Abstract
The embryonic development of four eriophyoid mite species, Cecidophyopsis ribis, Phytoptus avellanae, Oziella liroi and Loboquintus subsquamatus, has been studied with the use of fluorochrome DAPI and confocal microscopy. The first three nuclear divisions occur on the egg periphery (the groups of 2, 4, and 6 nuclei have been recorded), while the biggest part of yolk remains undivided. After four or five nuclear divisions all nuclei are situated only in one sector of the embryo, while other sectors contain only yolk suggesting possible meroblastic cleavage. Later, the formation of superficial blastoderm takes place. A few large yolk cells are situated inside the embryo. Germ band formation initiates as funnel-like cell invagination and leads to formation of a typical stage with four paired prosomal buds (chelicerae, palps, legs I and II). Each palp contains two lobes (anterior and posterior), the adult subcapitulum is presumably a fusion product of the anterior pair of the lobes. Neither rudiments of legs III and IV, traces of opisthosomal segments nor remnants of the prelarval exuvium under the egg shell were detected. Overall, the pattern of embryonic development in eriophyoids re-emphasizes the peculiarity of this ancient group of miniaturized phytoparasitic animals, and invites researches to pursue a deeper investigation of various fundamental aspects of this aberrant group of Acari. Further studies using various fluorescent dyes and transmission electron microscopy are needed to visualize plasma membranes and clarify the pattern of early cleavage of eriophyoids.
Similar content being viewed by others

Avoid common mistakes on your manuscript.
Introduction
Up to now, several acarine species have been studied with respect to early embryology. Among them are trombidiform mites—Cheyletus eruditus (Hafiz 1935; Edwards 1958), Knemidocoptes mutants (Langenscheidt 1958), Tetranychus urticae (Dittrich 1968; Dearden et al. 2002; Grbic et al. 2007)—and sarcoptiform mites, Tyroglyphus farinae (Hughes 1950; Sokolov 1952) and Archegozetes longisetosus (Laumann et al. 2010a, b). Early development of ticks Boophilus calcaratus (Wagner 1894), Ornithodorus moubata (Aeschlimann 1958; Fagotto et al. 1988), Hyalomma dromedarii (El Kammah et al. 1982), Rhipicephalus (Boophilus) microplus (Santos et al. 2013), and a few other ixodid and gamasid species (Yastrebtsov 1992), were also analyzed.
Traditionally, there were two points of view on the general pattern of early cleavage in the embryos of mites and ticks. Several authors (Hafiz 1935; Aeschlimann 1958; Langenscheidt 1958) discovered the occurrence of a superficial meroblastic cleavage, while the others (Hughes 1950; Sokolov 1952; Edwards 1958; Dittrich 1968) described the earliest two or three cleavage divisions as total (holoblastic) followed by a later series of superficial cleavage divisions on the periphery of inner yolk mass. This discrepancy stimulated further investigations (e.g., El Kammah et al. 1982; Fagotto et al. 1988; Yastrebtsov 1992; Gotoh et al. 1994), and recent publications (Laumann et al. 2010a, b; Scholtz and Wolff 2013) have favored the holoblastic cleavage pattern as a general character of Acari. Therefore, these recent works suggest that there is no diversity of cleavage patterns among mites and ticks. Laumann et al. (2010b) noted that acariform mites exhibit a specialized form of total cleavage with differentiation of blastomeres into micro- and macromeres, and involving unequal yolk distribution into micromeres and macromeres. They also assumed that total cleavage with blastomere differentiation may represent the cleavage ground-plan for all Acari.
It should be remarked, though, that among Acari there are a number of aberrant taxa, strongly different in their organization from other acarine groups. The phytoparasitic eriophyoid mites (Acariformes: Trombidiformes: Eupodina: Eriophyoidea) represent one of those aberrant taxa. Their evolution was characterized by miniaturization and great structural simplification (Nuzzaci and Alberti 1996; Lindquist 1996a). As a result, they acquired a morphology that is absolutely untypical for acariform mites: microscopic worm-like animals with two legs of 100–300 µm length and mouth parts highly adapted for piercing epidermal cells and sucking plant sap (Lindquist 1996a, b; Chetverikov and Craemer 2015). Although these mites are economically important (Lindquist and Amrine 1996), many fundamental aspects, including their early embryonic development, have never been studied. In the meantime, research in this direction might be helpful for elucidating the origin of their strange morphology and understanding the evolutionary trends in the group.
