Abstract
Research suggest that in autism spectrum disorder (ASD) a disturbance in the coordinated interactions of neurons within local networks gives rise to abnormal patterns of brainwave activity in the gamma bandwidth. Low frequency transcranial magnetic stimulation (TMS) over the dorsolateral prefrontal cortex (DLPFC) has been proven to normalize gamma oscillation abnormalities, executive functions, and repetitive behaviors in high functioning ASD individuals. In this study, gamma frequency oscillations in response to a visual classification task (Kanizsa figures) were analyzed and compared in 19 ASD (ADI-R diagnosed, 14.2 ± 3.61 years old, 5 girls) and 19 (14.8 ± 3.67 years old, 5 girls) age/gender matched neurotypical individuals. The ASD group was treated with low frequency TMS (1.0 Hz, 90% motor threshold, 18 weekly sessions) targeting the DLPFC. In autistic subjects, as compared to neurotypicals, significant differences in event-related gamma oscillations were evident in amplitude (higher) pre-TMS. In addition, recordings after TMS treatment in our autistic subjects revealed a significant reduction in the time period to reach peak amplitude and an increase in the decay phase (settling time). The use of a novel metric for gamma oscillations. i.e., envelope analysis, and measurements of its ringing decay allowed us to characterize the impedance of the originating neuronal circuit. The ringing decay or dampening of gamma oscillations is dependent on the inhibitory tone generated by networks of interneurons. The results suggest that the ringing decay of gamma oscillations may provide a biomarker reflective of the excitatory/inhibitory balance of the cortex and a putative outcome measure for interventions in autism.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Neurodevelopmental disorders are the result of impairments in the orderly sequence of events that coordinate the growth and development of the central nervous system. In autism spectrum disorder (ASD), a group of neurodevelopmental conditions have been nosologically grouped together based on commonalities in observable behavioral characteristics that include difficulties with social communication and interactions, and restricted patterns of interests and activities (APA, 2013). Multiple lines of evidence suggest that the underlying developmental disorder in ASD involves abnormalities in the fundamental processes that govern neuronal migration. The presence of neuronal heterotopias, the accumulation of neurons at the gray white matter junction, and morphometric findings indicative of a minicolumnopathy are all suggestive of a defect during corticogenesis that impairs the synchronization between the streams of radially and tangentially migrating neuroblasts (Bailey et al., 1998; Casanova, 2007, 2014; Casanova, El-Baz, et al., 2013a; Hutsler & Casanova, 2016; Wegiel et al., 2010). These migratory streams guide neuroblasts to their appropriate place in the cortical plate where they will coordinate their activities and establish functional dyads of pyramidal cells and interneurons (Marin-Padilla, 2011; Wong et al., 2018). In this regard, faulty neuronal migration results in an excitatory/inhibitory imbalance capable of explaining some of the core cognitive, sensory and neurological symptoms of the condition, including the increased prevalence of seizures (Casanova et al., 2003).
Several lines of evidence suggest that the cortical excitatory/inhibitory imbalance observed in ASD is the result of an inhibitory deficit. In vivo magnetic resonance spectroscopy (MRS) studies show a direct correlation between reduced gamma aminobutyric acid (GABA) levels and clinical features that help define the ASD phenotype (e.g., social cognition, motor stereotypies) (Cochran et al., 2015; Gaetz et al., 2014). Postmortem studies, sampling both the cerebral cortex and cerebellum, have shown a reduction of GABA(A) receptors in ASD individuals as compared to controls (Dickinson et al., 2016; Fatemi et al., 2009). Immunocytochemical studies have further characterized the inhibitory deficit as one due to a reduction in the number of neurons that express the calcium-binding protein parvalbumin (PV) (Hashemi et al., 2018).
PV-positive neurons normally drive experience-dependent circuit refinement and information processing during sensory perception, motor behavior, and memory formation (Hensch, 2005; Kann et al., 2014). Their numbers are reduced in the prefrontal cortex of numerous psychiatric conditions (e.g., schizophrenia, Alzheimer disease, bipolar disorder) (Berridge, 2013; Ferguson & Gao, 2018). Research studies have consistently revealed a reduced number of PV positive cells in animal models of ASD. These findings are of clinical relevance as the aforementioned pathological deficits have been found to correlate with behavioral abnormalities characteristic of the ASD phenotype (Gogolla et al., 2009; Saunders et al., 2013; Wöhr et al., 2015). Indeed, pharmacological interventions that rescue parvalbumin-immunoreactive neurons ameliorate deficits in prepulse inhibition (an operational measure of sensorimotor gating), relieve the reduction in phase-locked gamma oscillations, and ameliorate social behavioral deficits that are characteristic of a significant number of patients within the autism spectrum (Nakamura et al., 2015).
GABAergic PV-positive cells, comprising approximately 40% of all interneurons, provide primarily for perisomatic “basket” inhibition. Synchronization of neurons is achieved much more effectively by perisomatic inhibitory postsynaptic potentials than by dendritic excitatory postsynaptic potentials (Lytton & Sejnowski, 1991). Indeed, PV interneurons are capable of generating action potentials at high frequencies thus helping to coordinate the actions of neural network activities by rhythmic (“clockwork”) inhibition (Kann et al., 2014). This clockwork activity by PV-positive neurons shows its strongest coupling to the gamma oscillation cycle (Klausberger & Somogyi, 2008; Tukker et al., 2007; see also Tremblay et al., 2016). The bandwidth of these oscillations has been variously described as between 30 and 90 Hz. In schizophrenia, loss of PV-positive interneurons is believed to underlie reported abnormalities of gamma oscillations and related symptoms of executive dysfunction (e.g., conceptualization, cognitive flexibility, planning) (Curley & Lewis, 2012; Dienel & Lewis, 2019; Gonzalez-Burgos & Lewis, 2008; Gonzalez-Burgos et al., 2015; Lewis et al., 2012). Given the above information, it is unsurprising that ASD researchers have gone as far as to propose the use of gamma-band based metrics as a possible mean for subtyping clinical populations and as severity dependent measure of treatment outcome (Rojas & Wilson, 2014).
Inhibitory interneurons acting as GABA-gated pacemakers are critically involved in gamma oscillations (Grothe & Klump, 2000; Rossignol, 2011; Whittington et al., 2000). These oscillations can be induced by tasks involving the top-down integration of features, as for example, visual cognitive tasks using Kanizsa illusory features (Kanizsa, 1976). Our group has used oddball task paradigms of target classification and discrimination, requiring a response to target Kanizsa squares among nontarget Kanizsa triangles and other non-Kanizsa distractor figures, to examine gamma-band EEG activity (vide infra). The gamma waveforms elicited by this task exhibit a characteristic dampening after peak amplitude in which the outer envelope of successive peaks traces a decay curve that persists until baseline. In a variety of physical systems, the characteristics which determine dampening of sinusoidal oscillations are intrinsic properties of the object related to its impedance (i.e., the effective resistance of a circuit) (Brewer, 2012; Dobbs, 2001; Silver & Tiedemann, 1978; Trickey et al., 1977). In the brain, interneurons delay the firing of neuronal assemblies in ways that can either prevent or facilitate the transition of oscillatory patterns (Lin et al., 2019). After resonance, the ringing decay may therefore serve to monitoring the impedance provided by inhibitory neurons on gamma oscillations.
