Abstract
The two-spotted spider mite Tetranychus urticae is an important pest of strawberry crops in Brazil and many other countries. Focus for biocontrol studies involving entomopathogenic fungi has been on three species from the genus Metarhizium: M. anisopliae sensu stricto (s.s.), M. brunneum and M. robertsii. Also, the species Beauveria bassiana has been studied for spider mite control and one isolate (ESALQPL63) is commercially available in Brazil. New and undescribed Metarhizium species have been found recently in Brazil and provide a pool of isolates with potential for biocontrol in Brazil and probably also elsewhere. The mortality of adult females of T. urticae when exposed to four new Brazilian species of Metarhizium was compared to the mortality when exposed to M. anisopliae s.s., M. brunneum, M. pingshaense, M. robertsii and Beauveria bassiana ESALQPL63. Fungal suspensions were sprayed onto mites at 107 conidia/mL with 0.05% Tween 80 in laboratory bio-assays. We measured total mortality and percentage sporulating cadavers 10 days after exposure and calculated median lethal time (LT50). The lowest LT50 (4.0 ± 0.17) was observed for mites treated with Metarhizium sp. Indet. 1 (ESALQ1638), which also performed well with respect to mortality after 10 days and capacity to sporulate from cadavers. Among the other little studied species tested, M. pingshaense (ESALQ3069 and ESALQ3222) and Metarhizium Indet. 2 (ESALQ1476) performed well and were comparable to B. bassiana (ESALQPL63). The new Metarhizium isolates and species thus showed potential for biological control.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The two-spotted spider mite, Tetranychus urticae Koch (Acari: Tetranychidae), is one of the main pests of horticultural crops worldwide (Attia et al. 2013), and it is a main pest of strawberry crops in Brazil (Iwassaki et al. 2015). This spider mite attack affects these crops by feeding on the undersurface of leaves, causing curling and discoloration, which lead to reduced photosynthetic activity and leaf abscission (Attia et al. 2013; de Moraes and Flechtmann 2008). Furthermore, T. urticae has developed resistance to many chemical pesticides (Nicastro et al. 2010; Stumpf and Nauen 2001). According to Wu et al. (2016), widespread insecticide/acaricide usage has significantly reduced the number of the mites’ natural enemies in crops, thus allowing T. urticae populations in the crops to grow.
One alternative to chemical pesticides is biological control with entomopathogenic fungi, in particular species from the phylum Ascomycota. Entomopathogenic fungi from the genus Metarhizium are produced and marketed for biological control of various pests (Faria and Wraight 2007; Vega et al. 2012). Maniania et al. (2008) highlighted the importance of testing more isolates of entomopathogenic fungi to evaluate whether among the tested isolates were some with high potential for biocontrol. Whereas some species from the genus Metarhizium (M. anisopliae s.s., M. brunneum, and M. robertsii) have been subjected to several studies about virulence and other features, other described species from that genus (for example M. pingshaense) are more or less unknown with respect to biological properties. Also, new Metarhizium species have recently been discovered in Brazil (Rezende et al. 2015), so far referred to as Metarhizium Indet. 1, 2, 3, 4 and 5.
In this study, we aimed to compare the virulence (as measured by mortality) to T. urticae of 14 Brazilian Metarhizium isolates. We included Metarhizium Indet. 1, 2, 4 and 5, M. pingshaense, M. anisoliae s.s., M. brunneum, and M. robertsii and used a commercial isolate of Beauveria bassiana as a comparison. Also, the ability to sporulate from infected and killed mites was measured.
Materials and methods
Fungal origin
Two isolates each were selected of M. robertsii, M. anisopliae s.s., M. brunneum, M. pingshaense, and one isolate each of Metarhizium Indet. sp. 2 and 5 obtained from strawberry crop soils in southern Minas Gerais State in Brazil between 2012 and 2013 (Table 1) from the Collection of Entomopathogenic Microorganisms of ESALQ-USP. These were compared to two isolates each of Metarhizium sp. Indet. 1 and 4 (A.B.R. Zanardo unpubl. data) from the same collection. Beauveria bassiana (ESALQPL63), commercially available in Brazil for the control of this pest, was used as control. All fungi used were molecularly identified using TEF marker as suggested by Bischoff et al. (2009).
Tetranychus urticae stock colony
Tetranychus urticae was reared on Jack bean plants Canavalia ensiformis (Fabaceae) in a room at 25 °C, 60% RH, and 12 h photoperiod at the University of São Paulo (ESALQ-USP) since 2014. The plants were irrigated 3 × a week. Old and highly infested plants were replaced by new ones as required.
