Abstract
Haemaphysalis qinghaiensis, a prevalent tick species in China, is an ectoparasite that preferentially infests small ruminants and can transmit Theileria sp. and Babesia sp. In this study, we evaluated the pathogenicity of individual and mixed infections of the fungi Beauveria bassiana and Metarhizium anisopliae to H. qinghaiensis nymphs. The estimated LC50 for ticks immersed in solutions of B. bassiana, M. anisopliae and a mixture thereof were: 5.88056 × 104, 2.65 × 104, and 2.85 × 104 conidia mL−1 respectively, and the nymphal mortality ranged from 52 to 100 %. Thus, these results suggest a potential approach for the biocontrol of H. qinghaiensis.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ticks are important hematophagous arthropods that are distributed almost worldwide, but particularly in tropical and subtropical areas. They can have direct deleterious effects on their hosts, including damage to skin, blood loss, decreased milk yield, and loss of weight. In addition, and perhaps more seriously, ticks can also transmit various pathogens to their host animals and also to humans. They can also result in economic losses, particularly in the tanning industry, given their effects on the hides of their hosts. Ticks are considered to be second only to mosquitoes as vectors of human disease (Zhou et al. 2009). The annual worldwide cost of the control of ticks and tickborne diseases (TTBDs) has been estimated to be between US$13.9 and $18.7 billion (Angel-Sahagun et al. 2010).
The three-host tick, Haemaphysalis qinghaiensis, is a species endemic to China, where it is widely distributed on the western plateau. It preferentially infests small ruminants and can transmit Theileria sp. and Babesia sp. (Yin et al. 2002). Haemaphysalis qinghaiensis, and the protozoal diseases that it transmits, result in significant economic losses to livestock production in China.
Chemical acaricides, which are the primary and most commonly used methods to control tick populations, have many disadvantages, including environmental pollution, food contamination, appearance of acaricide-resistant ticks, increasing costs, and impacts on nontarget organisms (You and Fujisaki 2009; Pourseyed et al. 2010). These drawbacks are driving research into alternative, sustainable strategies for more efficient tick control (Bharadwaj and Stafford 2010).
Entomopathogenic fungi infect arachnids by direct penetration of the cuticle and, thus, can be used to control sucking arthropod pests (Shang et al. 2012). Given their global dispersal, relatively low risk to humans, animals and ecosystems, high virulence against ticks, and the fact that they are easy to produce commercially, entomopathogenic fungi are considered to be acceptable biological control agents (Leemon and Jonsson 2008). Recently, interesting results (Camargo et al. 2014; Golo et al. 2015) have been published relating to the use of the fungi Beauveria bassiana and Metarhizium anisopliae to control ticks. B. bassiana and M. anisopliae are the predominant fungal species infecting ticks; they have a broad host range and the ability to penetrate the arthropod cuticle. Different strains of B. bassiana and M. anisopliae are pathogenic to several kinds of tick.
Although there have been various studies of the individual effects of B. bassiana and M. anisopliae on ticks, few studies have considered the pathogenicity of their synergistic effects. Thus, with the aim of developing a new tick control method, we evaluated the virulence of B. bassiana, M. anisopliae, and the synergism thereof, on H. qinghaiensis nymphs.
Materials and methods
Ticks
For laboratory experiments, unfed H. qinghaiensis nymphs were collected from naturally infested sheep. After collection, the ticks were maintained in an incubator at 28 ± 2 °C and 80 ± 5 % relative humidity (RH) in glass tubes sealed with hydrophilic cotton. Two to three-week-old unfed nymphs were randomly divided into five groups of ten ticks for each treatment.
Fungal growth and preparation of conidial suspensions
Isolates of B. bassiana and M. anisopliae were originally obtained from soil samples collected in China. The fungi were maintained on potato dextrose agar (PDA) slopes and kept at 4 °C. Fungal isolates were cultured with fresh PDA Petri plates in an incubator at 26–28 °C and 75–85 % RH. Spores were harvested into sterilize aqueous 0.05 % Tween 80 solution by scraping the surface of the plate after 12 days; the suspension was then homogenized on a vortex mixer. Conidia concentrations were determined by direct count using a hemocytometer.
After homogenization, the concentration of conidia was determined with a hemocytometer and adjusted to concentrations of 105, 106, 107, 108, and 109 conidia mL−1 with 0.05 % Tween 80 in distilled water. In preparing the suspension of the fungal cocktails, co-formulations of 109 conidia mL−1 of B. bassiana and M. anisopliae were mixed at ratios of 1:1. The adjusted final concentrations were 105, 106, 107, 108, and 109 conidia mL−1.