In this paper we were primarily aimed to describe the early development of eriophyoid mites and answer the question: does it correspond or not to the recently proposed ground-plan by Laumann et al. (2010b)? We were also focused on finding typical and chronologically distant embryonic stages to compare those in representatives of phylogenetically remote lineages of Eriophyoidea in order to estimate the uniformity of the developmental patterns among this group of mites. Finally, we were interested to trace the steps of formation of the prosomal limbs (chelicerae, palps, legs I and II) including possible buds of legs III or IV and check the presence of the putative prelarval exuvia (see Shevchenko 1961; Lindquist 1996b for detail).
Materials and methods
Sampling (Table 1)
We studied early development of four species: Cecidophyopsis ribis (Westwood) form buds of Ribes alpinum L., Phytoptus avellanae Nalepa from buds of Corylus avellana L., Oziella liroi (Roiveinen) from leave sheaths of Carex leporina L., and early derivative mite Loboquintus subsquamatus Chetverikov and Petanović from under bark scale of young twigs of Cupressus sempevirens L. These species belong to the three different lineages of eriophyoids, which were revealed through a recent molecular phylogenetic analysis (Chetverikov et al. 2015b). Main data on the embryonic development of eriophyoids were obtained from a study of C. ribis, whereas the three other species were primarily used to confirm the presence of the typical developmental stages revealed in the embryos of C. ribis. It should be noted that we had no possible way of obtaining precise information on the age of the embryos; we also had no information on the location of the pole of the egg.
Collecting mites
Buds of alpine currant and hazel nut (containing mites C. ribis and P. avellanae, respectively) were cut into 2–3 parts by a blade, dissected by pincers, put in Eppendorf tubes containing 1 ml PBTFootnote 1 and slightly shaken by hand for 2–3 min. Thereafter the material was poured through a sieve (0.01 mm diameter) in a small cup and large pieces of plant tissue were removed. Applying this technique, we collected thousands of eggs (Table 1). The eggs of Loboquintus and Oziella were less numerous. We used a stereomicroscope, for detailed observation, and a fine needle to collect them from under the bark twig scales of cypress or within leaf sheaths of the sedge. As with C. ribis and P. avellanae, the eggs of Loboquintus and Oziella were transferred in a small cup with PBT and incubated at room temperature for about 10 min until all the eggs settled at the bottom.
DAPI staining
Prior to fixation, all the PBT was sucked by pipette and 10–15 drops of commercial bleach were added for 2–3 min to dechorionize the eggs (as was suggested by Laumann et al. 2010a and Barnett and Thomas 2013). After that the eggs were rinsed twice (at intervals of 20 min) in PBT. After the first rinsing all the eggs are usually clustered on the bottom of a cup, forming a rounded “ruft”. The eggs were fixed for 24 h in two ways: #1 most of the samples were fixed in 8 % PFA and kept at +4 °C; #2 several samples were fixed in cold methanol (−25 °C) in a freezer. After that, the eggs were twice rinsed in PBT and incubated for 3 h with DAPI solution (0.001 % in PBT). The stained eggs were thereafter rinsed once in PBT for 30–40 min, mounted on microscopic slides in pure glycerine, sealed with nail polish to avoid evaporating of glycerin, and kept in a refrigerator (+4 °C) prior to observation with a confocal laser scanning microscope.
CLSM technique and image processing
CLSM acquisition was carried out using Spectral Confocal and Multiphoton System Leica TCS SP2 with following adjustments: excitation wavelength of 405 nm, emission wavelength range of 415–470 nm, blue laser 405 nm at 90 % intensity. Both transmitted and excited (fluorescent) light was recorded in two different channels. Acquisition resolution was 1024 × 1024 pixels, level of gain 650–750, frame averaging of 1 (no averaging, i.e. a single frame) to scan images, and zoom range of 1.5–2.9 times. Stacks of 22–92 optical slices were recorded digitally from each of the 168 studied embryos. Most images showing different stages of embryonic development are maximum intensity projections (MIP) obtained with ImageJ free software (http://rsb.info.nih.gov/ij/); in the overlay images gray colour corresponds to transmission light and cyan to DAPI staining. Volume rendering and reconstructions of the advanced embryos of Loboquintus were carried out using Amira® 5.3.2 software and images were recorded using the “Snapshot” command in Amira® 5.3.2. Those images were obtained applying “volrenred” colormap of Amira®, the colour corresponds to DAPI signal.
