Abstract
We investigated oribatid mite communities in a Sphagnum-dominated boreal peatland system characterised by a mosaic of oligotrophic and meso-eutrophic areas. We aimed to determine the relative importance of environmental factors (pH, Sphagnum nutrient content, water table level, diversity of vascular plants and bryophytes in the surrounding plant community) and spatial variation in influencing abundance, diversity and community composition of aquatic and terrestrial oribatid mites. Among environmental variables, water table level (micro-topography), pH, and K in Sphagnum tissues were the main predictors of Oribatida community structure. Aquatic species were associated with pools; two terrestrial species—Hoplophthiracarus illinoisensis and Nothrus pratensis—were associated with oligotrophic hummocks; the rest of terrestrial species were associated with dryer mesotrophic and eutrophic habitats. Low water table depth (hummocks), high local plant diversity, and high P in Sphagnum tissues were predictors of high abundance of terrestrial Oribatida. Species richness of terrestrial Oribatida was linked with low water table and high plant diversity. For aquatic Oribatida abundance, water table depth was the single most important predictor variable. Plot trophic class (its status on the peatland poor-rich gradient assigned based on plant indicator species) was also a significant predictor of terrestrial Oribatida abundance, richness, and community structure. Spatial structuring was important for terrestrial Oribatida community composition, weak (P < 0.10) for terrestrial Oribatida abundance and richness, and not significant for aquatic Oribatida.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Sphagnum peatlands (raised bogs and transitional mires) occupy extensive areas in the boreal and subarctic regions of Eurasia and North America (Tarnocai and Stolbovoy 2006; Rydin and Jeglum 2013). Precipitation-fed (ombrotrophic) raised bogs generally lack any other hydrological inputs, resulting in acidic and nutrient-poor (oligotrophic) conditions. These environments are characterized by dominance of Sphagnum mosses, low pH (< 5.0), and scarcity of nutrients (Wheeler and Proctor 2000). Some areas of a peatland may receive supplementary nutrient input from mineral soil, e.g., from ground water seepage (Eurola and Holappa 1985); such areas form patches of nutrient enrichment and provide niches to meso-eutrophic vegetation (Ruuhijärvi and Lindholm 2006). As a result, patches of oligotrophic and meso-eutrophic habitats in a peatland can be complex.
Hydrology and nutrient availability exert a major control on distribution of oligotrophic and meso-eutrophic patches and on patterns of plant and animal diversity in peatlands. Peatland environments are typically described along a poor-rich (acidity–alkalinity) gradient (Ruuhijärvi and Lindholm 2006); it can also be referred to as an oligotrophy–eutrophy gradient (Eurola and Huttunen 2006). This gradient primarily reflects the degree of inflow of mineral soil water, and is best characterised by the pH, with peatland vegetation forming a pH-driven continuum of plant communities (Wheeler and Proctor 2000; Tahvanainen 2004). Perhaps confusingly, ‘oligotrophy–eutrophy’ terminology is also applied to describe the nutrient gradient, which reflects the availability of N, P and K (Ruuhijärvi and Lindholm 2006). The pH gradient and the nutrient availability gradient in peatlands usually coincide, but may be decoupled (Wheeler and Proctor 2000; Bragazza and Gerdol 2002). Different Sphagnum and vascular plant species occur at different positions along the pH and nutrient gradients (Limpens et al. 2003; Rydin and Jeglum 2013). Nutrient content in Sphagnum tissues has been found to co-vary with local nutrient input within the peatland (Wheeler and Proctor 2000; Limpens et al. 2017; van den Elzen et al. 2018).
Another major gradient in a peatland is the micro-topographic structure of hummocks, hollows, and intermediate lawns. Micro-topography gives peatlands their characteristic patterning and is linked to hydrology, biodiversity and carbon sequestration (Hajkova and Hajek 2007). Different Sphagnum species occupy different micro-topographic positions (Gunnarsson 2005; Johnson et al. 2014).
Peatlands contain specialized and highly distinctive assemblages of plants and invertebrates (Tahvanainen 2004; Spitzer and Danks 2006). Oribatid mites are an abundant group of arthropods in boreal peatlands, and their fauna in European peat bogs is well studied (e.g., Tarras-Wahlberg 1961; Markkula 1986; Weigmann 1991; Borcard and von Ballmoos 1997; Stary 2006; Seniczak 2011; Seniczak et al. 2014). At a site level, diversity patterns of Oribatida are influenced by the factors that are recognized as biodiversity drivers for broader groups of peatland organisms, such as ground water level, pH and habitat heterogeneity (Donaldson 1996; Minor et al. 2016).
The drivers of species distribution across environmental gradients continue to be a challenging question in ecology (Sutherland et al. 2013). Environmental conditions, spatial and temporal environmental heterogeneity, biotic interactions, and historical processes are all known to influence the taxonomic and functional structure of communities (Chesson 2000), but the relative contribution of these factors cannot be easily generalised (HilleRisLambers et al. 2012; Cadotte and Tucker 2017). In this study, we measured a set of variables expected to influence patterns of diversity in Oribatida communities in a relatively undisturbed boreal mire system characterised by a mosaic of oligotrophic and meso-eutrophic areas. We hypothesised that environmental factors such as pH, Sphagnum nutrient content, water table level, and plant diversity in the sampling plot will be important contributors to Oribatida diversity patterns, and aimed to quantify relative contribution of these factors to shaping oribatid mite communities.