Fungal production
All selected isolates were grown in Petri dishes (90 × 15 mm) at 25 ± 1 °C on PDA medium (Potato Dextrose Agar, Difco™, USA). Conidia were harvested by scraping the surface of 2-week-old sporulating cultures and then suspended in 5 mL sterile distilled water containing 0.05% Tween 80 (Oxiteno, São Paulo, Brazil) in a 40-mL flat bottom glass tube (stock suspension). The conidial suspension was vortexed for 1 min to produce a homogenous conidial suspension and counted on Neubauer chamber (K5-0111 model, KASVI, Brazil) to obtain a 107 conidia/mL suspension of each isolate.
Bioassay
Petri Dishes (3.5 cm diameter, 1.5 cm high) were filled with 3 mL of 0.5% water agar and a Jack Bean disc leaf (2 cm diameter) was placed on top of the agar with the abaxial surface up. Then, 12 T. urticae females were transferred with a fine paintbrush to the center of the leaf disc. Each Petri dish was sprayed with 1 mL of a 107 viable conidia/mL suspension of one isolate with 0.05% Tween 80 using a Potter Spray Tower (Burkard Manufacturing, Rickmansworth, UK). The device was adjusted to a pressure of 0.7 psi, with a coverage of 1.5 mg/cm2. To avoid contamination between treatments, the sprayer was cleaned with alcohol and washed 3 × with distilled water, and the first spray of each treatment was discarded. A control with distilled water plus 0.05% Tween 80 was included. Five replicates (Petri dishes) were sprayed separately with each concentration. The entire experiment was repeated 3 × yielding 180 mites per treatment; in total 2880 mites were used in the entire experiment. The data from the three experiments were analyzed together.
Mortality was recorded daily for 10 days. Dead mites were individually placed in 24-cell well culture plates with a lid on, containing moistened cotton with sterile distilled water to ensure conditions of high relative humidity to allow fungus growth on the surface of the cadaver. Mortality caused by fungus was confirmed by scraping the surface of the mite with a sterile loop to collect the fungi and microscopic examination of this material.
Statistical analysis
The survival parameters of infected mites were analyzed using the Kaplan–Meier survival analysis using Log Rank (Mantel–Cox) test in v. 22.0.0 SPSS (2013). Quasi-binomial models were fitted to the total mortality and sporulated cadaver’s data separately; F-tests were performed to assess significance of effects using a GLM procedure (multcomp R package) through ANOVA. Treatment differences were tested using 95% confidence intervals using Tukey HSD contrasts. Analyses were performed using the R statistical software environment (R Development Core Team 2015).
Results
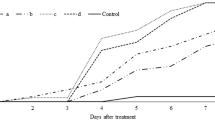
All Metarhizium isolates were pathogenic to T. urticae and mortality of mites differed significantly between untreated control and fungus treated specimens in the survival analysis (Fig. 1). The survival curve of the mites treated with our control isolate, B. bassiana ESALQPL63, was similar to Metarhizium sp. Indet. 1 (ESALQ1608 and ESALQ1638), and M. pingshaense (ESALQ3069 and ESALQ3222) but differed from all other isolates [Log Rank (Mantel–Cox) p < 0.05], confirming that Metarhizium sp. Indet. 1 and M. pingshaense were as efficient as B. bassiana. Metarhizium sp. Indet. 4 (ESALQ1660 and ESALQ1684), M. brunneum (ESALQ2623) and Metarhizium sp. Indet. 5 (ESALQ3140) were all pathogenic, but showed a lower mortality on the exposed mites than the other isolates.
The only isolate standing out with respect to low LT50 was Metarhizium sp. Indet. 1 ESALQ1638 (Table 1): 4.0 ± 0.2 days (95% CI 3.7–4.3). In the other end of the range, mites treated with Metarhizium sp. Indet. 4 ESALQ1660 had a LT50 of 8.0 ± 1.5 (95% CI 5.1–10.9).
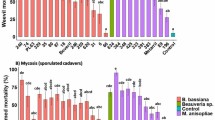
Mortality after 10 days differed significantly between untreated control and fungus treated specimens (Fig. 2). The mortality of mites treated with B. bassiana ESALQPL63 showed a mortality of 93.0 ± 1.0% 10 days after exposure and was similar to that of eight other isolates. Beauveria bassiana ESALQPL63 and Metarhizium sp. Indet. 1 (ESALQ1608 and ESALQ1638), differed (F1,15 = 29.92; p < 0.001) from M. anisopliae s.s. (ESALQ2651 and ESALQ3054), M. brunneum (ESALQ2623), Metarhizium sp. Indet. 4 (ESALQ1660 and ESALQ1684) (56 ± 9.7 and 59 ± 2.8%) and Metarhizium sp. Indet. 5 (ESALQ3140), the isolates resulting in lowest mite mortalities.