Transcranial magnetic stimulation (TMS) over the dorsolateral prefrontal cortex has been used to normalize gamma oscillations and enhance cognitive performance in schizophrenia (Farzan et al., 2012). These effects appear to be selective for oscillations in the gamma bandwidth (Barr et al., 2009) and are probably mediated by cortical changes that increase the levels of GABA (Feng et al., 2019). In animal models of ASD, TMS leads to normalization of social behavior deficits (Selimbeyoglu et al., 2017). In humans, the first clinical trial using TMS in ASD was reported by Sokhadze and associates (Sokhadze et al., 2009; for reviews of TMS studies in autism see Casanova et al., 2015, 2019; Cole et al., 2019; Oberman et al., 2016). Given the nature of the inhibitory deficits, the researchers decided on using low frequency TMS (0.5 Hz; trying to build inhibition) over the dorsolateral prefrontal cortex (DLPFC) in their inceptive experimental design. Thirteen patients (ADOS and ADI-R diagnosed) and an equal number of neurotypicals (wait list controls) participated in the study. Gamma power and behavioral screening were used as outcome measures. Results showed that at baseline, the gamma power was higher and of shorter latency in the ASD group as compared to controls. After treatment, the active group showed a wider difference in gamma power when comparing target and non-target stimuli for all recording sites. The findings for gamma oscillations were subsequently reproduced in studies using different populations of patients and number of TMS sessions (Baruth, Casanova, et al., 2010a, Baruth, Sokhadze, et al., 2010b; 2011; Casanova et al., 2012; Sokhadze et al., 2014).
The research findings linking gamma oscillations to the pathology of ASD led us to consider performing a spectral time analysis of gamma oscillations in ASD both before and after a series of TMS sessions. To this effect we introduced envelope analysis as a novel metric for characterizing gamma oscillations in ASD. We believed that the information derived from demodulating the EEG waveform, along with quantitative features of the ringing decay of gamma oscillations, could provide a biomarker closely tied to the neuropathology (inhibitory deficit) of the autistic disorder.
Methods
Subjects
Participants with ASD were recruited through referrals from several pediatric clinics. All patients (N = 19 mean age, 14.4 ± 3.61 years old, 5 females) were diagnosed according to the Diagnostic and Statistical Manual of Mental Disorders (DSM-IVTR) and/or DSM-5 (APA, 2013). Diagnosis of autism was further ascertained with the Autism Diagnostic Interview-Revised (ADI-R) (LeCouteur et al., 2003). A developmental pediatrician evaluated the patients, ascertained them to be in good health, had normal hearing, and were willing to participate in lab testing. Patients were excluded if they had a history of seizures, impairment of vision, genetic disorders, and/or brain abnormalities based on neuroimaging studies. Exclusionary criteria for this group were as follows: (a) current diagnosis of any Axis I psychiatric disorder, such as psychosis, bipolar disorder, and schizophrenia; (b) current psychiatric symptoms requiring medication other than those for ADHD; (c) severe medical, cognitive or psychiatric impairments that would preclude from cooperation with the study protocol; and (d) inability to read, write, or speak English. The EEG test procedures also required the following exclusionary criteria: (1) impaired, non-correctable vision or hearing; (2) significant neurological disorder (epilepsy, encephalitis) or head injury. Enrolled subjects were high-functioning children or adolescents with a full-scale Intelligence Quotient (IQ) of more than 80 according to evaluations using the Wechsler Intelligence Scale for Children, Fourth Edition (WISC-IV, [Wechsler, 2003]) or the Wechsler Abbreviated Scale of Intelligence (WASI, [Wechsler, 1999]). Children with an ASD diagnosis who were on stimulant medication were included in this study only if they were taken off medication on the day of the lab visit for testing.
Typically developing children (i.e., control subjects, CNT group, N = 19, 14.8 ± 3.67 years old, 5 females) were recruited through advertisements in the local media. All control participants were free of neurological or significant medical disorders, had normal hearing and vision, and were free of psychiatric, learning, or developmental disorders based on self- and parental reports. Subjects were screened for a history of psychiatric or neurological diagnosis using the Structured Clinical Interview for DSM-IV, Research version, Non-Patient Edition (SCID-NP, First et al., 2002). Participants within the control and ASD groups were matched by age, full scale IQ, and socioeconomic status (based on parental level of education and annual household income) of their family.
The study was conducted in accordance with relevant national regulations and institutional policies and complied with the Helsinki declaration. The protocol of the study including informed consent and assent forms that were reviewed and received approval of our university’s IRB. Children and their family representatives (either parents or legal guardians) received detailed information about the specifics of this research study, including its purpose, responsibilities, reimbursement rate, risk vs. benefits evaluation, etc. The participants were reimbursed only for oddball tests ($25 for each procedure) and did not receive any reimbursement for the TMS treatment. Investigators provided consent and assent forms to all families who expressed interest in participation in this treatment research study, allowed them to review the documents and answered all questions. If the child and family member agreed to take part in this study and confirmed their commitment, both child and parent were requested to sign and date the consent and assent forms and then received a copy co-signed by the study investigator.
Event-Related Gamma Oscillations Recording
The dense-array (128 channel) electroencephalogram (EEG) was recorded with an Electrical Geodesics Inc. Netstation system (EGI-Philips, Eugene, Oregon). Experimental control (e.g., stimulus presentation, reaction time) was executed using E-prime software (Psychological Software Tools [PST], Inc., Pittsburg, PA). Visual stimuli were presented on a monitor located in front of the subject, while motor responses were recorded with a 4-button keypad (PST’s Serial Box). EEG was recorded with 512 Hz sampling rate, analog Notch (60 Hz, IIR, 5th order) filter and analog bandpass elliptical filters set at 0.1 to 100 Hz range. Electrodes impedance was kept under 40 KΩ. According to the Electrical Geodesics (2003) impedances < 50 KΩs are sufficient.
for recording quality EEG data (note: Ferree et al. (2001) have suggested that modern high input impedance amplifiers and accurate digital filters for power noise provide excellent EEG signals in conjunction with above scalp impedances). Raw EEG recordings were segmented off-line spanning 200 ms pre-stimulus baseline and 800 ms epoch post-stimulus. EEG data was screened for artifacts and all trials that had eye blinks, gross movements and other artifacts were removed using Netstation artifact rejection tools (Luu et al., 2001; Srinivasan et al., 1998). Other details of our experimental procedure and EEG data acquisition, pre-processing and analysis can be found in our prior studies using the same methodology (Baruth et al., Baruth, Casanova, et al., 2010, Baruth, Sokhadze, et al., 2010; Casanova et al., 2012, 2014; Sokhadze et al., 2014, 2016). In our gamma oscillation analysis, stimulus-locked dependent EEG variables for the frontal (F3, F4, F7, F8) and parietal (P3, P4) sites-of-interest were referenced to vertex (Cz) and nasion as a ground or common reference point.
EEG Analysis Description
An equiripple bandpass FIR filter with a passband ripple of 1 dB and stopband attenuation of 60 dB was used to extract the gamma wave frequency components within the frequency range from 35 to 45 Hz. EEG signal was sampled at a rate of 500 samples/second.
Zero crossings were defined at the time location where the sign of the gamma wave changed from the positive to the negative and vice versa. Zero crossings were classified into two types: upward zero crossings and downward zero crossings. An upward zero crossing was found when the gamma wave changes from a negative to a positive value while a downward zero crossing was located where the gamma wave changed from a positive to a negative value. Both upward zero crossing (\({Z}_{upward}\)) and downward zero crossing (\({Z}_{downward})\) were defined as follows:
where \(G\left(n\right)\) is the gamma wave time sample located at n.
A peak amplitude was defined as the maximum value of the set of values within the time interval defined between an upward zero crossing and a downward zero crossings. Peaks (\(P(\overline{n})\)) were represented using the following mathematical equation:
The major peak (PM) of the burst of gamma waves was defined as the maximum value of all the peaks ( \(P(\overline{n})\)) located within the corresponding time frame.
where \({\overline{n}}_{1}\) and \({\overline{n}}_{2}\) are time location of the starting and ending peaks of the gamma waves.
Latencies were defined as the time difference between the major peak time location and the time location of the lowest peak values at the beginning and at the end of the gamma wave time intervals (i.e., \({\overline{n}}_{1}\) and \({\overline{n}}_{2}\) respectively).