Laboratory bioassays
Suspensions ranging from 105 to 109 conidia mL−1 of B. bassiana, M. anisopliae, and B. bassiana + M. anisopliae (Bb + Ma) were prepared. Ten H. qinghaiensis nymphs per treatment were immersed for 30 s in each conidial suspension and eliminated the excess suspension. As controls, ticks were immerged in the same volume of sterile water containing 0.05 % Tween 80. Each trial was conducted with five repetitions. After the treatment, the treatment ticks were placed individually in a Petri dish and kept in an incubator at 28 ± 2 °C and 80 ± 5 % RH. The ticks were observed every 72 h to check for mortality.
Data analysis
To evaluate the mortality and LC50 of H. qinghaiensis nymphs for each treatment, mortality data were analysed using a one-way analysis of variance (ANOVA) via SPSS. The data collected were based on the percentage mortality 21 days after treatment. The median lethal concentrations (LC50), respective confidence limits, regression equation, and resistance ratios were determined using the probit-analysis method.
Results
The mortality of the H. qinghaiensis nymphs immersed in the different fungal strains are shown in Table 1. The mortalities of B. bassiana, M. anisopliae, and Bb + Ma were not significantly different from each other, whereas any group treated with any fungal strain showed significant differences compared with the control treatments. All these three treatment groups were pathogenic to H. qinghaiensis at concentration of 105, 106, 107, 108, and 109 conidia mL−1 in the laboratory, but the highest mortality (92–100 %) was obtained at the concentration of 109 conidia mL−1, mortalities were lower at the other concentrations. The mixture of strains (Bb + Ma) showed 96 % efficacy compared with control unfed nymphs at the same concentration, and no dead ticks were observed in the control groups. The mortality of the ticks in the mixed-infection treatments were consistently the intermediate of the two single infection treatments.
The LC50 of B. bassiana, M. anisopliae, and Bb + Ma on H. qinghaiensis nymphs are shown in Table 2. The estimated LC50 for ticks immersed in B. bassiana, M. anisopliae and Bb + Ma were 5.88 × 104, 2.65 × 104, and 2.85 × 104 conidia mL−1, respectively. This indicates a lower LC50 for M. anisopliae and the mixture of both than for B. bassiana.
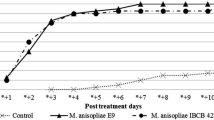
The temporal mortality of unfed H. qinghaiensis nymphs infected with fungal conidia is presented in Fig. 1. All three treatments resulted in >50 % mortality at 9 days post-inoculation (DPI) at a concentration of 108 conidia mL−1. The fungal cocktail (Bb + Ma) resulted in 96 % mortality at 18 DPI for 109 conidia mL−1.
Discussion
Entomopathogenic fungi are major pathogens of ticks. Given their ability to penetrate the external cuticle of arthropods, their wide distribution, wide host range, low risk to humans and animals, ease of production, and environmental safety, entomopathogenic fungi are frequently evaluated as biocontrol agents (Hussain et al. 2014). Studies showed that entomopathogenic fungi are potential pathogens to use for the control of ticks (Ojeda-Chi et al. 2010). Among the entomopathogenic fungi examined for pathogenicity against ticks, M. anisopliae and B. bassiana are the most commonly studied species (Fernandes et al. 2012).
However, few studies have examined the virulence of B. bassiana and M. anisopliae, either individually or in combination, to ticks. Thus, in this study, we examined the virulence of the individual strains and a mix of B. bassiana and M. anisopliae to H. qinghaiensis nymphs. The data demonstrated the susceptibility of H. qinghaiensis to isolates of B. bassiana and M. anisopliae and that this susceptibility was similar to that of a combination of the two species. This result contrasts with a previous investigation of the mortality of the tick Amblyomma variegatum resulting from fungal suspensions of B. bassiana and M. anisopliae, which indicated that the fungal cocktails induced higher mortalities than each fungi alone, although the differences were mostly not statistically significant (Maranga et al. 2005).
In conclusion, this study suggests a new approach for the biocontrol of H. qinghaiensis ticks using fungal strains. However, subsequent studies are needed to improve the synergistic effects of B. bassiana and M. anisopliae against these ticks. The pathogenicity of B. bassiana and M. anisopliae should then be followed up by field studies, under the various climatic conditions of the geographical distribution area of the tick.