Methods
Study site
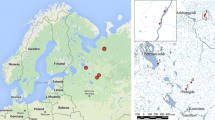
The sampled mire system (‘Boloto Shichengskoe’, 59°56′30.4″N, 41°16′57.1″E) is located in Vologda oblast in the north-western Russia (Fig. 1a). The climate in the region is humid continental (Köppen climate classification: Dfb), with long moderately cold winter (mean temperature of January − 12 °C) and short warm summer (mean temperature of July 16–17 °C). Annual precipitation is 500–650 mm as rain and snow; snow cover lasts 165–170 days of the year. The mire is a large (15,900 ha) wetland system of predominantly lacustrine origin, formed in the large glacial lake basin (Philippov 2015). The central part of the mire is occupied by a shallow oligotrophic lake Shichengskoe (1060 ha). The mire occupies a transitional-accumulative position on the local landscapes, with distribution of ombrotrophic and minerotrophic areas in the mire system reflecting the flows of gravitational and ground water (Fig. 1b). Significant area of the mire is occupied by the ombrotrophic peat bog, dominated by Pinus-shrublets-Sphagnum and Eriophorum-shrublets-Sphagnum associations. The bog ridges and hummocks are dominated by Sphagnum fuscum, less frequently by Sphagnum angustifolium and S. medium; hollows are occupied by S. cuspidatum, S. balticum, less frequently by S. majus and S. fallax; nine other species of Sphagnum occur sporadically (Philippov and Boychuk 2015). Within the ombrotophic bog there are several ground water seeps, usually associated with forested mineral islands, the seeps are visible in Fig. 1b. Present in or near the seeps are meso-and eutrophic Sphagnum mosses. The south-eastern part of the mire system, where three rivers flow, is meso- and eutrophic. The eutrophic edges of the mire are forested with Betula pubescens, Salix pentandra, and Alnus glutinosa (Philippov 2015).
Sampling
Sampling was conducted in July 2016 and limited to Sphagnum-dominated communities in ombrotrophic (western) part of the mire. Samples were collected from 48 plots (1 m−2), each plot dominated by one of the 16 Sphagnum species present in the mire, with three replicated plots per Sphagnum species. The stratification by Sphagnum species was used to achieve balanced sampling. The minimum distance between some conspecific Sphagnum plots was 5–7 m, but usually over 30 m. Sphagnum mosses were identified using Ignatov and Ignatova (2003), and classified into phylogenetic sections and preferred habitat types (Table 1); nomenclature follows Ignatov et al. (2006). In each sampling plot GPS coordinates, Sphagnum moss identity, and plant diversity (as simple species richness of vascular plants, bryophytes and liverworts) were recorded.
Other variables recorded at a plot level were water table level, water pH, and Sphagnum nutrient content (C, N, P, K). Water table depth was measured in each sampling plot using temporary wells. The upper layers of peat were removed and the water level allowed to stabilize; the depth from the water table to the top of Sphagnum capitula was measured with a ruler. Water pH samples (one compound sample per plot, squeezed from the relevant Sphagnum moss species) were collected in 15 ml plastic vials into the portable chiller, stored in the dark at 4 °C and analysed the next day in the lab using Sartorius PB-11 benchtop pH meter. Nutrient content (C, N, P, K) of Sphagnum moss from all plots was analysed in duplicate samples. The moss samples were air-dried at room temperature and homogenised. Total organically bound carbon (TOC) and nitrogen (TNb) were measured using ‘Vario TOC cube’ analyser (Elementar Analysensysteme). Phosphorous (as Р2О5) was quantified colorimetrically in a spectrophotometer using molybdenum-blue method from samples prepared by wet digestion (H2SO4 plus 30% H2O2 with Se as a catalyst). Potassium (as K2O) was measured using atomic absorption spectrophotometer AA-6300 (Shimadzu) from samples prepared by wet digestion (same method as for P). All nutrient content results were converted to dry-matter basis (determined by drying subsamples at 105 °C to a constant weight).
To characterise the status of each sampled peatland plot on the poor-rich continuum, each plot was assigned to a qualitative ‘trophic class’ based on plant community features (Rydin et al. 1999; Eurola and Huttunen 2006). We used oligotrophy-eutrophy terminology and included intermediate categories to separate five trophic classes: oligotrophic, oligo-mesotrophic, mesotrophic, meso-eutrophic, eutrophic. Note that ‘trophic class’ here does not directly reflect nutrient status, but instead is based on plant indicator species of the poor-rich (acidity–alkalinity) gradient; we then compared resulting trophic classes with pH and Sphagnum nutrient content measurements.
Sphagnum moss for mite extraction was collected as 10 × 10 cm samples to the depth of living moss plants (including capitula and the length of stems). Mites from moss samples were extracted in modified Berlese funnels until samples were fully dry. Adult Oribatida were counted, identified to a species level, and classified into two functional groups—‘aquatic’ (species living on submerged vegetation in freshwater habitats, following Weigmann and Deichsel 2006; Seniczak 2011), and ‘terrestrial’ (all other species). Nomenclature system is mostly following Subías (2018).
Statistical analysis
A multicollinearity analysis (SAS v.9.4, proc reg and proc corr) was done to check simple correlations and variance inflation factors for environmental variables. Plot trophic class (an ordinal variable) was represented by a numerical dummy variable in this analysis. Although different Sphagnum species were sampled as a stratification measure, we did not focus on the effect of Sphagnum species identity on Oribatida due to (1) low replication for Sphagnum species, and (2) strong confounding effect of environmental variables for Sphagnum species (see Table 1; Fig. 2a).
