reddy SV, Thirumala-Devi K, Chander Rao S, Manoj Kumar V et al (2003) The host range of tobacco streak virus in India and transmission by thrips. Ann Appl Biol 142:365–368
Reddy GPV, Prasad VD, Rao RS (1992) Relative resistance in chilli thrips, Scirtothrips dorsalis Hood populations in Andhra Pradesh to some conventional insecticides. Indian J Plant Prot 20:218–222
Sabelis MW (1990) How to analyse prey preference when prey density varies? A new method to discriminate between the effects of gut fullness and prey type composition. Oecologia 82:289–298
Samaras K, Pappas ML, Fytas E, Broufas GD (2015) Pollen suitability for the development and reproduction of Amblydromalus limonicus (Acari: Phytoseiidae). Biocontrol 60:773–782
Seal DR, Kumar V (2010) Biological responses of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), to various regimes of chemical and biorational insecticides. Crop Prot 29:1241–1247
Seal DR, Ciomperlik M, Richards ML, Klassen W (2006) Comparative effectiveness of chemical insecticides against the chilli thrips Scirtothrips dorsalis Hood (Thysanoptera: Thripidae), on pepper and their compatibility with natural enemies. Crop Prot 25:949–955
Seal DR, Klassen W, Sabines C (2007) Efficacy of Botanigard®, TriCon®, and Metarhizium anisopliae treatments for the control of chilli thrips, Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) in the greenhouse. Proc Carib Food Crops Soc 43:30–38
Silagyi AJ, Dixon WN (2006) Assessment of chilli thrips, Scirtothrips dorsalis Hood, in Florida. Florida Cooperative Agricultural Pest Survey, Program report # 2006-08-SDS-01
Sridhar V, Rani BJ (2003) Relative resistance in open and greenhouse populations of Scirtothrips dorsalis Hood (Thysanoptera: Thripidae) on rose to dimethoate and acephate. Res Pest Manage News Lett 12:62–64
van Houten YM, van Rijn PCJ, Tanigoshi LK, van Stratum P, Bruin J (1995) Preselection of predatory mites to improve year-round biological control of western flower thrips in greenhouse crops. Entomol Exp Appl 74:225–234
van Houten YM, Rothe J, Bolckmans KJF (2008) The generalist predator Typhlodromalus limonicus (Acari: Phytoseiidae): a potential biological control agent of thrips and whiteflies. IOBC-WPRS Bull 32:237–240
van Houten YM, Hoogerbrugge H, Oude Lenferink K, Knapp M, Bolckmans KJF (2016) Evaluation of Euseius gallicus as a biological control agent of western flower thrips and greenhouse whitefly in rose. J Acarol Soc Jpn 25(S1):147–159
van Maanen R, Vila E, Sabelis MW, Janssen A (2010) Biological control of broad mites (Polyphagotarsonemus latus) with the generalist predator Amblyseius swirskii. Exp Appl Acarol 52:29–34
Vangansbeke D, Nguyen DT, Audenaert J, Verhoeven R, Deforce K, Gobin B, Tirry L, De Clercq P (2014) Diet-dependent cannibalism in the omnivorous phytoseiid mite Amblydromalus limonicus. Biol Control 74:30–35
Vervoort M, Melis P, Hanssens J, Craeye S, Pisman M, Smagghe G, Clymans R, Belien T (2017) Thrips control with predatory mites Amblydromalus limonicus and Amblyseius swirskii in different strawberry cultivation systems. Acta Horticult 1156:833–842
Wimmer D, Hoffmann D, Schausberger P (2008) Prey suitability of western flower thrips, Frankliniella occidentalis, and onion thrips, Thrips tabaci, for the predatory mite Amblyseius swirskii. Biocontrol Sci Technol 18:533–542
Xiao YF, Avery P, Chen JJ, McKenzie C, Osborne LS (2012) Ornamental pepper as banker plants for establishment of Amblyseius swirskii (Acari: Phytoseiidae) for biological control of multiple pests in greenhouse vegetable production. Biol Control 63:279–286
Funding
Funding for this research was provided by the USDA-ARS Floriculture and Nursery Research Initiative (Grant No. #58-6618-5-0248), USDA-APHIS Farm Bill (Grant No. #AP18PPQS) and (Grant No. #T00C089), and support from CSREES/NIFA CRIS IPM Technologies for Subtropical Insect Pests (Grant No. #66182200003700D).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Schoeller, E.N., McKenzie, C.L. & Osborne, L.S. Comparison of the phytoseiid mites Amblyseius swirskii and Amblydromalus limonicus for biological control of chilli thrips, Scirtothrips dorsalis (Thysanoptera: Thripidae). Exp Appl Acarol 82, 309–318 (2020). https://doi.org/10.1007/s10493-020-00556-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-020-00556-5