Results
Earliest cleavage stages (Fig. 1)
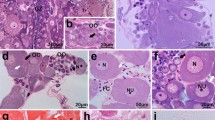
The eggs of all the studied species are slightly elongated and evenly filled with numerous yolk droplets. The eggs of L. subsquamatus are notably larger than those of the three other species: about twice as long as the eggs of C. ribis and about 1.5 time as long as the eggs of P. avellanae and O. liroi (Table 2). The earliest cleavage stages observed have 2, 4, and 6 small nuclei (Fig. 1) situated on the periphery of the embryos. Quite expectedly, cell borders were not visible during early cleavage, because DAPI does not stain plasma membranes. However, these embryos were not abundant: eight, four and three embryos of Cecidophyopsis, Phytoptus and Oziella, respectively; such early stages were not found in the case of Loboquintus. The occurrence of the stage with three (not shown) and six nuclei (Fig. 1f) was observed suggesting an early loss of division synchrony. However, additional material and observations would be necessary to finally prove this point. We did not see mitotic figures during the early cleavage stages (perhaps due to the brevity of nuclear divisions). The stained nuclei are bright, small (about 1.6 long and 1.2 wide in average), usually elongated, and with distinct margins. A dark halo (an area devoid of yolk droplets) around the nuclei were observed in P. avellanae (Fig. 1a, arrow) and C. ribis (not shown).
CLSM MIP images showing the earliest cleavage stages of Phytoptus avellanae (a) and Oziella liroi (b, d) and Cecidophyopsis ribis (c, e, f). Scale bar 10 μm. Note that the peripheral position of nuclei during the early cleavage in these three species is in accord with our data (Chetverikov et al. 2015a, Fig. 7) concerning the peripheral position of the nucleus in mature eggs of another eriophyoid mite, Neoprothrix hibiscus Reis and Navia
Sectoral growth of blastomeres inside yolk and problem of cellularization (Figs. 2, 3)
We did not observe embryos with between 6 and 20 nuclei. Further divisions result in the formation of a relatively compact group of nuclei (up to 50) situated closer to the egg surface than to the egg center (Fig. 2). At the stage of 60–80 nuclei, they grow into the yolk, and at a later stage (>100 nuclei) they start to spread under the surface so that the strands of nuclei envelop the yolk. After at least four or five nuclear divisions (a stage of 20–30 nuclei), the putative cell borders are visible in some embryos fixed in PFA (Fig. 3e, f, h, i); the yolk is mainly outside the cells and not within the homogenously stained cytoplasm surrounding the nuclei. Several arguments favor the meroblastic pattern of cleavage: #1 in all observations, all nuclei are situated only in one sector of the embryo, while several other sectors of the same embryo contain only yolk with no trace of cleavage furrows (Figs. 2, 3); #2 under transmission light the sector of the embryo containing nuclei is homogenously grey and without any bubbles or droplets (Fig. 2e, f, g, h asterisk) whereas the remaining part of the egg is heterogeneous and contains a lot of yolk droplets; #3 when observing the CLSM stacks in 3D editor, the number of cells separated by dark furrows (putatively indicate cell membranes), each containing a stained nucleus in DAPI images, directly correlates with the number of cells observable on the transmission light images (e.g. Figs. 3e, f, g, 4a, b, c). Additionally, we observed several embryos with quite distinct borders between blastomeres (Fig. 3h, i). Cell borders in Fig. 3 might have become visible because chromatin stained with DAPI partially leaked to the cytoplasm making it glowing. Another possible explanation is that in PFA fixed material DAPI sometimes marks not only chromatin but also brightly stains cytoplasm. However it does not contradict to the assumption that the lipid droplets are not included in the blastomeres, because the space between the droplets is not homogenously stained (like the putative cytoplasm around the nuclei in the Fig. 3) suggesting absence of such a material (cytoplasm) between the droplets.