Slopes were calculated between the major peak and the time location of the lowest peak values mentioned above. A positive slope (\({S}_{+}\)) was defined between the lowest peak at the beginning of the gamma wave time interval (\({\overline{n}}_{1}\)) and the major peak while a negative slope (\({S}_{-}\)) was defined between the major peak and the lowest peak at the end of the gamma wave time interval (\({\overline{n}}_{2}\)).
Amplitude of the gamma oscillation peaks (within 100–300 ms window post-stimulus) and differences of the latencies of ascending and descending slopes of gamma were used as main outcomes in the study. One more variable of interest was positive–minus–negative slope latency difference.
Transcranial Magnetic Stimulation
For repetitive TMS administration we used a Magstim Rapid device (Magstim Co., Sheffield, UK) with a 70-mm wingspan figure-eight coil. To identify resting motor threshold (MT) for each hemisphere the output of the magnetic stimulator was increased by 5% steps until a 50 μV deflection of the electromyogram (EMG) or a visible twitch in the First Dorsal Interosseous (FDI) muscle was detected in at least 2 or 3 trials of TMS delivered over the contralateral motor cortex. The electromyogram was recorded with a portable C-2 J&J Engineering Inc. physiological monitor using USE-3 software and Physiodata applications (J&J Engineering, Inc., Poulsbo, WA).
The rTMS was administered on a weekly basis with the following stimulation parameters: 1.0 Hz frequency, 90% MT, 180 pulses per session with 9 trains of 20 pulses each with 20–30 s intervals between the trains. The initial six weekly rTMS session were administered over the left DLPC, followed by 6 sessions targeting the right DLPC, and an additional 6 treatments done bilaterally (over the left and right DLPFC). The procedure for stimulation placed the TMS coil 5 cm anterior, and in a parasagittal plane, to the site of maximal FDI response as judged by the FDI EMG response. A swimming cap was used to ensure better positioning of the TMS coil. Positioning of the TMS coil followed recommendations that take into consideration anatomical landmarks (Mir-Moghtadaei et al., 2015; Pommier et al., 2017) that could be approximately described as the scalp region used for F3 and F4 EEG electrode placements in the 10–20 International System.
Selection of 90% of the MT was based on data from prior studies where low frequency rTMS was used for the stimulation of DLPFC in various neurological and psychiatric disorders (Daskalakis et al., 2002; Gershon et al., 2003; Loo & Mitchell, 2005; Pascual-Leone et al., 2000; Wassermann & Lisanby, 2001; Wassermann & Zimmermann, 2012). Furthermore, we decided to have the stimulation power below MT as a safety precaution meant to lower the probability of seizure risk in this study population. The decision to use low frequency (1 Hz) was based on the finding that at this frequency range rTMS exerts an inhibitory influence on the stimulated cortex (Maeda et al., 2000).
Behavioral and Social Functioning Evaluation
Social and behavioral functioning was evaluated using caregiver (parent or guardian) reports. Participant in each group were evaluated before TMS course and within a week following treatment. Aberrant Behavior Checklist (ABC, Aman & Singh, 1994) is a rating scale to assess Irritability, Lethargy/Social Withdrawal, Stereotypy, Hyperactivity, and Inappropriate Speech based on parent/caregiver report. Repetitive Behavior Scale—Revised (RBS-R, Bodfish et al., 1999) is a caregiver completed rating scale to assess stereotyped, self-injurious, compulsive, ritualistic, sameness, and restricted range.
Statistical Analysis
Repeated measure ANOVA and paired sample t-test (2-tailed) were the primary models for statistical analyses of subject-averaged amplitude and ascending and descending slope latencies of gamma oscillations. Dependent stimulus-locked gamma variables were amplitude and latency of differences of positive and negative slopes of gamma oscillations at pre-determined frontal and parietal EEG sites (F3, F4, F7, F8 and P3, P4). The within-participant factors in the ASD group were the following: Stimulus (Target Kanizsa, Non-target Kanizsa, Standard non-Kanizsa), Hemisphere (Left, Right), and Time Point (Baseline, Post-TMS treatment). The between-subject factor was Group (ASD vs. CNT). Histograms with distribution curves were obtained for each dependent variable to determine normality of distribution and appropriateness of data for ANOVA and post-hoc t-tests. For normality analysis we used the Shapiro–Wilk test. All dependent variables in the study had normal distribution. Greenhouse–Geisser (GG) corrected p-values were employed where appropriate in all ANOVAs. For the estimation of the effect size we used a Partial Eta Squared (ηp2) measure (Richardson, 2011). Behavioral data (reaction time, accuracy) and parental behavioral rating scores were analyzed using paired sample 2-tailed t-test. IBM SPSS 26 and Sigma Stat 9.1 statistical software were used for data analysis.
Results
Peak Amplitude
Autism (ASD) vs. Control (CNT) Group Comparisons
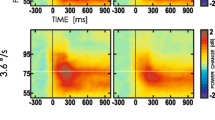
Amplitude of evoked gamma oscillation peaks showed Stimulus (target Kanizsa, non-target Kanizsa, non-Kanizsa standard) x Group (ASD, CNT) interaction at the frontal sites (e.g., at F7 and F8 sites, F2,38 = 4.49, p = 0.014, ηp2 = 0.106). In particular, at these EEG sites peak amplitude of responses to targets was lower as compared to both non-target stimuli in the ASD group, while in the CNT group amplitude to targets (TRG) was higher as compared to non-target Kanizsa (NTG) stimuli. Post-hoc t-test showed higher amplitude to TRG, NTG and non-Kanizsa standards (NOK) stimuli in the ASD group at all frontal sites of interest (F3, F4, F7, F8) but not at the parietal sites (P3, P4). The results of group differences at the frontal sites are depicted in Table 1 and Fig. 1.
Event-related 40 Hz-centered gamma oscillations in response to standard non-Kanizsa, non-target Kanizsa and target Kanizsa stimuli in visual oddball task with illusory figures in children with ASD and in neurotypical children (N = 19/per group). Children in the ASD group showed higher amplitude of the gamma oscillations in response to all three type of stimuli. Neurotypical children had higher amplitude of response to target rather than task-irrelevant stimuli. Grand average for the left frontal sites (F3, F7)
TMS Treatment Effects on Evoked Peak Amplitude
Repeated measures analysis did not show any Stimulus × Treatment (pre-, post-TMS) interactions. At the frontal sites 2-tailed paired t-test showed statistical increase in response to targets (F3, by 1.79 ± 3.51 μV, t(18) = 2.29, p = 0.034; F4, by 1.30 ± 2.76 μV, t(18) = 2.16, p = 0.044; F7, 1.55 ± 2.89 μV, t(18) = 2.34, p = 0.031). TMS resulted in an increase to non-target stimuli only at one right frontal site (F4, 1.34 ± 2.51 μV, t(18) = 2.39, p = 0.029). Responses to the non-Kanizsa stimuli were not changed post-TMS except, again, only at the F4 site (1.44 ± 2.69 μV, t(18) = 2.39, p = 0.027, Fig. 2).
Effects of rTMS on event-related gamma oscillations in 19 children with ASD in response to standard non-Kanizsa, non-target Kanizsa and target Kanizsa figures. Magnetic stimulation therapy resulted in decreased responses to both non-target stimuli, more pronounce for non-Kanizsa standards at the right frontal sites (F4, F8)
Positive–Minus–Negative Slope Latency Difference
Autism (ASD) vs. Control (CNT) Group
Difference between positive and negative slope latencies was compared between the ASD and CNT groups, and in the ASD group post-TMS treatment. Comparison of slope differences for the target and non-target Kanizsa stimuli revealed Stimulus (TRG, NTG) x Group (ASD, CNT) interaction (F3, F4, F2,38 = 4.17, p = 0.048, ηp2 = 0.099), where the CNT group had larger difference for non-target stimuli. T-test did not yield any group differences in response to TRG stimuli but revealed statistical differences in response to both NTG and NOK stimuli at the frontal sites. For instance, at the F4 site difference for NTG stimuli was shorter in the ASD group by 8.23 ± 0.61 ms, t(18) = 3.37, p = 0.003, and at the F7 in response to NOK stimuli it was shorter by 9.35 ± 16.29 ms, t(18) = 2.51, p = 0.022.