Ecological gradients in the mire: a distribution of the three major Sphagnum phylogenetic clades (sections) along the pH-water table gradients, symbol size represents N:P ratio in Sphagnum tissues. b N and K in Sphagnum tissues along the pH gradient, OLS reg (N) r2 = 0.09, P < 0.005, (K) r2 = 0.21, P < 0.001. Note: there was no significant relationship between P in Sphagnum tissues and the pH gradient. c Plant diversity (symbol size) in sampling plots of different trophic classes along the pH-water table gradients, OLS reg (pH) r2 = 0.17, P = 0.003, (water table) r2 = 0.02, P = 0.33. Plot trophic class (‘oligotrophic-eutrophic’) is based on plant indicator species and reflects plot status on the peatland poor-rich gradient. (color figure online)
The environmental variables best explaining the abundance of aquatic and terrestrial Oribatida and species richness of terrestrial Oribatida were identified using random forests regression (randomForest package in R) (Genuer et al. 2010); richness for aquatic Oribatida was not analysed due to the overall low number of aquatic species. Random forests (RF) is a classification and regression tree method that makes no distributional assumptions, has the ability to incorporate non-linear relationships between dependent and independent variables, can combine both quantitative (e.g., environmental) and qualitative (e.g., plot trophic class) variables, and is suitable for ecological data which are correlated. The RF creates multiple regression trees (the default is 500 trees, we adjusted this number for each model) fitting each tree to a randomly selected sample of the data; all trees are then averaged. The output provides the total % variance explained, and the importance score for each explanatory variable. The importance scores can be used to select the variables most important for interpretation of observed patterns. The significance of RF forests models was tested using permutation procedure (rfUtilities package; Murphy et al. 2010). Random forests can be also used for developing predictive models, but in our case the sample number is too low to train this algorithm to develop meaningful prediction, and the method was used for variable selection. Since consistency of results across statistical methods gives indication of robustness, we used the generalized linear regression (gamma distribution with log-link, SAS v.9.4, proc hpgenselect) to check for variable significance for top five most important variables identified by random forests.
Since the contribution of abiotic and biotic factors can be complex, we used path analysis to quantify relative contribution of environmental variables which form a network of causal relationships (Hatcher 1996). We constructed a hypothetical model of causal relationships underlying the observed diversity patterns in oribatid mites (see examples in Fig. 4) and used path analysis to calculate coefficients associated with each path in the diagram (proc CALIS in SAS v.9.4). Abundance of terrestrial and aquatic Oribatida, and species richness of terrestrial Oribatida were modelled; count variables were √x transformed for this analysis.
Principal coordinate analysis (Bray–Curtis distance on square root transformed species abundance counts) was used to construct ordination plot of samples and environmental variables in species space. Because Oribatida communities are affected not only by environmental gradients, but also by autocorrelation, we checked for spatial structuring in Oribatida communities using the Principal Coordinates of Neighbouring Matrices (PCNM) method (Borcard et al. 2004). The PCNM was applied to the matrix of pairwise Euclidean distances between sampling plots calculated from GPS coordinates, resulting in two orthogonal vectors (PCNM1, PCNM2) which were used to represent independent spatial variables. The distance-based linear modelling (DistLM) in PRIMER 7 (Clarke et al. 2014) with step-wise variable selection using AICc criterion was used to test which variables best explain Oribatida community composition. Taxa with low abundance (less than 5 individuals from all samples) were excluded from community analysis. Significance level α = 0.05 was used for all statistical tests.
Results
Environmental gradients in the mire
Sphagnum mosses and their related environmental and biological variables are reported in Table 1. Figure 2 outlines the main ecological gradients in the mire. There was a considerable correlation between taxonomic sections within the genus Sphagnum and their position along the pH-wetness gradients (Fig. 2a): species from Cuspidata section occupied the wetter positions (hollows and lawns), while Acutifolia and Sphagnum sections occurred at the dry end of the gradient (hummocks), which is a known trend (Anderson et al. 1995; Johnson et al. 2014). The exception was S. warnstorfii (Acutifolia section), which was only found in eutrophic lawns. A multicollinearity analysis (SAS v.9.4, proc reg) checking simple correlations and variance inflation factors for plot-level variables showed that pH was significantly correlated with water table level (in drier habitats pH was more acidic, r = 0.51, P < 0.001), with N (0.30, P = 0.037) and K (0.4, P = 0.001) in Sphagnum tissues (Fig. 2b), and with plot-level plant diversity (0.35, P = 0.015) (Fig. 2c). The variance inflation factor 3.05 for pH was only moderate; other variables were not significantly collinear.
Plot trophic class index, based on plant indicator species, was significantly correlated with local environmental variables representing both the acidity–alkalinity (pH) gradient and the nutrient availability (N–P–K) gradient in the mire, but was not related to the water table (micro-topography) gradient (Table 2).
Oribatida community composition, abundance and species richness
We collected 8048 adult Oribatida individuals, representing 52 species (Table S1). Water table level, pH, and K in Sphagnum tissues were three best environmental predictors of Oribatida assemblages, explaining 50.6% of the variance in community structure (combined aquatic and terrestrial species) (Table 3; Fig. 3). Aquatic species were associated with pools; two terrestrial species—Hoplophthiracarus illinoisensis and Nothrus pratensis—were associated with dry oligotrophic hummocks; the rest of terrestrial species were associated with drier mesotrophic and eutrophic habitats (Fig. 3). Plot trophic class by itself explained 14.7% of the variance in community structure (pseudo-F = 7.93, P = 0.002). Spatial structuring in Oribatida communities was significant but explained only a small proportion of the total variability in community structure (Table 3). However, for terrestrial Oribatida spatial variables accounted for 16.6% of community variability, and three terrestrial species (H. illinoisensis, Adoristes ovatus, Parachipteria punctata) showed strong correlation (> 0.55) with spatial variables. There was no significant spatial structuring in communities of aquatic Oribatida.