CLSM overlay (a, b, c, d) and corresponding transmission light (e, f, g, h) images showing different stages of cleavage in four embryos of Cecidophyopsis ribis. a 22 nuclei, b 32–34 nuclei, c 48–50 nuclei; d 60–64 nuclei; images on the right (e–h) are MIPs of a single slice from the middle of the same embryos shown in the images on the left (a–d). The asterisks on the transmission light images indicate the homogenous area devoid of yolk droplets where the cleavage presumably appears; the arrows indicate nuclei. Scale bar 10 μm
CLSM images (a, b, c, d, g overlay, e, h, i fluorescence; f transmission channel) showing putative blastomeres with glowing cytoplasm (ct) and yolk droplets (yd) inside the cleaving eggs of Cecidophyopsis ribis (a–h) and Phytoptus avellanae (i). Scale bar a–g 10 μm, h, i 20 μm. Images a, b, c, d are MIPs of single slices (##3, 7, 28, 34) from one stack. Images e and g are composite images to show the glowing cytoplasm (obtained from more superficial slices) and corresponding nuclei (obtained from deeper slices) from the same stack as for images a–d. Images h and i represent two series of slices from deeper part of two embryos to more superficial part showing a sector of cleaving blastomeres. Paired arrows in c and d indicate two pairs of bright round young nuclei (similar to those observed in earlier stages, see Fig. 1) underwent division
Superficial blastoderm, yolk cells and the “funnel” (Figs. 4, 5)
Cells are tightly packed, forming a single superficial cellular layer (blastoderm), surrounding the yolk (Fig. 4). The superficial borders of blastodermal cells are distinctly visible under a transmission light microscope (Fig. 4b). Among blastodermal nuclei, there are groups of compacted elongated brightly stained nuclei (probably engaged in mitotic divisions) (Fig. 4a, d, e) hinting at a possibility of “proliferation waves”. Note that mitotic waves have been detected in Drosophila melanogaster embryos during syncytial blastoderm development (Foe and Alberts 1983; Tomer et al. 2012).
Inside blastulae of all three species, there are individual large nuclei among yolk droplets (Fig. 5), while cell borders cannot be seen. These cells seemed to penetrate inside the embryo at the stage of advanced cleavage (Fig. 2a–d), and they can be seen at all subsequent stages up to the formed larva within the egg (not shown here). According to our preliminary data, the number of these cells is essentially the same during embryonic and larval development of all studied species: approximately 20–25. We will conventionally call them the “yolk cells”. At a later stage, a funnel-shaped invagination appears (Fig. 5c–f). In all studied species it forms near one end of the elongated egg, at first as a small group of cells (Fig. 5b), and later as a funnel-shaped invagination (Fig. 5c, d, e). Finally, in one egg of C. ribis and two eggs of P. avellanae, we have seen that this structure is already rod-shaped and connects the opposite sides of the blastoderm (Fig. 5f). When viewed with the 3D editor, the rod seemed to be hollow and with thin walls.
Germ band, furrows and limb buds (Fig. 6)
Further embryo development includes the formation of the germ band and prosoma appendages. In the eriophyoid mites, germ band development occurs in a close link with the above mentioned funnel. After the funnel reaches the opposite end of the egg, several cellular clusters are formed, which connect the funnel with the superficial embryonic tissue (not shown here). Among these clusters, the conglomerations of cells appear which grow and form the paired bulges on the ventral surface of embryo. Already at this stage, a middle longitudinal furrow is distinct and easy to see. This structure seems to correspond to the anterior/posterior embryonic axis (Fig. 6a, b arrow). Several transverse furrows, dividing the paired buds of prosomal limbs (Fig. 6c arrows), are also clearly visible at this stage. The differentiation of appendages seems to proceed from front to back: at first, the anlagen of chelicerae and palps, and then the anlagen of legs I and II (Fig. 6f). We did not observe any anlagen of legs III, IV or any protuberances, which might be considered rudiments of these legs (Fig. 6g, h, i). The longitudinal sections definitely show that a minimum of one large (“yolk”?) cell (Fig. 6d arrows) resides within each prosomal bud (corresponding to a chelicera, a palp, and legs I, II). In the light of the recent work on Drosophila (Huelsmann et al. 2006), it is possible that these yolk cells are migrating embryonic macrophages. Nonetheless, the yolk cells inside blastulae (see above) seem to be vitellophages. In the beginning, each palp bud is entire (Fig. 6f), but later two small protuberances appear (Fig. 6g, asterisks), and soon the palp resembles a spanner as it bears two lobes: anterior (Fig. 6h lobe) and posterior (Fig. 6h black arrow).