TMS Effects on Positive–Minus–Negative Slope Latency Differences
There were no Stimulus × Treatment (pre-, post-TMS) interactions found for this slope latency difference measure. The effects of TMS treatment on differences between positive and negative slope latencies were significantly decreased at practically all EEG sites. For example, difference became shorter at F3 site in response to TRG by 12.8 ms (t = 6.50, p < 001), to NTG by 7.3 ms (t = 2.16, p = 0.016), and to NOK stimuli by 10.8 ms (t = 4.33, < 0.001). Results of the paired sample t-test for TRG, NTG and NOK stimuli at F3, F4, F7, F8, and P3, P4 EEG sites are summarized in the Table 2.
Behavioral Responses (Reaction Time and Accuracy)
Reaction Time (RT) and Accuracy
Differences between the ASD and CNT groups at the baseline were statistically significant only for total error percentage (7.89 ± 7.91% in ASD vs 0.62 ± 0.71 in CNT, t(18) = 4.12, p = 0.001). The course of rTMS did not affect RT but resulted in a decrease of the total error rate (from 7.89 ± 7.91% down to 2.80 ± 3.04% post-TMS, t(18) = 2.30, p = 0.033).
Behavioral Evaluations Post-TMS
As expected, based on our prior studies, the ABC and RBS-R parental behavioral checklists rating changes showed statistically significant improvements in several domains. Irritability subscale of the ABC rating decreased (mean − 2.79 ± 4.89, t(18) = − 2.84, p = 0.023), and Lethargy/Social Withdrawal subscale showed a significant score reduction (mean decrease − 1.79 ± 3.20, t(18) = − 2.43, p = 0.026). Hyperactivity score of the ABC also showed reduction (− 3.94 ± 7.14, t(18) = − 2.40, p = 0.027).
We found a significant decrease in stereotypic, repetitive, and restricted behavior patterns following 18 sessions of rTMS as measured by the RBS-R. Stereotypic Behavior subscale showed a significant decrease (− 1.52 ± 1.50, t(18) = − 4.42, p = 0.001) and Ritualistic/Sameness Behavior Subscale scores showed a decrease (− 1.57 ± 2.41, t(18) = − 2.85, p = 0.011). Compulsive Behavior Subscale also demonstrated a significant decrease (− 1.42 ± 2.51, t(18) = − 2.47, p = 0.023).
Discussion
Some of our results reproduce prior findings regarding gamma oscillation abnormalities in autism. The reported increased amplitude and shortened latency of event-related gamma oscillations at baseline have been interpreted as a consequence of a reduced “signal to noise” ratio resulting from decreased inhibition during cortical processing (Brown et al., 2005; Casanova et al., 2012). Evoked gamma-band activity (latency of around 100 ms after stimulus) is thought to represent the early stage of sensory processing within the same cortical area while the induced component (latency of around 250 ms after stimulus) is thought to represent processing among networks of different cortical regions (Casanova, Baruth, et al., 2013). Excessive (higher amplitude) but unsynchronized gamma activity is possibly related to a reduction in the ability to focus attention on relevant stimuli. According to some researchers, in autism, uninhibited gamma activity suggests that none of the circuits of the brain comes to dominance as many of them are active simultaneously (Brown et al., 2005).
In autism, evidence of diminished stimulus discrimination is derived from augmented potentials to irrelevant visual stimuli that occur at early stages of visual processing (Casanova et al., 2012). Baseline evoked gamma activity in our autism group was not discriminative of stimulus type for the Kanizsa task employed. This is in contrast to recordings of significant differences between target and non-target stimuli in our neurotypical controls. Following TMS, autistic individuals displayed diminished amplitude to both distracters and standard stimuli, and a significant improvement in discriminating between relevant and irrelevant visual stimuli (Baruth, Casanova, et al., 2010a, Baruth, Sokhadze, et al., 2010b). Performance on visual oddball task post-TMS improved and resulted in lower error rate. Parental questionnaires showed improvements in several measures of aberrant behavior and repetitive and stereotype behaviors. Post-TMS treatment improvements in behavior point at the potential therapeutic utility and clinical relevance of neuromodulation in children with ASD.
In addition to the normal metrics of event-related gamma potentials, our study is the first to quantitate the envelope and settling time of gamma oscillations. Measurements of the ringing decay were significantly different when comparing autistics and neurotypical controls. In autism, baseline levels of gamma oscillations showed a shortened period of ringing decay. Short ringing times implies a system with lower sensitivity (Silver & Tiedemann, 1978); one that makes synchronization and integration of information among different neuronal networks imprecise or inefficient. The inhibitory deficit evidenced in neuropathological studies of ASD thus translates into a low sensitivity system seemingly overwhelmed by the background level of noise. In a previous study, modeling such a system gave rise to stochastic resonance; a phenomenon where a neural network embedded in a noisy background acquired, counterintuitively, enhanced sensitivity (Casanova et al., 2014). The phenomenon serves to explain the autistic emphasis for sameness (i.e., an adaptation to an optimal noise level) and the sensory peculiarities (hypo- and hypersensitivity) characteristic of the condition.
In modeling the activity of excitable membranes, resonance is achieved by the combined action of inductive and capacitive reactance (Gutfreund et al., 1995). These are intrinsic properties of passive elements within the membrane that serve to oppose the flow of current. The interaction of capacitance and inductance allows the membrane to act as an electrical resonator, one that preferentially oscillates at the circuit's resonant frequency. In biological systems, a resonant peak in the frequency domain implies a dampened oscillation in the time domain (Gutfreund et al., 1995). Some systems may have multiple, distinct resonance frequencies. The greatest response or amplitude is achieved for the least amount of dampening. Following an excitatory phase, wherein the system is stimulated into resonance, a free ringing decay ensues which provides a measure of impedance (Brewer, 2012). For brainwave forms, interneurons provide the resistive element necessary to elicit gamma oscillations. Detailed computational models of cortical circuitry have shown how downregulation of PV cells disinhibit networks and alter gamma oscillations in response to stimulation (Volman et al., 2011). Indeed, optogenetically inhibiting the action of PV cells suppresses gamma oscillations in vivo while activating these interneurons generates gamma oscillations (Sohal et al., 2009). In the end, excitation and inhibition of appropriate power alternate in order to establish the cyclic behavior of brainwave oscillations (Buzsáki & Wang, 2012). During this cyclic behavior excitation and amplification mark the time period to peak amplitude while inhibition characterizes the settling pace of the ensuing decay curve.
In any circuit, impedance is a complex function dependent on frequency. In neural circuits, there is a decline in impedance with increasing frequency wherein an oscillatory input current produces a smaller voltage response as the frequency rises (Hutcheon & Yarom, 2000). It may be that in autism, a system already taxed by an inhibitory deficit, abnormalities will become apparent at higher frequencies. In regard to brainwaves, the highest frequencies and largest bandwidth belongs to gamma oscillations. This makes event-related gamma activity an ideal scenario to study the excitatory/inhibitory balance of the cerebral cortex in autism, specially so when considering the strong coupling of PV interneurons to frequencies in this bandwidth (Klausberger & Somogyi, 2008; Tukker et al., 2007; see also Tremblay et al., 2016).