Mortality and sporulation from cadavers of Tetranychus urticae females at 25 °C and 12:12 L:D 10 days after being sprayed with 14 isolates of Metarhizium spp. or Beauveria bassiana (isolate PL 63) with 0.05% Tween-80. Different lower-case letters indicate treatment differences in sporulated cadavers; different upper-case letters indicate treatment differences in total mortality by quasi-binomial models and F-tests through ANOVA and Tukey HSD comparison (p < 0.05)
Sporulation was observed in approximately 80% of spider mite cadavers in all treatments. Beauveria bassiana (ESALQPL63), Metarhizium sp. Indet. 1 (ESALQ1608 and ESALQ1638), and Metarhizium pingshaense (ESALQ3222) showed the highest percentage of sporulation (F1,15 = 67.45, p < 0.001) compared with M. anisopliae s.s. (ESALQ2651 and ESALQ3054), M. brunneum (ESALQ2623), Metarhizium sp. Indet. 4 [ESALQ1660 (37 ± 1.0%) and ESALQ1684] and Metarhizium sp. Indet. 5 (ESALQ3140).
Discussion
This study revealed for the first time the potential of new and less studied species and isolates of Metarhizium as biocontrol agents of T. urticae. The mortality levels obtained in our study were in an overall sense similar to other studies using Metarhizium species against spider mites elsewhere. Chandler et al. (2005) showed reductions of all developmental stages (eggs, nymphs and adults) of T. urticae when M. anisopliae s.l. was applied; they also studied the efficacy of Naturalis-L (B. bassiana-based mycopesticide—Troy Biosciences, Phoenix, TX, USA) which reduced T. urticae populations by 97%. Up to 93 and 96% reductions in T. urticae population density on cucumber and tomato, respectively, were observed following application of Naturalis-L (Marcic et al. 2012). Bugeme et al. (2015) reported that M. anisopliae s.l. formulations were as effective as abamectin (chemical insecticide) in reducing T. urticae densities on common bean in both screenhouse and field experiments. An emulsifiable formulation of M. anisopliae s.l. was tested in the control of five Tetranychidae species including T. urticae, in irrigated cotton fields in a desert area in the Tarim Basin of northwestern China (Shi et al. 2008). A high potential for practical use in the management of the spider mites using this technique was reported by the authors.
Based on the 2009 classification of Metarhizium, the fungus previous known as M. anisopliae s.l. actually comprises complex of nine species: M. anisopliae s.s., M. guizhouense, M. pingshaense, M. acridum, M. majus, M. lepidiotae, M. brunneum, M. globosum and M. robertsii (Bischoff et al. 2009), so in literature before this year the name ‘Metarhizium anisopliae’ may refer to any of these species. In our studies, we had for the first time a possibility to compare virulence between eight different and partly unknown Metarhizium species determined by molecular methods, and have by that a solid background for choosing one or more isolate for further studies.
Although all evaluated fungal isolates were able to infect adult female of T. urticae mite in laboratory, there were significant variations amongst the isolates. These results revealed the potential of the unassigned new lineages of Metarhizium as biocontrol agents. Metarhizium sp. Indet. 1 ESALQ1638 stands out (based on survival curves of treated hosts, high mortality and sporulation on cadavers 10 days after exposure, low LT50, and sporulation) when compared with the other tested Metarhizium isolates, although differences were not big. Metarhizium sp. Indet. 1 was therefore in the same range as the isolate registered for control of mites, B. bassiana (ESALQPL63).
Metarhizium sp. Indet. 1 and the other new species of Metarhizium are here for the first time studied for their potential. Also, M. pingshaense is largely underexplored. In one study, an isolate of M. pingshaense (MGC02) caused more than 80% larval mortality of Anomala cincta (Coleoptera: Scarabeidae) in laboratory assays (Guzmán-Franco et al. 2011). Pena-Pena et al. (2015) reported that the same isolate was potentially useful in controlling root-feeding pests by seed inoculation, confirming its ability to endophytic colonize maize roots.
Subsequent studies may include more biological data in an exploration, if for example Metarhizium sp. Indet. 1 or M. pingshaense possess characters allowing them to be superior to B. bassiana, M. anisopliae, M. brunneum or M. robertsii for mite control. Data on sporulation, as in Fig. 2, are for example important as these data reveal information of the potential of the fungi to reproduce after application and by that infect more targets over time.