Partial maximum intensity projections (a–e) and volume rendering reconstructions (g–I) showing formation of prosomal limbs in Cecidophyopsis ribis (a, b, d, e, f), Phytoptus avellanae (c) and Loboquintus subsquamatus (g–i). Notation: Ch chelicera, P palp, LI leg I, LII leg II, lobe anterior lobe of the palp, furrow one of the transverse furrows delimiting limb buds. Arrows in a, b indicate longitudinal furrow, arrows on the d indicate the putative yolk cells within limb buds. Scale bar a–f 10 μm, g–i 20 μm
Body growth, folding the larva inside an egg
Intensive growth of the posterior part of the body (opisthosoma) begins after the formation of prosomal limbs. No external segmentation of the opisthosoma can be observed (Fig. 6i). The growth of the embryonic body occurs inside a restricted volume of the oblong egg, which leads to the folding and straightforward growth of the posterior body region. As a result, the embryo happens to be arranged in two layers and its legs embrace the posterior end of the body (e.g. CLSM image from Chetverikov 2012, p. 60, Fig. 2). A completely formed larva resides in the egg at this stage. Interestingly, we have never found any additional moulting exuvia inside the eggs. We did not plan to analyze organogenesis. Nonetheless, it is appropriate to remark that inside the DAPI stained embryos that have fully formed limbs and a mouth apparatus, 90 % of the anterior body region is represented by the putative CNS, which has similar structure to that in adult individuals (Nuzzaci and Alberti 1996). For all the species we have studied it is typical for larval eriophyoids to contain a huge amount of the yolk droplets. Large cells with shapeless nuclei are situated among these droplets. They seem to take part in the assimilation of “maternal” nutrient materials. This event might compensate for the frequent phenomenon of weak exotrophic nutrition of eriophyoid larvae after hatching and, in turn, enable the larva to accumulate enough metabolic resources for the nymphal transformation.
Discussion
Uniformity and atypical traits of eriophyoid embryonic development
Our study shows that eriophyoid mites possess an uncommon pattern of early development among the Acari. Additionally, this pattern demonstrates remarkable uniformity among the main phylogenetic lineages of the Eriophyoidea. The early cleavage pattern in eriophyoid mites (based on DAPI stained nuclei and confocal microscopy) seems to be meroblastic. This is in contrast with recent data on other groups of Acari (Grbic et al. 2007; Laumann et al. 2010a, b). The difference may represent a trait that is unique to eriophyoid mites. The evidence, so far, therefore indicates that there may be substantial variation in the early ontogenetic patterns within the Acari, which may represent comparable levels of developmental diversity to those observed within other groups of invertebrates, such as sea urchins (Raff 1987), ascidians (Jeffery 1997), and parasitic wasps (Mancini et al. 2013).
Certainly, further analyses of the embryonic sectors with nuclei and the parts containing only yolk with transmission electron microscopy would be of great importance for decisive elucidation of cleavage pattern. However, presuming that the cleavage of eriophyoids might be meroblastic it would be interesting to consider it in the context of the general scheme of arthropod cleavage phylogeny (Scholtz and Wolff 2013, Fig. 4.3, p. 70). In the future, phylogenetic data on the Acari will be helpful for discriminating between two hypotheses. #1 Despite a contemporary consensus on the phylogenetic position and origin of Eriophyoidea (Lindquist 1996b), our embryological data admit that the eriophyoid lineage might have diverged from very primitive Acari before the latter underwent a transition from superficial to total cleavage. As a result, the eriophyoid mites might have acquired their unusual meroblastic pattern distinguishing them from all other arthropods. #2 In accordance with the current view on eupodine origin of eriopyoids (Lindquist 1996b), the transition from the total cleavage (basic for all Acari) to the atypical meroblastic cleavage might have occurred during the lengthy divergence of eriophyoid mites from other eupodine mites, which might have been associated with the process of miniaturization.