Our study showed a normalization of time to peak amplitude and ringing decay of our autistic subjects after TMS therapy. The applied inductive pulses increased the sensitivity of evoked gamma activity (Du et al., 2013). By convention, repetitive TMS with a frequency less than 1 Hz is considered low frequency stimulation (Kozel et al., 2019; Klomjau et al., 2015). Previous studies by our group in ASD subjects using 1 Hz TMS stimulation reported significant changes in gamma oscillations. The latter experience along with the guiding hypothesis of an excitatory/inhibitory imbalance in ASD guided us to the use low frequency (1 Hz) TMS in this study. Models on long-term potentiation suggest that low frequency TMS is typically inhibitory while faster stimulation (≥ 5 Hz) is excitatory (Kozel et al., 2019; Pell et al., 2011; Wassermann et al., 1998). The difference in threshold may be due to the orientation selectivity of anatomical elements within the cerebral cortex and their sensitivity to magnetic stimulation (Fox et al., 2004). Lower frequencies stimulation may preferentially induce currents along longitudinally oriented elements traversing the flux lines of the magnetic field; that is, along axons rather than across the same (Fox et al., 2004). When higher frequencies are used (≥ 5 Hz) in a targeted cortical area, all neurons, regardless of their geometrical orientation, are stimulated. Since pyramidal cells comprise 70 to 90% of all neurons in the cerebral cortex the end result is that TMS becomes excitatory at higher stimulation frequencies. The geometrical disposition of interneurons and their projections within cortical minicolumns makes them especially susceptible to low frequency TMS stimulation. Indeed, Mountcastle who first described the functional nature of minicolumns described the arrangement of interneurons as one providing a strong vertical flow of inhibition (Mountcastle, 1998).
As one of the limitations of our study should be considered relying on “5 cm rule” for positioning TMS coil rather than using more advanced navigation systems such as BeamF3 (Beam et al., 2009; Mir-Moghtadaei et al., 2015) or Brainsight device (Rogue Research, Canada). TMS has effects in disparate areas linked to the stimulation site. We have discussed in previous publications the selection of the DLPFC for our studies based on its widespread connectivity. In effect, the DLPFC is interconnected with every other cortical region thus allowing its neural circuitry to engender integrated responses as required by higher cognitive functions. In this regard, we were hoping that by stimulating the DLPFC in ASD, downstream effects in areas connected to the DLPFC would become patent and benefit the patient. Unfortunately, we cannot speculate on these downstream effects in the present study. The topographical screening done with select electrodes placements was meant to examine the elicited gamma oscillations rather than to examine the widespread effect of TMS stimulation.
Electrophysiological studies of oscillatory activities in several frequency bands found significant modulation and interaction of oscillations in various frequency bands (Hyafil et al., 2015; Jensen & Colgin, 2007). Recently more interest was focused on coupling of certain slow and high frequency activities. In particular, it was shown that the power of gamma oscillations (30–80 Hz) is modulated by the phase of theta oscillations (5–8 Hz) (Jensen & Colgin, 2007). Given that the theta-gamma coupling might be especially important in individuals with autism where gamma activity is often is reported to be aberrant (An et al., 2018; Uzunova et al., 2016; Rojas & Wilson, 2014) and theta activity found to be excessive at the frontal sites. Our study investigated effects of rTMS only on sub-band of gamma oscillations without analysis of changes in theta oscillations and this is yet another limitation to mention. In the future theta-gamma coupling should be considered as an important factor affecting TMS-based neuromodulation effects in children with ASD.
In summary, this is the first study on the metrics of ringing decay of gamma oscillations in ASD. Salient differences were noted at baseline and normalized by low frequency (inhibitory) TMS therapy. At present we did not include the individual analysis of induced gamma band activity as these oscillations, in contrast to evoked gamma oscillations, are not phase-locked to the stimulus. Induced gamma oscillations are strongly jittering making it difficult to extract accurate measurements in the time domain. A weakness of any TMS study is the localization of the anatomical site for stimulation. None of the available techniques used to identify the DLPFC have been validated by cytoarchitectonics. It would be useful, in this regard, to compare our results by using other localization method, including neuronavigation. We also hope to expand on the findings in future studies specially in regard to the longitudinal benefit of the TMS intervention, the role of gamma asymmetries, possible behavioral correlates for both the excitatory phase and ringing decay, and their influence on some systemic manifestations of ASD presently regarded as comorbidities.
References
Aman, M. G., & Singh, N. N. (1994). Aberrant Behavior checklist—Community. Supplementary Manual. Slosson Educational Publications.
American Psychiatric Association. (2000). Diagnostic and statistical manual of mental disorders (DSM-IV-TR). (4th ed.). Author.
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-V). (5th ed.). Author.
An, K. M., Ikeda, T., Yoshimura, Y., Hasegawa, C., Saito, D. N., Kumazaki, H., Hirosawa, T., Minabe, Y., & Kikuchi, M. (2018). Altered gamma oscillations during motor control in children with autism spectrum disorder. The Journal of Neuroscience, 38(36), 7878–7886. https://doi.org/10.1523/JNEUROSCI.1229-18.2018
Bailey, A., Luthert, P., Dean, A., Harding, B., Janota, I., Montgomery, M., Rutter, M., & Lantos, P. (1998). A clinicopathological study of autism. Brain, 121(5), 889–905. https://doi.org/10.1093/brain/121.5.889
Barr, M. S., Farzan, F., Rusjan, P. M., Chen, R., Fitzgerald, P. B., & Daskalakis, Z. J. (2009). Potentiation of gamma oscillatory activity through repetitive transcranial magnetic stimulation of the dorsolateral prefrontal cortex. Neuropsychopharmacology, 34(11), 2359–2367. https://doi.org/10.1038/npp.2009.79
Baruth, J. M., Casanova, M. F., El-Baz, A., Horrell, T., Mathai, G., Sears, L., & Sokhadze, E. (2010). Low-frequency repetitive transcranial magnetic stimulation (rTMS) modulates evoked-gamma frequency oscillations in autism spectrum disorder (ASD). Journal of Neurotherapy, 14(3), 179–194. https://doi.org/10.1080/10874208.2010.501500
Baruth, J., Sokhadze, E., El-Baz, A., Mathai, G., Sears, L., & Casanova, M. F. (2010). Transcranial magnetic stimulation as a treatment for autism. In K. Siri & T. Lyons (Eds.), Cutting edge therapies for autism. (pp. 388–397). Skyhorse Publishing.
Baruth, J. M., Williams, E. L., Sokhadze, E. M., El-Baz, A. S., & Casanova, M. F. (2011). Beneficial effects of repetitive transcranial magnetic stimulation (rTMS) on behavioral outcome measures in autism spectrum disorder. Autism Science Digest, 1, 52–57
Beam, W., Borckardt, J., Reeves, S., & George, M. (2009). An efficient and accurate new method for locating the F3 position for prefrontal TMS applications. Brain Stimulation, 2, 50e4
Berridge, M. J. (2013). Dysregulation of neural calcium signaling in Alzheimer disease, bipolar disorder and schizophrenia. Prion, 7(1), 2–13. https://doi.org/10.4161/pri.21767
Bodfish, J. W., Symons, F. J., & Lewis, M. H. (1999). Repetitive Behavior Scale. Western Carolina Center Research Reports.
Brewer, D. F. (2012). Progress in low temperature physics. (Vol. XIII)North-Holland.
Brown, C. C., Gruber, T., Boucher, J., Rippon, G., & Brock, J. (2005). Gamma abnormalities during perception of illusory figures in autism. Cortex, 41(3), 364–376. https://doi.org/10.1016/s0010-9452(08)70273-9
Buzsáki, G., & Wang, X. J. (2012). Mechanisms of gamma oscillations. Annual Review of Neuroscience, 35, 203–225. https://doi.org/10.1146/annurev-neuro-062111-150444
Casanova, M. F. (2007). The neuropathology of autism. Brain Pathology, 17(4), 422–433. https://doi.org/10.1111/j.1750-3639.2007.00100.x
Casanova, M. F. (2014). Autism as a sequence: From heterochronic germinal cell divisions to abnormalities of cell migration and cortical dysplasias. Medical Hypotheses, 83(1), 32–38. https://doi.org/10.1016/j.mehy.2014.04.014
Casanova, M. F., Baruth, J., El-Baz, A. S., Sokhadze, G. E., Hensley, M., & Sokhadze, E. S. (2013). Evoked and induced gamma frequency oscillations in autism. In M. F. Casanova, A. S. El-Baz, & J. S. Suri (Eds.), Imaging the brain in autism. (pp. 87–106). Springer.