Ordination tri-plot (principal coordinate analysis, Bray–Curtis distance) for Oribatida assemblages, showing sampling plots (circles) in species space. Plot trophic class (‘oligotrophic–eutrophic’) is based on plant indicator species and reflects plot status on the peatland poor-rich (acidity–alkalinity) gradient. Mesotrophic and oligo-mesotrophic plots grouped as ‘mesotrophic’, eutrophic and meso-eutrophic plots grouped as ‘eutrophic’. Environmental variables are overlaid as vectors. Only species with > 50% correlation with axes are labelled: A. ard—Acrotritia ardua, A. str—Atropacarus striculus, H. ill—Hoplophthiracarus illinoisensis, L. alp—Liochthonius alpestris, L. cil—Limnozetes ciliatus, L. pal—Limnozetes palmerae, L. rug—Limnozetes rugosus, M. mon—Malaconothrus monodactylus, N. cor—Nanhermannia coronata, N. pra—Nothrus pratensis, O. nov—Oppiella nova, P. bor—Phthiracarus boresetosus, P. pun—Parachipteria punctata, S. cir—Scheloribates circumcarinatus, S. lae—Scheloribates laevigatus, S. pal—Suctobelbella palustris, T. fov—Trimalaconothrus foveolatus, T. lon—Trhypochthoniellus longisetus, T. mai—Tyrphonothrus maior, T. vel—Tectocepheus velatus. (Color figure online)
Among environmental variables, water table depth, local plant diversity, and P in Sphagnum tissues best explained the abundance of terrestrial Oribatida (Figs. 4a, 5a). Low water table depth and increased diversity of plants were also linked to increased richness of terrestrial Oribatida (Figs. 4b, 5b). Plot trophic class was a significant predictor of terrestrial Oribatida abundance and richness (Fig. 4a, b). For aquatic Oribatida abundance, high water table was the single most important predictor (Figs. 4c, 5c). Spatial variables were weakly significant for terrestrial Oribatida abundance and richness (P < 0.10), and not significant for aquatic Oribatida.
Environmental variables best explaining Oribatida abundance and species richness in Sphagnum samples: a terrestrial Oribatida abundance; b terrestrial Oribatida richness; c aquatic Oribatida abundance. The percentage in parentheses is the total % variance explained. Plotted are variable importance scores (% increase MSE) from random forests regressions; the variables with higher % increase MSE are more important in explaining observed patterns; all random forests models significant, P < 0.05 with 999 permutations. Variable significance checked using generalized linear regression: *P < 0.05, **P < 0.01, ***P < 0.001. Plant diversity refers to the number of species of vascular plants and bryophytes in 1 × 1 m sampling plots. Plot trophic class, based on plant indicator species, reflects plot status on the peatland poor-rich gradient. Nutrients (N, C, P and K) were measured in Sphagnum tissues. Richness of aquatic Oribatida was not analysed due to the overall low number of aquatic species
Standardized path coefficients for environmental factors affecting Oribatida diversity in Sphagnum samples: a terrestrial Oribatida abundance; b terrestrial Oribatida richness; c aquatic Oribatida abundance. Plant diversity refers to the number of species of vascular plants and bryophytes in 1 × 1 m sampling plots. Richness of aquatic Oribatida was not analysed due to the low number of aquatic species. Single-headed arrows represent paths; grey double-headed arrows represent correlations. Solid paths are significant, P < 0.05, dashed paths have P < 0.10; other paths not shown
Discussion
Oribatida diversity patterns in the Shichengskoe mire corresponded with environmental gradients such as ground water level, pH, plant diversity, nutrients, and were less influenced by spatial structuring, which was significant only for some terrestrial species.
Among measured environmental variables, abiotic factors (water table level and pH) were important in explaining Oribatida assemblages in mire environments. With respect to Oribatida assemblages, sampled habitats fell into three groups—(1) dry and oligotrophic hummocks, characterised by the presence of Sphagnum medium, S. fuscum and S. rubrum; characteristic Oribatida species were tyrphophylic N. pratensis and H. illinoisensis; (2) mesotrophic and eutrophic, with a gradient from dry hummocks (characterised by S. wulfianum and S. russowii) to wetter lawns (S. squarrosum, S. warnstorfii), and characterised by presence of tyrphophilic and eurybiontic Oribatida species; (3) pool communities, characterised by S. majus, S. subsecundum, S. lindbergii, S. flexuosum, S. balticum, S. cuspidatum, S. fallax; characteristic Oribatida were aquatic species Trimalaconothrus foveolatus, Trhypochthoniellus longisetus, Hydrozetes lacustris, Limnozetes spp., Malaconothrus spp. which typically inhabit submerged vegetation in European Sphagnum bogs (Weigmann 2006; Seniczak 2011; Seniczak et al. 2014).
The depth of the water table is one of the most ecologically significant hydrologic properties in peatlands (Lafleur et al. 2005). In our study at the wet end of the water table gradient Oribatida communities converge to a small set of aquatic species, presenting an example of environmental filtering. Environmental filtering (Keddy 1992; Lavorel and Garnier 2002) refers to species-sorting whereby an abiotic filter (here, the high water table) limits communities to species with specific tolerances (here, the ability to survive underwater). In drier environments (higher micro-topographic position), more species are able to pass through the filter, and the resulting outcome is a less predictable community, which reflects an interplay of all local biotic and abiotic factors (Comte et al. 2016). As a result, ‘dry’ Oribatida assemblages vary, but all ‘wet’ assemblages are strongly homogeneous, irrespectively of other local factors such as nutrient status (see Fig. 3). The concept of environmental filtering has been criticized (e.g., Kraft et al. 2014; Cadotte and Tucker 2017), who argued that it is not possible to conclude whether a species is excluded from a community due to an environmental filter, dispersal limitation, or local competition. However, we suggest that a strong abiotic gradient can have a deciding influence in species-sorting.