Enygmatic eriophyoid prelarva
In general, Acari may pass through up to six instars after eclosion: prelarva, larva, protonymph, deutonymph, tritonymph and adult (Walter and Krantz 2009, p. 59). Typically, the prelarva is a nonfeeding form that occurs not only in the Acari but in other arachnids as well. Contemporary known acarine prelarvae are quiescent and sequestered within the egg chorion (Walter and Proctor 1999). Shevchenko (1961) reported that he observed indistinct remnants in the eggshell of an eriophyoid mite Eriophyes laevis, which he interpreted as a putative prelarval exuvium. Similar observation belongs to Prof J. Amrine (pers. comm. 2013) who indicated indistinct squashed material in the egg shell of a hatching eriophyoid larva as prelarval vestiges. This was observed on one of the LTSEM images provided by Dr. R. Ochoa when they taught students attending the Acarology Summer Program at The Ohio State University (USA). The retention of the prelarval stage in the life cycle would reinforce the ancestry of the eriophyoid stalk, especially in the light of the recent finding of the eriophyoid-like triasacaroid mites in Triassic amber (Sidorchuk et al. 2015). Under CLSM we inspected hundreds of eggs containing differently developed embryos, including larvae which have already started slowly moving their legs under the egg shell. However, we did not detect any prelarval remnants, which implies that this stage is probably missed or greatly suppressed in Eriophyoidea.
Germ band, subcapitulum and reduction of legs III and IV in eriophyoids
The “funnel” (Fig. 5) observed in the eriophyoid embryo is one of the hard structures for interpretation. However, according to Walter (2009, p. 55), this might be a mark of gastrulation. He reports that «…during the early stages of gastrulation, a ventral germ disc … appears and develops as a funnel-shaped band in the yolk mass, eventually giving rise to the endo-mesoderm layers. The germ band elongates and gives rise to the cephalic lobes, after which the prosomatic segments with limb buds become distinct». Although we did not observe a distinct “ventral germ disc”, the short description by Walter precisely corresponds to what we observed in eriophyoid embryos, reinforcing similarities in the formation of the germ band in various groups of Acari. In all of the studied eriophyoid embryos, the early prosomal limbs appear as uniform symmetrical buds delimited by a longitudinal furrow, which starts between the bases of the chelicerae and passes between the bilobed palps and legs I and II (Fig. 6f, g). We suggest that, when the chelicerae elongate and take a terminal position (Fig. 6h, i), the anterior lobes of the palps appress to each other and presumably fuse medially, forming the subcapitulum enclosing the anterior portion of the gnathosomal stylets (see for details in Chetverikov and Craemer 2015). Further basal parts of the palps fused, forming the subcapitular plate, whereas each posterior lobe develops into a segmented palpal appendage. Overall, this draft scenario of the formation of the larval gnathosoma in eriophyoids is likely to be in accordance with the brief description by Walter (2009, p. 56) on the formation of subcapitulum in Acari and previous findings of the bilobed palps in the embryos of other chelicerates (e.g. Aeschlimann and Hess 1984; Grbic et al. 2007; Liu et al. 2009; Barnett and Thomas 2013).
Typically adult and nymphal Acari have four paired legs while the larva possesses only three pairs. However, the suppression of legs III and IV is characteristic of all stages of Eriophyoidea. Recent studies indicate that in spite of the 6-legged larval condition in embryos of acariform mites, the limb buds for legs IV are present (Barnett and Thomas 2013, Fig. 1), though external evidence for their existence soon disappears. One could expect to find the same situation in eriophyoids: limb buds for legs III develop early on but later dissolve. Our results suggest that in eriophyoid mites, only legs I and II develop, while no rudiments of limb buds III and IV have been visualized in CLSM reconstructions. The presence of the large cells migrating into each of the four prosomal limb buds (and thus “indicating” them, Fig. 6d) provide additional support for this hypothesis. If this is true, the question arises as to whether the formation of legs III and IV is only suppressed in eriophyoids, or is it that the metapodosomal segments do not develop at all? Precise comparative myoanatomy and studies of groups of genes involved in segmentation development, including Hox gene expression, could elucidate this question.
Notes
=PBS (1 tablet of phosphate buffer saline P4417 Sigma dissolved in 200 ml of purified water) with 4–5 drops of 0.1 % Triton X-100.