Casanova, M. F., Baruth, J. M., El-Baz, A., Tasman, A., Sears, L., & Sokhadze, E. (2012). Repetitive transcranial magnetic stimulation (rTMS) modulates event-related potential (ERP) indices of attention in autism. Translational Neuroscience, 3(2), 170–180. https://doi.org/10.2478/s13380-012-0022-0
Casanova, M. F., Buxhoeveden, D., & Gómez, J. (2003). Disruption in the inhibitory architecture of the cell minicolumns: Implications for autism. The Neuroscientist, 9(6), 496–507. https://doi.org/10.1177/1073858403253552
Casanova, M. F., El-Baz, A. S., Kamat, S. S., Dombroski, B. A., Khalifa, F., Elnakib, A., Soliman, A., Allison-McNutt, A., & Switala, A. E. (2013). Focal cortical dysplasias in autism spectrum disorders. Acta Neuropathologica Communications, 1, 67. https://doi.org/10.1186/2051-5960-1-67
Casanova, M. F., Hensley, M. K., Sokhadze, E. M., El-Baz, A. S., Wang, Y., Li, X., & Sears, L. (2014). Effects of weekly low frequency rTMS on autonomic measures in children with autism spectrum disorder. Frontiers in Human Neuroscience, 8, 851. https://doi.org/10.3389/fnhum.2014.00851
Casanova, M. F., Sokhadze, E. M., Opris, I., Li, X., & Casanova, E. L. (2019). Autism, transcranial magnetic stimulation, and gamma frequencies. In E. M. Sokhadze & M. F. Casanova (Eds.), Autism spectrum disorder: Neuromodulation, neurofeedback, and sensory integration approaches to research and treatment. (pp. 49–65). FNNR & BMED Press.
Casanova, M. F., Sokhadze, E., Opris, I., Wang, Y., & Li, X. (2015). Autism spectrum disorders: Linking neuropathological findings to treatment with transcranial magnetic stimulation. Acta Paediatrica, 104(4), 346–355. https://doi.org/10.1111/apa.12943
Cochran, D. M., Sikoglu, E. M., Hodge, S. M., Edden, R. A., Foley, A., Kennedy, D. N., Moore, C. M., & Frazier, J. A. (2015). Relationship among glutamine, γ-aminobutyric acid, and social cognition in autism spectrum disorders. Journal of Child and Adolescent Psychopharmacology, 25(4), 314–322. https://doi.org/10.1089/cap.2014.0112
Cole, E. J., Enticott, P. G., Oberman, L. M., Gwynette, M. F., Casanova, M. F., Jackson, S. L. J., Jannati, A., McPartland, J. C., Naples, A. J., Puts, N. A. J., rTMS in ASD Consensus Group. (2019). The potential of repetitive transcranial magnetic stimulation for autism spectrum disorder**: A consensus statement. Biological Psychiatry, 85(4), e21–e22. https://doi.org/10.1016/j.biopsych.2018.06.003
Curley, A. A., & Lewis, D. A. (2012). Cortical basket cell dysfunction in schizophrenia. Journal of Physiology, 590(4), 715–724. https://doi.org/10.1113/jphysiol.2011.224659
Daskalakis, Z. J., Christensen, B. K., Fitzgerald, P. B., & Chen, R. (2002). Transcranial magnetic stimulation: A new investigational and treatment tool in psychiatry. Journal of Neuropsychiatry and Clinical Neuroscience., 14, 406–415. https://doi.org/10.1176/appi.neuropsych.14.4.406
Dickinson, A., Jones, M., & Milne, E. (2016). Measuring neural excitation and inhibition in autism: Different approaches, different findings and different interpretations. Brain Research, 1648(A), 277–289. https://doi.org/10.1016/j.brainres.2016.07.011
Dienel, S. J., & Lewis, D. A. (2019). Alterations in cortical interneurons and cognitive function in schizophrenia. Neurobiology of Disease, 131, 104208. https://doi.org/10.1016/j.nbd.2018.06.020
Dobbs, E. R. (2001). Helium three (International Series of Monographs on Physics). Oxford University Press.
Du, L., Zhu, X., Han, Y., Zhao, L., & Zhe, J. (2013). Improving the sensitivity of an inductive pulse sensor for detection of metallic wear debris in lubricants using parallel LC resonance method. Measurement Science and Technology. https://doi.org/10.1088/0957-0233/24/7/075106
Electrical Geodesics. (2003). Users manual for EGI netstation 4.0. Eugene, OR
Farzan, F., Barr, M. S., Sun, Y., Fitzgerald, P. B., & Daskalakis, Z. J. (2012). Transcranial magnetic stimulation on the modulation of gamma oscillations in schizophrenia. Annals of the New York Academy of Sciences, 1265, 25–35. https://doi.org/10.1111/j.1749-6632.2012.06543.x
Fatemi, S. H., Reutiman, T. J., Folsom, T. D., & Thuras, P. D. (2009). GABA(A) receptor downregulation in brains of subjects with autism. Journal of Autism and Developmental Disorders, 39(2), 223–230. https://doi.org/10.1007/s10803-008-0646-7
Feng, J., Zhang, Q., Zhang, C., Wen, Z., & Zhou, X. (2019). The effect of sequential bilateral low frequency rTMS over dorsolateral prefrontal cortex on serum level of BDNF and GABA in patients with primary insomnia. Brain and Behavior, 9(2), e01206. https://doi.org/10.1002/brb3.1206
Ferguson, B. R., & Gao, W. J. (2018). PV interneurons: Critical regulators of E/I balance for prefrontal cortex-dependent behavior and psychiatric disorders. Frontiers in Neural Circuits, 12, 37. https://doi.org/10.3389/fncir.2018.00037
Ferree, T. C., Luu, P., Russell, G. S., & Tucker, D. M. (2001). Scalp electrode impedance, infection risk, and EEG data quality. Clinical Neurophysiology, 112(3), 444–536. https://doi.org/10.1016/s1388-2457(00)00533-2
First, M. B., Spitzer, R. L., Gibbon, M., & Williams, J. B. W. (2002). Structured clinical interview for DSM-IV-TR axis I disorders, research version (SCID-I/NP) (non-patient ed.). New York State Psychiatric Institute.