Our results demonstrate that abundance and species richness of terrestrial Oribatida in the Shichengskoe mire can be linked to local plant diversity. More diverse plant communities create greater small-scale heterogeneity, which can provide a richer habitat structure and a more diverse range of food resources to soil fauna (Perez-Harguindeguy et al. 2000; Hättenschwiler et al. 2005; Nielsen et al. 2010). Vegetation composition and type have been shown to have strong influence on Oribatida communities (Nielsen et al. 2010, 2012; Minor et al. 2016). Greater litter diversity has been associated with greater species richness (but not abundance) of oribatid mites (Hansen and Coleman 1998; Kaneko and Salamanca 1999). However, some studies showed no correlation between plant or litter diversity and species richness or community composition of oribatid mites (Kaneko et al. 2005; Winkler et al. 2018).
Our results also identify P as is an important element in predicting terrestrial Oribatida abundance in the mire. Strong links (both positive and negative) between P and abundance of bog Oribatida species have been observed elsewhere (Seniczak 2011). Both N and P are important nutrients affecting growth of Sphagnum and vascular plants in peatlands (Limpens et al. 2004; Hill et al. 2014; Toberman et al. 2015). In ombrotrophic peat bogs the input of P is limited to dry atmospheric deposition and biological sources (Rydin and Jeglum 2013; Tipping et al. 2014). As a result, P is an important limiting element in N cycling in bogs, controlling net primary production (Limpens et al. 2004), organic N mineralization (White and Reddy 2000) and microbial N-fixation (Toberman et al. 2015; van den Elzen et al. 2017). Local P enrichment, for example, can increase N uptake and lead to higher N concentrations in Sphagnum tissue, which in turn promotes faster Sphagnum litter decomposition and stimulates local nutrient cycling (Gerdol et al. 2007).
The P input within the Shichengskoe mire is linked to the land relief and the local hydrology. Within the oligotrophic ombrotrophic bog there are several ground water seeps associated with forested mineral islands. Such seeps of ground water provide sources of nutrient enrichment in an otherwise oligotrophic environment (Ruuhijärvi and Lindholm 2006). Ground water seeps of the Shichegskoe mire create localised patches of nutrient enrichment within the oligotrophic bog, with higher pH, eutrophic and mesotrophic Sphagnum species, and characteristic plant species (e.g., Equisetum spp.) more common for fens. When combined with higher micro-topographic position, these seeps provide niches for more abundant and diverse Oribatida communities.
The relationship between local pH, nutrient availability, and Oribatida diversity patterns can reflect indirect effects on the local food web structure. Recent stable isotope data (Lehmitz and Maraun 2016) show that the majority of Oribatida typical for Sphagnum peatlands are primary and secondary decomposers with heterogeneous δ15N values, suggesting that they use a variety of resources, including plant fragments and associated bacteria, fungi, micro-algae and protozoa. Some species (aquatic Limnozetes ciliatus and H. lacustris, terrestrial Acrotritia ardua and Pergalumna nervosa) had wide trophic niches, indicating feeding on more than one trophic level. Abundant in our samples Tyrphonothrus maior had δ13C signal close to that of Sphagnum, which suggested that it feeds directly on Sphagnum. Species such as Limnozetes rugosus and H. lacustris were enriched in δ15N, suggesting they are predators or scavengers. At all trophic levels the δ15N values of oribatid mites were significantly correlated with the d15N values of the Sphagnum in respective samples, indicating that the local food web is based on Sphagnum moss, with bacteria, fungi and microfauna that oribatid mites consume also deriving their nutrients from Sphagnum.
Bacteria, microalgae and protozoa make up the majority of microbial biomass in Sphagnum peatlands (Gilbert et al. 1998; Jassey et al. 2013) and are sensitive to pH, water table depth, and nutrient availability (Andersen et al. 2013). Nutrient enrichment can lead to an overall increase in microbial biomass (Gilbert et al. 1998), algal biomass (Guasch et al. 1995), and change the taxonomic composition and functioning of peatland microbial community (Andersen et al. 2013; Mieczan et al. 2015), which then affects Oribatida through bottom–up interactions.
The effects of acidity, nutrient availability and habitat heterogeneity are difficult to separate in a peatland. Sphagnum mosses are adapted for high uptake and retention of N, and in nutrient-poor ombrotrophic bog they are able to outcompete other plants (Hájek 2009; Turetsky et al. 2012). Additional input of nutrients (e.g., from ground water seeps), especially in the rhizosphere, depresses Sphagnum growth and favours vascular plants, therefore increasing habitat heterogeneity (Berendse et al. 2001; Bragazza et al. 2006; Limpens et al. 2011). Our data show that within the Shichengskoe mire system the pH gradient and the nutrient availability gradient were linked, which is common but not universal in peatlands (Wheeler and Proctor 2000; Bragazza and Gerdol 2002). This is highlighted by the result that the trophic class of sampled plots (based on plant community features) was correlated to both pH and nutrient measurements, and was one of the best predictors of abundance and species richness of terrestrial Oribatida. Other indirect factors may have a role in shaping local communities—for example, litter type (i.e., plant species) was the main factor affecting activity of aerobic decomposers in a boreal peatland, more important than local nutrients and water table regime (Straková et al. 2011).