References
Aeschlimann A (1958) Développement embryonnaire d’Ornithodorus moubata (Murray) et transmission transovarienne de Borrelia duttoni. Acta Trop 15:15–64
Aeschlimann A, Hess E (1984) What is our current knowledge of acarine embryology? In: Griffiths DA, Bowman CE (eds) Acarology 6(1):90–99
Barnett AA, Thomas RH (2013) The expression of limb gap genes in the mite Archegozetes longisetosus reveals differential patterning mechanisms in chelicerates. Evol Dev 15(4):280–292
Chetverikov PE (2012) Confocal laser scanning microscopy technique for the study of internal genitalia and external morphology of eriophyoid mites (Acari: Eriophyoidea). Zootaxa 3453:56–68
Chetverikov PE, Craemer C (2015) Gnathosomal interlocking apparatus and remarks on functional morphology of frontal lobes of eriophyoid mites (Acariformes, Eriophyoidea). Exp Appl Acarol 66(2):187–202. doi:10.1007/s10493-015-9906-3
Chetverikov PE, Desnitskiy AG, Navia D (2015a) Confocal microscopy refines generic concept of a problematic taxon: rediagnosis of the genus Neoprothrix and remarks on female anatomy of eriophyoids (Acari: Eriophyoidea). Zootaxa 3919(1):179–191
Chetverikov PE, Cvrković T, Makunin A, Sukhareva S, Vidović B, Petanović R (2015b) Basal divergence of Eriophyoidea (Acariformes, Eupodina) inferred from combined partial COI and 28S gene sequences and CLSM genital anatomy. Exp Appl Acarol 67(2):219–245. doi:10.1007/s10493-015-9945-9
Dearden PK, Donly C, Grbic M (2002) Expression of pair-rule gene homologues in a chelicerate: early patterning of the two-spotted spider mite Tetranychus urticae. Development 129:5461–5472
Dittrich V (1968) Die Embryonalentwicklung von Tetranychus urticae Koch in der Auflichtmikroskopie. Z Angew Entomol 61:142–153
Edwards AR (1958) Cleavage in Cheyletus eruditus (Acarina). Nature 181:1409–1410
El Kammah KM, Adham FK, Tadross NR, Osman M (1982) Embryonic development of the camel tick Hyalomma dromedarii (Ixodoidea: Ixodidae). Int J Acarol 8:47–54
Fagotto F, Hess E, Aeschlimann A (1988) The early embryonic development of the argasid tick Ornithodorus moubata (Acarina: Ixodoidea: Argasidae). Entomol Gener 13:1–8
Foe VE, Alberts BM (1983) Studies of nuclear and cytoplasmic behaviour during the five mitotic cycles that precede gastrulation in Drosophila embryogenesis. J Cell Sci 61:31–70
Gotoh T, Kamoto T, Hatakeyama M, Gomi K (1994) Embryonic development and diapause stage in Panonychus Mites (Acari: Tetranychidae). Appl Entomol Zool 29:507–515
Grbic M, Khila A, Lee K-Z, Bjelica A, Grbic V, Whistlecraft J, Verdon L, Navajas M, Nagy L (2007) Mity model: Tetranychus urticae, a candidate for chelicerate model organism. BioEssays 29:489–496
Hafiz HA (1935) The embryological development of Cheyletus eruditus (a mite). Proc R Soc Lond Ser B 117:174–201
Huelsmann S, Hepper C, Marchese D, Knöll C, Reuter R (2006) The PDZ-GEF Dizzy regulates cell shape of migrating macrophages via Rap1 and integrins in the Drosophila embryo. Development 133:2915–2924
Hughes TE (1950) The embryonic development of the mite Tyroglyphus farinae Linnaeus 1758. Proc Zool Soc Lond 119:873–886
Jeffery WR (1997) Evolution of ascidian development. BioScience 47:417–425
Langenscheidt M (1958) Embryologische, morphologische und histologische Untersuchungen an Knemidocoptes mutants (Robin et Lanquetin). Z Parasitenkd 18:349–385
Laumann M, Bergmann P, Norton RA, Heethoff M (2010a) First cleavages, preblastula and blastula in the parthenogenetic mite Archegozetes longisetosus (Acari, Oribatida) indicate holoblastic rather than superficial cleavage. Arthropod Struct Dev 39:276–286
Laumann M, Norton RA, Heethoff M (2010b) Acarine embryology: inconsistencies, artificial results and misinterpretations. Soil Organ 82:217–235
Lindquist EE (1996a) 1.1.1. External anatomy and notation of structures. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid Mites: their biology, natural enemies and control. World crop pests, vol 6. Elsevier Science Publishers, Amsterdam, The Netherlands, pp 3–31