Fox, P. T., Narayana, S., Tandon, N., Sandoval, H., Fox, S. P., Kochunov, P., & Lancaster, J. L. (2004). Column-based model of electric field excitation of cerebral cortex. Human Brain Mapping, 22(1), 1–16. https://doi.org/10.1002/hbm.20006
Gaetz, W., Bloy, L., Wang, D. J., Port, R. G., Blaskey, L., Levy, S. E., & Roberts, T. P. L. (2014). GABA estimation in the brains of children on the autism spectrum: Measurement precision and regional cortical variation. NeuroImage, 86, 1–9. https://doi.org/10.1016/j.neuroimage.2013.05.068
Gershon, A. A., Dannon, P. N., & Grunhaus, L. (2003). Transcranial magnetic stimulation in the treatment of depression. American Journal of Psychiatry, 160(5), 835–845. https://doi.org/10.1176/appi.ajp.160.5.835
Gogolla, N., Leblanc, J. L., Quast, K. B., Südhof, T. C., Fagiolini, M., & Takao, K. (2009). Common circuit defect of excitatory-inhibitory balance in mouse models of autism. Journal of Neurodevelopmental Disorders, 1, 172–181. https://doi.org/10.1007/s11689-009-9023-x
Gonzalez-Burgos, G., Cho, R. Y., & Lewis, D. A. (2015). Alterations in cortical network oscillations and parvalbumin neurons in schizophrenia. Biological Psychiatry, 77(12), 1031–1040. https://doi.org/10.1016/j.biopsych.2015.03.010
Gonzalez-Burgos, G., & Lewis, D. A. (2008). GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophrenia Bulletin, 34(5), 944–961. https://doi.org/10.1093/schbul/sbn070
Grothe, B., & Klump, G. M. (2000). Temporal processing in sensory systems. Current Opinion in Neurobiology, 10(4), 467–473. https://doi.org/10.1016/s0959-4388(00)00115-x
Gutfreund, Y., Yarom, Y., & Segev, I. (1995). Subthreshold oscillations and resonant frequency in guinea-pig cortical neurons: Physiology and modelling. Journal of Physiology, 483(3), 621–640. https://doi.org/10.1113/jphysiol.1995.sp020611
Hashemi, E., Ariza, J., Rogers, H., Noctor, S. C., & Martinez-Cerdeño, V. (2018). The number of parvalbumin-expressing interneurons is decreased in the prefrontal cortex in autism. Cerebral Cortex, 28(2), 690. https://doi.org/10.1093/cercor/bhx063
Hensch, T. K. (2005). Critical period plasticity in local cortical circuits. Nature Reviews. Neuroscience, 6(11), 877–888. https://doi.org/10.1038/nrn1787
Hutcheon, B., & Yarom, Y. (2000). Resonance, oscillation, and the intrinsic frequency preferences of neurons. Trends in Neurosciences, 23(5), 216–222. https://doi.org/10.1016/S0166-2236(00)01547-2
Hutsler, J. J., & Casanova, M. F. (2016). Review: Cortical construction in autism spectrum disorder: Columns, connectivity and the subplate. Neuropathology and Applied Neurobiology, 42(2), 115–134. https://doi.org/10.1111/nan.12227
Hyafil, A., Giraud, A. L., Fontolan, L., & Gutkin, B. (2015). Neural cross-frequency coupling: Connecting architectures, mechanisms, and functions. Trends in Neurosciences, 38(11), 725–740
Jensen, O., & Colgin, L. L. (2007). Cross-frequency coupling between neuronal oscillations. Trends in Cognitive Sciences, 11(7), 267–269
Kanizsa, G. (1976). Subjective contours. Scientific American, 234(4), 48–52. https://doi.org/10.1038/scientificamerican0476-48
Kann, O., Papageorgiou, I. E., & Draguhn, A. (2014). Highly energized inhibitory interneurons are a central element for information processing in cortical networks. Journal of Cerebral Blood Flow and Metabolism, 34(8), 1270–1282. https://doi.org/10.1038/jcbfm.2014.104
Klausberger, T., & Somogyi, P. (2008). Neuronal diversity and temporal dynamics: The unity of hippocampal circuit operations. Science, 321(5885), 53–57. https://doi.org/10.1126/science.1149381
Klomjau, W., Katz, R., & Lckmy-Vallée, A. (2015). Basic principles of transcranial magnetic stimulation (TMS) and repetitive TMS (rTMS). Annals of Physical and Rehabilitation Medicine, 58(4), 208–213
Kozel, F. A., Van Trees, K., Larson, V., Phillips, S., Hashimie, J., Gadbois, B., Johnson, S., Gallinati, J., Barrett, B., Toyinbo, P., Weisman, M., Centorino, M., Gibson, C. A., & Catalano, G. (2019). One hertz versus ten hertz repetitive TMS treatment of PTSD: A randomized clinical trial. Psychiatry Research, 273, 153–162. https://doi.org/10.1016/j.psychres.2019.01.004
LeCouteur, A., Lord, C., & Rutter, M. (2003). The autism diagnostic interview—revised (ADI-R). Western Psychological Services.
Lewis, D. A., Curley, A. A., Glusier, J. R., & Volk, D. W. (2012). Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends in Neurosciences, 35(1), 57–67. https://doi.org/10.1016/j.tins.2011.10.004
Lin, P. X., Wang, C. Y., & Wu, Z. X. (2019). Two-fold effects of inhibitory neurons on the onset of synchronization in Izhikevich neuronal networks. The European Physical Journal B, 92, 113. https://doi.org/10.1140/epjb/e2019-100009-2
Loo, C. K., & Mitchell, P. B. (2005). A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. Journal of Affective Disorders, 88(3), 255–267. https://doi.org/10.1016/j.jad.2005.08.001
Luu, P., Tucker, D. M., Englander, R., Lockfeld, A., Lutsep, H., & Oken, B. (2001). Localizing acute stroke-related EEG changes: Assessing the effects of spatial undersampling. Journal of Clinical Neurophysiology, 18(4), 302–317. https://doi.org/10.1097/00004691-200107000-00002
Lytton, W. W., & Sejnowski, T. J. (1991). Simulations of cortical pyramidal neurons synchronized by inhibitory interneurons. Journal of Neurophysiology, 66(3), 1059–1079. https://doi.org/10.1152/jn.1991.66.3.1059
Maeda, F., Keenan, J. P., Tormos, J. M., Topka, H., & Pascual-Leone, A. (2000). Modulation of corticospinal excitability by repetitive transcranial magnetic stimulation. Clinical Neurophysiology, 111(5), 800–805. https://doi.org/10.1016/s1388-2457(99)00323-5
Marin-Padilla, M. (2011). The human brain: Prenatal development and structure. Springer.
Mir-Moghtadaei, A., Caballero, R., Fried, P., Fox, M. D., Lee, K., Giacobbe, P., Daskalakis, Z. J., Blumberger, D. M., & Downar, J. (2015). Concordance between BeamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimulation, 8(5), 965–973. https://doi.org/10.1016/j.brs.2015.05.008
Mountcastle, V. B. (1998). Perceptual neuroscience: The cerebral cortex. Harvard University Press.
Nakamura, T., Matsumoto, J., Takamura, Y., Ishii, Y., Sasahara, M., Ono, T., & Nishijo, H. (2015). Relationships among parvalbumin-immunoreactive neuron density, phase-locked gamma oscillations, and autistic/schizophrenic symptoms in PDGFR-B knock-out and control mice. PLoS ONE, 10(3), 0119258. https://doi.org/10.1371/journal.pone.0119258
Oberman, L. M., Enticott, P. G., Casanova, M. F., Rotenberg, A., Pascual-Leone, A., & McCracken, J. T., TMS in ASD Consensus Group. (2016). Transcranial magnetic stimulation in autism spectrum disorder: ***Challenges, promise, and roadmap for future research. Autism Research, 9(2), 184–203. https://doi.org/10.1002/aur.1567
Pascual-Leone, A., Walsh, V., & Rothwell, J. (2000). Transcranial magnetic stimulation in cognitive neuroscience–Virtual lesion, chronometry, and functional connectivity. Current Opinion in Neurobiology, 10(2), 232–237. https://doi.org/10.1016/s0959-4388(00)00081-7
Pell, G. S., Roth, Y., & Zangen, A. (2011). Modulation of cortical excitability induced by repetitive transcranial magnetic stimulation: Influence of timing and geometrical parameters and underlying mechanisms. Progress in Neurobiology, 93(1), 59–98. https://doi.org/10.1016/j.pneurobio.2010.10.003
Pommier, B., Vassal, F., Boutet, C., Jeannin, S., Peyron, R., & Faillenot, I. (2017). Easy methods to make the neuronavigated targeting of DLPFC accurate and routinely accessible for rTMS. Neurophysiologie Clinique, 7(1), 35–46. https://doi.org/10.1016/j.neucli.2017.01.007
Richardson, J. T. E. (2011). Eta squared and partial eta squared as measures of effect size in educational research. Educational Research Review, 6(2), 135–147. https://doi.org/10.1016/j.edurev.2010.12.001
Rojas, D. C., & Wilson, L. B. (2014). Gamma-band abnormalities as markers of autism spectrum disorders. Biomarkers in Medicine, 8(3), 353–368. https://doi.org/10.2217/bmm.14.15
Rossignol, E. (2011). Genetics and function of neocortical GABAergic interneurons in neurodevelopmental disorders. Neural plasticity, 2011, 649325. https://doi.org/10.1155/2011/649325
Saunders, J. A., Tatard-Leitman, V. M., Suh, J., Billingslea, E. N., Roberts, T. P., & Siegel, S. J. (2013). Knockout of NMDA receptors in parvalbumin interneurons recreates autism-like phenotypes. Autism Research, 6(2), 69–77. https://doi.org/10.1002/aur.1264
Selimbeyoglu, A., Kim, C. K., Inoue, M., Lee, S. Y., Hong, A. S. O., Kauvar, I., Ramakrishnan, C., Fenno, L. E., Davidson, T. J., Wright, M., & Deisseroth, K. (2017). Modulation of prefrontal cortex excitation/inhibition balance rescues social behavior in CNTNAP2-deficient mice. Science Translational Medicine, 9(401), eaah6733. https://doi.org/10.1126/scitranslmed.aah6733
Silver, M. L., & Tiedemann, D. (1978). Dynamic geotechnical testing. ASTM International.