To conclude, if we were to suggest best predictors of Oribatida community structure, abundance and diversity in the Shichengskoe mire, it would be two variables—water table depth (micro-topography) and plot trophic class (combined pH and nutrient availability gradients). With their defined environmental gradients and well-known assemblages of plants and animals, peatlands are unique model systems for investigating questions related to the community assembly rules and niche-based species coexistence. Information on functional traits of individual Oribatida species (e.g., Lehmitz and Maraun 2016) offers a further opportunity for understanding the species coexistence processes and hyperdiversity among Oribatida.
References
Andersen R, Chapman SJ, Artz RRE (2013) Microbial communities in natural and disturbed peatlands: a review. Soil Biol Biochem 57:979–994
Anderson AS, Davis RB, Janssens JA (1995) Relationships of bryophytes and lichens to environmental gradients in Maine peatlands. Vegetatio 120:147–259
Berendse F, Van Breemen N, Rydin H, Buttler A, Heijmans M, Hoosbeek MR, Lee JA, Mitchell E, Saarinen T, Vasander H, Wallen B (2001) Raised atmospheric CO2 levels and increased N deposition cause shifts in plant species composition and production in Sphagnum bogs. Glob Change Biol 7:591–598
Borcard D, von Ballmoos VC (1997) Oribatid mites (Acari, Oribatida) of a primary peat bog pasture transition in the Swiss Jura Mountains. Ecoscience 4:470–479
Borcard D, Legendre P, Avois-Jacquet C, Tuomisto H (2004) Dissecting the spatial structures of ecological data at all scales. Ecology 85:1826–1832
Bragazza L, Gerdol R (2002) Are nutrient availability and acidity-alkalinity gradients related in Sphagnum-dominated peatlands? J Veg Sci 13:473–482
Bragazza L, Freeman C, Jones T et al (2006) Atmospheric nitrogen deposition promotes carbon loss from peat bogs. PNAS 103:19386–19389
Cadotte MW, Tucker CM (2017) Should environmental filtering be abandoned? Trends Ecol Evol. https://doi.org/10.1016/j.tree.2017.03.004
Chesson P (2000) Mechanisms of maintenance of species diversity. Annu Rev Ecol Syst 31:343–366
Clarke KR, Gorley RN, Somerfield PJ, Warwick RM (2014) Change in marine communities: an approach to statistical analysis and interpretation, 3rd edn. PRIMER-E, Plymouth
Comte L, Cucherousset J, Boulêtreau S, Olden JD (2016). Resource partitioning and functional diversity of worldwide freshwater fish communities. Ecosphere. https://doi.org/10.1002/ecs2.1356
Donaldson GM (1996) Oribatida (Acari) associated with three species of Sphagnum at Spruce Hole Bog, New Hampshire, USA. Can J Zool 74:1713–1720
Eurola S, Holappa K (1985) The Finnish mire type system. Aquilo Ser Bot 21:101–110
Eurola S, Huttunen A (2006) Mire plant species and their ecology in Finland. In: Lindholm T, Heikkilä R (eds) Finland—land of mires. The Finnish environment 23/2006. Finnish Environment Institute, Helsinki, pp 127–144
Genuer R, Poggi J-M, Tuleau-Malot C (2010) Variable selection using random forests. Pattern Recognit Lett 31:2225–2236
Gerdol R, Petraglia A, Bragazza L, Iacumin P, Brancaleoni L (2007) Nitrogen deposition interacts with climate in affecting production and decomposition rates in Sphagnum mosses. Glob Change Biol 13:1810–1821
Gilbert D, Amblard C, Bourdier G, Francez A-J (1998) Short-term effect of nitrogen enrichment on the microbial communities of a peatland. Hydrobiologia 373:111–119
Guasch H, Marti E, Sabater S (1995) Nutrient enrichment effects on biofilm metabolism in a Mediterranean stream. Freshw Biol 33:373–383
Gunnarsson U (2005) Global patterns of Sphagnum productivity. J Bryol 27:269–279
Hájek T (2009) Habitat and species controls on Sphagnum production and decomposition in a mountain raised bog. Bor Environ Res 14:947–958
Hajkova P, Hajek M (2007) Sphagnum distribution patterns along environmental gradients in Bulgaria. J Bryol 29:18–26
Hansen RA, Coleman DC (1998) Litter complexity and composition are determinants of the diversity and species composition of oribatid mites (Acari:Oribatida) in litterbags. Appl Soil Ecol 9:17–23
Hatcher L (1996) Using SAS® PROC CALIS for path analysis: an introduction. Struct Equ Model 3:176–192
Hättenschwiler S, Tiunov AV, Scheu S (2005) Biodiversity and litter decomposition in terrestrial ecosystems. Annu Rev Ecol Evol Syst 36:191–218
Hill BH, Elonen CM, Jicha TM, Kolka RK, Lehto LLP, Sebestyen SD, Seifert-Monson LR (2014) Ecoenzymatic stoichiometry and microbial processing of organic matter in northern bogs and fens reveals a common P-limitation between peatland types. Biogeochemistry 120:203–224
HilleRisLambers J, Adler PB, Harpole WS, Levine JM, Mayfield MM (2012) Rethinking community assembly through the lens of coexistence theory. Annu Rev Ecol Evol Syst 43:227–248
Ignatov MS, Ignatova EA (2003) Moss flora of the Middle European Russia. Sphagnaceae—Hedwigiaceae. Arctoa 1(suppl 1):1–608 (In Russian)