Lindquist EE (1996b) 1.5.2 Phylogenetic relationships. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid Mites: their biology, natural enemies and control. World crop pests, vol 6. Elsevier Science Publishers, Amsterdam, The Netherlands, pp 301–327
Lindquist EE, Amrine JW (1996) 1.1.2 Systematics, diagnoses for major taxa, and keys to families and genera with species on plants of economic importance. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid Mites: their biology, natural enemies and control. World crop pests, vol 6. Elsevier Science Publishers, Amsterdam, The Netherlands, pp 33–87
Liu Y, Maas A, Waloszek D (2009) Early development of the anterior body region of the grey widow spider Latrodectus geometricus Koch, 1841 (Theridiidae, Araneae). Arthropod Struct Dev 38(5):401–416
Mancini D, Garonna AP, Pedata PA (2013) A new embryonic pattern in parasitic wasps: divergence in early development may not be associated with lifestyle. Evol Dev 15:418–425
Nuzzaci G, Alberti G (1996) Internal anatomy and physiology. In: Lindquist EE, Sabelis MW, Bruin J (eds) Eriophyoid Mites: their biology, natural enemies and control. World crop pests, vol 6. Elsevier Science Publishers, Amsterdam, The Netherlands, pp 101–150
Raff RA (1987) Constraint, flexibility, and phylogenetic history in the evolution of direct development in sea urchins. Dev Biol 119:6–19
Santos VT, Ribeiro L, Fraga A, de Barros CM, Campos E, Moraes J, Fontenele MR, Araujo HM, Feitosa NM, Logullo C, da Fonseca RN (2013) The embryogenesis of the tick Rhipicephalus (Boophilus) microplus: the establishment of a new chelicerate model system. Genesis 51:803–818
Scholtz G, Wolff C (2013) Arthropod embryology: cleavage and germ band development. In: Minelli A, Boxshall G, Fusco G (eds) Arthropod biology and evolution: molecules, development, morphology. Springer, Berlin, pp 63–89
Shevchenko VG (1961) Osobennosti postembrional’nogo razvitiya chetyrekhnogikh kleshchei-galloobrazovatelei (Acariformes, Eriophyidae) i nekotorye zamechaniya po sistematike Eriophyes laevis (Nal., 1889). Zool Zh 40:1143–1158
Sidorchuk EA, Schmidt AR, Ragazzi E, Roghi G, Lindquist EE (2015) Plant-feeding mite diversity in Triassic amber (Acari: Tetrapodili). J Syst Paleontol 13(2):129–151. doi:10.1080/14772019.2013.867373
Sokolov II (1952) Observations on the embryonic development of the granary mites. I. Construction of the egg and segmentation. Trudy Leningrad. Soc Nat 71:245–260 (in Russian)
Tomer R, Khairy K, Amat F, Keller PJ (2012) Quantitative high-speed imaging of entire developing embryos with simultaneous multiview light-sheet microscopy. Nat Methods 9:755–763
Wagner J (1894) Die Embryonalentwicklung von Ixodes calcaratus. Trav Soc Nat St Petersburg 24:214–246
Walter DE (2009) Reproduction and embryogenesis. In: Krantz GW, Walter DE (eds) A manual of acarology, 3rd edn. Texas Tech University Press, Lubbock, TX, pp 54–56
Walter DE, Krantz GW (2009) Oviposition and life stages. In: Krantz GW, Walter DE (eds) A manual of acarology, 3rd edn. Texas Tech University Press, Lubbock, TX, pp 57–63
Walter DE, Proctor HC (1999) Mites: ecology evolution and behavior, 1st edn. UNSW Press, Sydney
Yastrebtsov A (1992) Embryonic development of gamasid mites (Parasitiformes: Gamasida). Int J Acarol 18:121–141
Acknowledgments
We are grateful to Dr. S. Bolton (Ohio State University, USA) and three anonymous reviewers for their valuable comments and linguistic corrections. CLSM studies of the mites were performed at the Center for Molecular and Cell Technologies and Center for Microscopy and Microanalysis of Research park of St. Petersburg State University. Collecting, staining and microscopy studies of the embryos were supported by the Russian Science Foundation (RSCF Grant #14-14-00621 to the first author).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chetverikov, P.E., Desnitskiy, A.G. A study of embryonic development in eriophyoid mites (Acariformes, Eriophyoidea) with the use of the fluorochrome DAPI and confocal microscopy. Exp Appl Acarol 68, 97–111 (2016). https://doi.org/10.1007/s10493-015-9982-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10493-015-9982-4