Sohal, V. S., Zhang, F., Yizhar, O., & Deisseroth, K. (2009). Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature, 459(7247), 698–702. https://doi.org/10.1038/nature07991
Sokhadze, E. M., Casanova, M. F., El-Baz, A. S., Farag, H. E., Li, X., & Wang, Y. (2016). TMS-based neuromodulation of evoked and induced gamma oscillations and event-related potentials in children with autism. NeuroRegulation, 3(3), 101–126. https://doi.org/10.15540/nr.3.3.101
Sokhadze, E. M., El-Baz, A., Baruth, J., Mathai, G., Sears, L., & Casanova, M. F. (2009). Effects of low frequency repetitive transcranial magnetic stimulation (rTMS) on gamma frequency oscillations and event-related potentials during processing of illusory figures in autism. Journal of Autism and Developmental Disorders, 39(4), 619–634. https://doi.org/10.1007/s10803-008-0662-7
Sokhadze, E. M., El-Baz, A. S., Sears, L. L., Opris, I., & Casanova, M. F. (2014). rTMS neuromodulation improves electrocortical functional measures of information processing and behavioral responses in autism. Frontiers in Systems Neuroscience, 8, 134. https://doi.org/10.3389/fnsys.2014.00134
Srinivasan, R., Tucker, D. M., & Murias, M. (1998). Estimating the spatial Nyquist of the human EEG. Behavior Research Methods, Instruments, & Computers, 30, 8–19. https://doi.org/10.3758/BF03209412
Tremblay, R., Lee, S., & Rudy, B. (2016). GABAergic interneurons in the neocortex: From cellular properties to circuits. Neuron, 91(2), 260–292. https://doi.org/10.1016/j.neuron.2016.06.033
Trickey, S. B., Adams, E. D., & Dufty, J. W. (Eds.). (1977). Quantum fluids and solids. Plenum Press.
Tukker, J. J., Fuentealba, P., Hartwich, K., Somogyi, P., & Klausberger, T. (2007). Cell type-specific tuning of hippocampal interneuron firing during gamma oscillations in vivo. Journal of Neuroscience, 27(31), 8184–8189. https://doi.org/10.1523/JNEUROSCI.1685-07.2007
Uzunova, G., Pallanti, S., & Hollander, E. (2016). Excitatory/inhibitory imbalance in autism spectrum disorders: implications for interventions and therapeutics. World Journal of Biological Psychiatry, 17(3), 174–186. https://doi.org/10.3109/15622975.2015.1085597
Volman, V., Behrens, M. M., & Sejnowski, T. J. (2011). Downregulation of parvalbumin at cortical GABA synapses reduced network gamma oscillatory activity. Journal of Neuroscience, 31(49), 18137–18148. https://doi.org/10.1523/JNEUROSCI.3041-11.2011
Wassermann, E. M., & Lisanby, S. H. (2001). Therapeutic application of repetitive transcranial magnetic stimulation: A review. Clinical Neurophysiology, 112(8), 1367–1377. https://doi.org/10.1016/s1388-2457(01)00585-5
Wassermann, E. M., Wedegaertner, F. R., Ziemann, U., George, M. S., & Chen, R. (1998). Crossed reduction of motor cortex excitability by 1 Hz transcranial magnetic stimulation. Neuroscience Letters, 250(3), 141–144. https://doi.org/10.1016/s0304-3940(98)00437-6
Wassermann, E. M., & Zimmermann, T. (2012). Transcranial magnetic stimulation: Therapeutic promises and scientific gaps. Pharmacology & Therapeutics, 133(1), 98–107. https://doi.org/10.1016/j.pharmthera.2011.09.003
Wechsler, D. (1999). Wechsler abbreviated scale of intelligence (WASI). Harcourt Assessment Inc.
Wechsler, D. (2003). Wechsler intelligence scale for children (4th ed.) (WISC-IV). Harcourt Assessment, Inc.
Wegiel, J., Kuchna, I., Nowicki, K., Imaki, H., Wegiel, J., Marchi, E., Ma, S. Y., Chauhan, A., Chauhan, V., Bobrowicz, T. W., de Leon, M., Saint Louis, L. A., Cohen, I. L., London, E., Brown, W. T., & Wisniewski, T. (2010). The neuropathology of autism: Defects of neurogenesis and neuronal migration, and dysplastic changes. Acta Neuropathologica, 119(6), 755–770. https://doi.org/10.1007/s00401-010-0655-4
Whittington, M. A., Traub, R. D., Kopell, N., Ermentrout, B., & Buhl, E. H. (2000). Inhibition-based rhythms: Experimental and mathematical observations on network dynamics. International Journal of Psychophysiology, 38(3), 315–336. https://doi.org/10.1016/s0167-8760(00)00173-2
Wöhr, M., Orduz, D., Gregory, P., Moreno, H., Khan, U., Vörckel, K. J., Wolfer, D. P., Welzl, H., Gall, D., Schiffmann, S. N., & Schwaller, B. (2015). Lack of parvalbumin in mice leads to behavioral deficits relevant to all human autism core symptoms and related neural morphofunctional abnormalities. Translational Psychiatry, 5(3), e525. https://doi.org/10.1038/tp.2015.19
Wong, F. K., Bercsenyi, K., Sreenivasan, V., Portales, A., Fernandez-Otero, M., & Marin, O. (2018). Pyramidal cell regulation of interneuron survival sculpts cortical networks. Nature, 557(7707), 668–6738. https://doi.org/10.1038/s41586-018-0139-6
Acknowledgements
The study was partially supported by National Institutes of Health Eureka R01 grant MH86784 to Manuel F. Casanova, M.D.
Author information
Authors and Affiliations
Contributions
All authors listed have made a substantial, direct, and intellectual contribution to the work, and approved it for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Ethical Approval
This human subject study was approved by the University of Louisville Institutional Review Board and was performed according with all required best practices for human subjects in the United States and in compliance with Helsinki Declaration.
Informed Consent
All participant and their parents gave signed informed consent/assent approved by the IRB prior to participation in this research study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Casanova, M.F., Shaban, M., Ghazal, M. et al. Ringing Decay of Gamma Oscillations and Transcranial Magnetic Stimulation Therapy in Autism Spectrum Disorder. Appl Psychophysiol Biofeedback 46, 161–173 (2021). https://doi.org/10.1007/s10484-021-09509-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-021-09509-z