References
Angel-Sahagun CA, Lezama-Gutierrez R, Molina-Ochoa J, Pescador-Rubio A, Skoda SR, Cruz-Vazquez C, Lorenzoni AG, Galindo-Velasco E, Fragoso-Sanchez H, Foster JE (2010) Virulence of Mexican isolates of entomopathogenic fungi (Hypocreales: Clavicipitaceae) upon Rhipicephalus = Boophilus microplus (Acari: Ixodidae) larvae and the efficacy of conidia formulations to reduce larval tick density under field conditions. Vet Parasitol 170:278–286
Bharadwaj A, Stafford KR (2010) Evaluation of Metarhizium anisopliae strain F52 (Hypocreales: Clavicipitaceae) for control of Ixodes scapularis (Acari: Ixodidae). J Med Entomol 47:862–867
Camargo MG, Marciano AF, Sa FA, Perinotto WM, Quinelato S, Golo PS, Angelo IC, Prata MC, Bittencourt VR (2014) Commercial formulation of Metarhizium anisopliae for the control of Rhipicephalus microplus in a pen study. Vet Parasitol 205(1–2):271–276
Fernandes EK, Bittencourt VR, Roberts DW (2012) Perspectives on the potential of entomopathogenic fungi in biological control of ticks. Exp Parasitol 130:300–305
Golo PS, Santos HA, Perinotto WM, Quinelato S, Angelo IC, Camargo MG, Sa FA, Massard CL, Fernandes EK, Roberts DW, Bittencourt VR (2015) The influence of conidial Pr1 protease on pathogenicity potential of Metarhizium anisopliae senso latu to ticks. Parasitol Res 114:2309–2315
Hussain A, Rizwan-Ul-Haq M, Al-Ayedh H, Al-Jabr AM (2014) Mycoinsecticides: potential and future perspective. Recent Pat Food Nutr Agric 6:45–53
Leemon DM, Jonsson NN (2008) Laboratory studies on Australian isolates of Metarhizium anisopliae as a biopesticide for the cattle tick Boophilus microplus. J Invertebr Pathol 97:40–49
Maranga RO, Kaaya GP, Mueke JM, Hassanali A (2005) Effects of combining the fungi Beauveria bassiana and Metarhizium anisopliae on the mortality of the tick Amblyomma variegatum (Ixodidae) in relation to seasonal changes. Mycopathologia 159:527–532
Ojeda-Chi MM, Rodriguez-Vivas RI, Galindo-Velasco E, Lezama-Gutierrrez R (2010) Laboratory and field evaluation of Metarhizium anisopliae (Deuteromycotina: Hyphomycetes) for the control of Rhipicephalus microplus (Acari: Ixodidae) in the Mexican tropics. Vet Parasitol 170:348–354
Pourseyed SH, Tavassoli M, Bernousi I, Mardani K (2010) Metarhizium anisopliae (Ascomycota: Hypocreales): an effective alternative to chemical acaricides against different developmental stages of fowl tick Argas persicus (Acari: Argasidae). Vet Parasitol 172:305–310
Shang Y, Duan Z, Huang W, Gao Q, Wang C (2012) Improving UV resistance and virulence of Beauveria bassiana by genetic engineering with an exogenous tyrosinase gene. J Invertebr Pathol 109:105–109
Yin H, Guan G, Ma M, Luo J, Lu B, Yuan G, Bai Q, Lu C, Yuan Z, Preston P (2002) Haemaphysalis qinghaiensis ticks transmit at least two different Theileria species: one is infective to yaks, one is infective to sheep. Vet Parasitol 107:29–35
You M, Fujisaki K (2009) Vaccination effects of recombinant chitinase protein from the hard tick Haemaphysalis longicornis (Acari: Ixodidae). J Vet Med Sci 71:709–712
Zhou J, Liao M, Ueda M, Gong H, Xuan X, Fujisaki K (2009) Characterization of an intracellular cystatin homolog from the tick Haemaphysalis longicornis. Vet Parasitol 160:180–183
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Ren, Q., Chen, Z., Luo, J. et al. Laboratory evaluation of Beauveria bassiana and Metarhizium anisopliae in the control of Haemaphysalis qinghaiensis in China. Exp Appl Acarol 69, 233–238 (2016). https://doi.org/10.1007/s10493-016-0033-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-016-0033-6