Ignatov MS, Afonina OM, Ignatova EA et al (2006) Check-list of mosses of East Europe and North Asia. Arctoa 15:1–130. https://doi.org/10.15298/arctoa.15.01
Jassey VEJ, Meyer C, Dupuy C, Bernard N, Mitchell EAD, Toussaint M-L, Metian M, Chatelain AP, Gilbert D (2013) To what extent do food preferences explain the trophic position of heterotrophic and mixotrophic microbial consumers in a sphagnum peatland? Microb Ecol 66:571e580
Johnson MG, Granath G, Tahvanainen T, Pouliot R, Stenøien HK, Rochefort L, Rydin H, Shaw AJ (2014) Evolution of niche preference in Sphagnum peat mosses. Evolution 69:90–103
Kaneko N, Salamanca E (1999) Mixed leaf litter effects on decomposition rates and soil microarthropod communities in an oak–pine stand in Japan. Ecol Res 14:131–138
Kaneko N, Sugawara Y, Miyamoto T, Hasegawa M, Hiura T (2005) Oribatid mite community structure and tree species diversity: a link? Pedobiologia 49:521–528
Keddy PA (1992) Assembly and response rules: two goals for predictive community ecology. J Veg Sci 3:157–164
Kraft NJB, Adler PB, Godoy O, James EC, Fuller S, Levine JM (2014) Community assembly, coexistence and the environmental filtering metaphor. Funct Ecol. https://doi.org/10.1111/1365-2435.12345
Lafleur PM, Hember RA, Admiral SM, Roulet NT (2005) Annual and seasonal variability in evapotranspiration and water table at a shrub-covered bog in southern Ontario, Canada. Hydrol Process 19:3533–3550
Lavorel S, Garnier E (2002) Predicting changes in community composition and ecosystem functioning from plant traits: revisiting the Holy Grail. Funct Ecol 16:545–556
Lehmitz R, Maraun M (2016) Small-scale spatial heterogeneity of stable isotopes signatures (d15N, d13C) in Sphagnum sp. transfers to all trophic levels in oribatid mites. Soil Biol Biochem 100:242–251
Limpens J, Berendse F, Klees H (2003) N deposition affects N availability in interstitial water, growth of Sphagnum and invasion of vascular plants in bog vegetation. New Phytol 157:339–347
Limpens J, Berendse F, Klees H (2004) How phosphorus availability affects the impact of nitrogen deposition on Sphagnum and vascular plants in bogs. Ecosystems 7:793–804
Limpens J, Granath G, Gunnarsson U et al (2011) Climatic modifiers of the response to nitrogen deposition in peat-forming Sphagnum mosses: a meta-analysis. New Phytol 191:496–507
Limpens J, Bohlin E, Nilsson MB (2017) Phylogenetic or environmental control on the elemental and organo-chemical composition of Sphagnum mosses? Plant Soil 417:69–85
Markkula I (1986) Comparison of the communities of oribatids (Acari: Cryptostigmata) of virgin and forest ameliorated pine bogs. Ann Zool Fennici 23:33–38
Mieczan T, Adamczuk M, Pawlik-Skowrońska B, Toporowska M (2015) Eutrophication of peatbogs: consequences of P and N enrichment for microbial and metazoan communities in mesocosm experiments. Aquat Microb Ecol. https://doi.org/10.3354/ame01727
Minor MA, Ermilov SG, Philippov DA, Prokin AA (2016) Relative importance of local habitat complexity and regional factors for assemblages of oribatid mites (Acari: Oribatida) in Sphagnum peat bogs. Exp Appl Acarol. https://doi.org/10.1007/s10493-016-0075-9
Murphy MA, Evans JS, Storfer AS (2010) Quantifying Bufo boreas connectivity in Yellowstone National Park with landscape genetics. Ecology 91:252–261
Nielsen UN, Osler GHR, Campbell CD, Neilson R, Burslem DFRP, van der Wal R (2010) The enigma of soil animal species diversity revisited: the role of small-scale heterogeneity. PLoS One 5(7):e11567
Nielsen UN, Osler GHR, Campbell CD, Burslem DFRP, van der Wal R (2012) Predictors of fine-scale spatial variation in soil mite and microbe community composition differ between biotic groups and habitats. Pedobiologia 55:83–91
Perez-Harguindeguy N, Diaz S, Cornelissen JH, Venramini F, Cabido M, Castellanos A (2000) Chemistry and toughness predict leaf litter decomposition rates over a wide spectrum of functional types and taxa in central Argentina. Plant Soil 218:21–30
Philippov DA (2015) Flora Shichengskogo vodno-bolotnogo ugodya Vologodskaya oblast [Flora of wetland ‘Shichengskoe’ (Vologda Region, Russia)]. Phytodiversity of Eastern Europe IX. pp 86–117 (in Russian with English summary)
Philippov DA, Boychuk MA (2015) Mkhi Shichengskogo landshaftnogo zakaznika (Vologodskaya oblast) [Mosses of the Shichengskiy Landscape Reserve (Vologda Region)]. Vestnik of Northern (Arctic) Federal University, ser. Nat Sci 2:80–89 (in Russian with English summary)
Ruuhijärvi R, Lindholm T (2006) Ecological gradients as the basis of Finnish mire site type system. In: Lindholm T, Heikkilä R (eds) Finland—land of mires. The Finnish Environment 23/2006. Finnish Environment Institute, Helsinki, pp 119–126
Rydin H, Jeglum JK (2013) The biology of peatlands, 2nd edn. Oxford University Press, Oxford
Rydin H, Sjörs H, Löfroth M (1999) Mires. Acta Phytogeogr Suec 84:91–112