References
Attia S, Grissa KL, Lognay G, Bitume E, Hance T, Mailleux AC (2013) A review of the major biological approaches to control the worldwide pest Tetranychus urticae (Acari: Tetranychidae) with special reference to natural pesticides. J Pest Sci 86:361–386
Bischoff JF, Rehner SA, Humber RA (2009) A multilocus phylogeny of the Metarhizium anisopliae lineage. Mycologia 101:512–530
Bugeme DM, Knapp M, Ekesi S, Chabi-Olaye A, Boga HI, Maniania NK (2015) Efficacy of Metarhizium anisopliae in controlling the two-spotted spider mite Tetranychus urticae on common bean in screenhouse and field experiments. Insect Sci 22:121–128
Chandler D, Davidson G, Jacobson RJ (2005) Laboratory and glasshouse evaluation of entomopathogenic fungi against the two spotted spider mite, Tetranychus urticae (Acari: Tetranychidae), on tomato, Lycopersicon esculentum. Biocontrol Sci Technol 15:37–54
de Moraes GJ, Flechtmann CHW (2008) Manual de acarologia. Acarologia básica e ácaros de plantas cultivadas no brasil, vol 1. Ribeirão Preto, Holos
Faria MRD, Wraight SP (2007) Mycoinsecticides and mycoacaricides: a comprehensive list with worldwide coverage and international classification of formulation types. Biol Control 43:237–256
Guzmán-Franco AW, Hernández-López J, Enríquez-Vara JN, Alatorre-Rosas R, Tamayo-Mejía F, Ortega-Arenas LD (2011) Susceptibility of Phyllophaga polyphylla and Anomala cincta larvae to Beauveria bassiana and Metarhizium anisopliae isolates, and the interaction with soil properties. Biocontrol 57:553–563
Iwassaki LA, Sato ME, Calegario FF, Poletti M, Maia Ade H (2015) Comparison of conventional and integrated programs for control of Tetranychus urticae (Acari: Tetranychidae). Exp Appl Acarol 65:205–217
Maniania NK, Bugeme DM, Wekesa VW, Delalibera I Jr, Knapp M (2008) Role of entomopathogenic fungi in the control of Tetranychus evansi and Tetranychus urticae (Acari: Tetranychidae), pests of horticultural crops. Exp Appl Acarol 46:259–274
Marcic D, Prijovic M, Drobnjakovic T, Medjo I, Peric P, Milenkovic S (2012) Greenhouse and field evaluation of two biopesticides against Tetranychus urticae and Panonychus ulmi (Acari: Tetranychidae). Pesticidi i Fitomedicina 27:313–320
Nicastro RL, Sato ME, Da Silva MZ (2010) Milbemectin resistance in Tetranychus urticae (Acari: Tetranychidae): Selection, stability and cross-resistance to abamectin. Exp Appl Acarol 50:231–241
Pena-Pena AJ, Santillan-Galicia MT, Hernandez-Lopez J, Guzman-Franco AW (2015) Metarhizium pingshaense applied as a seed treatment induces fungal infection in larvae of the white grub Anomala cincta. J Invertebr Pathol 130:9–12
Rezende JM, Zanardo ABR, Lopes MD, Delalibera I, Rehner SA (2015) Phylogenetic diversity of Brazilian Metarhizium associated with sugarcane agriculture. Biocontrol 60:495–505
R Development Core Team (2015) R: a language and environment for statistical computing, 3.2.2 ed. Vienna, Austria. Retrived from http://www.R-project.org/. 5 Feb 2016. R Foundation for Statistical Computing
Shi W-B, Zhang L-L, Feng M-G (2008) Field trials of four formulations of Beauveria bassiana and Metarhizium anisoplae for control of cotton spider mites (Acari: Tetranychidae) in the Tarim Basin of China. Biol Control 45:48–55
Stumpf N, Nauen R (2001) Cross-resistance, inheritance, and biochemistry of mitochondrial electron transport inhibitor-acaricide resistance in Tetranychus urticae (Acari: Tetranychidae). J Econ Entomol 94:1577–1583
Vega FE, Meyling NV, Luangsa JJ, Blackwell M (2012) Fungal entomopathogens. In: Vega FE, Kaya HK (eds) Insect Pathology. Academic Press, San Diego, pp 171–220
Wu S, Xie H, Li M, Xu X, Lei Z (2016) Highly virulent Beauveria bassiana strains against the two-spotted spider mite, Tetranychus urticae, show no pathogenicity against five phytoseiid mite species. Exp Appl Acarol 70(4):421–435
Acknowledgements
This research was funded by the Innovation Fund Denmark and The São Paulo Research Foundation (through the Project IMBICONT: ‘Improved biological control for IPM in fruits and berries’, Project Numbers 0603-00486B and 2011/51556-3 respectively). The first author is a recipient of scholarships from the São Paulo Research Foundation (FAPESP, Project Numbers 2013/24430-4 and 2013/10517-0).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Castro, T., Eilenberg, J. & Delalibera, I. Exploring virulence of new and less studied species of Metarhizium spp. from Brazil for two-spotted spider mite control. Exp Appl Acarol 74, 139–146 (2018). https://doi.org/10.1007/s10493-018-0222-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-018-0222-6