Seniczak A (2011) Oribatid mites (Acari, Oribatida) and their seasonal dynamics in a floating bog mat in Jeziorka Kozie Reserve, Tuchola Forest (Poland). Biol Lett 48:3–11
Seniczak A, Seniczak S, Kowalski J, Graczyk R, Mistrzak M (2014) Mites (Acari) at the edges of bog pools in Orawa–Nowy-Targ Basin (S Poland), with particular reference to the Oribatida. Biol Lett 51:93–102
Spitzer K, Danks HV (2006) Insect biodiversity of boreal peat bogs. Annu Rev Entomol 51:137–161
Stary J (2006) Contribution to the knowledge of the oribatid mite fauna (Acari, Oribatida) of peat bogs in Bohemian Forest. Silva Gabreta 12:35–47
Strakova P, Niemi RM, Freeman C, Peltoniemi K, Toberman H, Heiskanen I, Fritze H, Laiho R (2011) Litter type affects the activity of aerobic decomposers in a boreal peatland more than site nutrient and water table regimes. Biogeosciences 8:2741–2755
Subías LS (2018) Listado sistemático, sinonímico y biogeográfico de los ácaros oribátidos (Acariformes: Oribatida) del mundo (excepto fósiles). p 605. http://bba.bioucm.es/cont/docs/RO_1.pdf. Accessed 05 Dec 2018
Sutherland WJ, Freckleton RP, Godfray HCJ et al (2013) Identification of 100 fundamental ecological questions. J Ecol. https://doi.org/10.1111/1365-2745.12025
Tahvanainen T (2004) Water chemistry of mires in relation to the poor-rich vegetation gradient and contrasting geochemical zones of the north-eastern Fennoscandian Shield. Folia Geobot 39:353–369
Tarnocai C, Stolbovoy V (2006) Northern peatlands: their characteristics, development and sensitivity to climate change. Dev Earth Surf Proc 9:17–51
Tarras-Wahlberg N (1961) The Oribatei of a central Swedish bog and their environment. Oikos 4:1–56
Tipping E, Benham S, Boyle JF, Crow P, Davies J, Fischer U, Guyatt H, Helliwell R, Jackson-Blake L, Lawlor AJ, Monteith DT, Rowe EC, Toberman H (2014) Atmospheric deposition of phosphorus to land and freshwater. Environ Sci Proc Impacts 16:1608–1617
Toberman H, Tipping E, Boyle JF, Helliwell RC, Lilly A, Henrys PA (2015) Dependence of ombrotrophic peat nitrogen on phosphorus and climate. Biogeochemistry 125:11–20
Turetsky MR, Bond-Lamberty B, Euskirchen E, Talbot J, Frolking S, McGuire AD, Tuiitla E-S (2012) The resilience and functional role of moss in boreal and arctic ecosystems. New Phytol 196:49–67
van den Elzen E, Kox MAR, Harpenslager SF, Hensgens G, Fritz C, Jetten MSM, Ettwig KF, Lamers LPM (2017) Symbiosis revisited: phosphorus and acid buffering stimulate N2 fixation but not Sphagnum growth. Biogeosciences 14:1111–1122
van den Elzen E, van den Berg LJL, van der Weijden B, Fritz C, Sheppard LJ, Lamers LPM (2018) Effects of airborne ammonium and nitrate pollution strongly differ in peat bogs, but symbiotic nitrogen fixation remains unaffected. Sci Total Environ. https://doi.org/10.1016/j.scitotenv.2017.08.102
Weigmann G (1991) Oribatid communities in transects from bogs to forests in Berlin indicating the biotope qualities. Mod Acarol 1:359–364
Weigmann G (2006) Hornmilben (Oribatida). Die Tierwelt Deutschalnds. 76 Teil. Goecke and Evers, Keltern
Weigmann G, Deichsel R (2006) Acari: limnic Oribatida. In: Gerecke R (ed) Chelicerata: Araneae, Acari I. Susswasserfauna von Mitteleuropa, Band 7/2-1. Spektrum Akademischer Verlag, Heidelberg, pp 89–112
Wheeler BD, Proctor MCF (2000) Ecological gradients, subdivisions and terminology of north-west European mires. J Ecol 88:187–203
White JR, Reddy KR (2000) Influence of phosphorus loading on organic nitrogen mineralization of everglades soils. Soil Sci Soc Am J 64:1525–1534
Winkler M, Illmer P, Querner P, Fischer BM, Hofmann K, Lamprecht A, Praeg N, Schied J, Steinbauer K, Pauli H (2018) Side by side? Vascular plant, invertebrate, and microorganism distribution patterns along an alpine to nival elevation gradient. Arct Antarct Alp Res 50:1–13
Acknowledgements
We thank Alexander A. Prokin (Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, Russia) for funding chemical analysis, Victoria V. Yurchenko (Papanin Institute for Biology of Inland Waters, Russian Academy of Sciences, Borok, Russia) for pH analysis, and Dr. Gillian Rapson (School of Agriculture and Environment, Massey University, New Zealand) for discussion of Sphagnum bogs. We also thank the anonymous reviewers for helpful suggestions which improved the paper. Fieldwork was carried out as a part of the Russian Science Foundation Grant no. 14-14-01134. Work by D. A. Philippov was supported within the framework of the state assignments from the Russian Federal Agency for Scientific Organizations (IBIW RAS theme no. AAAA-A18-118012690099-2).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Minor, M.A., Ermilov, S.G. & Philippov, D.А. Hydrology-driven environmental variability determines abiotic characteristics and Oribatida diversity patterns in a Sphagnum peatland system. Exp Appl Acarol 77, 43–58 (2019). https://doi.org/10.1007/s10493-018-0332-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0332-1