Abstract
Laboratory stress tasks induce strong changes in linear and non-linear indices of heart rate variability (HRV) among healthy adults, due to a task-induced parasympathetic withdrawal. Previous findings suggested that negative affectivity and its correlates (i.e., depressive symptoms, anxiety, hostility, type D personality, and situational stress) could profoundly affect autonomic activity. However, to date no studies considered these psychological dimensions simultaneously while trying to disentangle their acute effects on HRV during a laboratory stress task. A total of 65 healthy participants completed a battery of questionnaires and later underwent a psychosocial stress protocol, which involves a stressful and a non-stressful mental arithmetic task, with the latter serving as a control condition for the former. During the entire procedure, autonomic activity was recorded through a portable ECG device. We analysed longitudinal changes in HRV indices using Mixed Models, taking into account respiration rates and the associations between psychophysiological variables through bivariate Pearson’s r (partial) correlation indices. We found significant changes in linear (e.g., HF power, RMSSD) and non-linear (e.g., Poincaré Plot and Correlation Dimension D2) HRV indices during the procedure, with the lowest point reached during the stressful mental arithmetic task. Interestingly, only depressive symptomatology was significantly and positively related to a higher resting-state HRV and to a blunted reactivity to the stress task, even after controlling for baseline values. Results suggest that healthy individuals with higher levels of depressive symptoms could experience atypical cardiovascular responses to stressful events: several speculative interpretations, considering autonomic, behavioral, and motivational dysregulations, are discussed.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
There is a wealth of evidence indicating that psychosocial stress can increase the odds of developing cardiovascular diseases (CVD), which are considered one of the leading causes of morbidity and mortality worldwide (Lagraauw et al. 2015). In particular, an exaggerated reactivity to a stressor could trigger acute cardiovascular events through activation of the Autonomic Nervous System (ANS; Carroll et al. 2012). Even if autonomic responses to laboratory stress tasks and the mediating effect of psychological factors on them have been widely explored in both patients with CVD and healthy adults in recent years, no consensus has been achieved.
Under optimal conditions, both sympathetic and parasympathetic branches of ANS are recruited by a central autonomic network to support the physiological and behavioural responses to any event that disrupts the homeostatic balance (i.e., the fight-or-flight reaction; Carroll et al. 2017). The complex interactions between the two branches of ANS can be described along two dimensions, namely coactivity and reciprocity (for a review of the bivariate “autonomic space model”, see Berntson et al. 2008). Thanks to these interactions, ANS can dynamically adapt itself to changing environmental circumstances by detecting and quickly responding to unpredictable stimuli, for example through an acute modulation of the heart’s rhythm (Acharya et al. 2006). A physiological marker of ANS is heart rate variability (HRV), defined as the heart’s variation over time of the beat-to-beat interval (Acharya et al. 2006). HRV has been extensively investigated using time- and frequency-domain analyses; the former are simple techniques of analysis applied straight to the series of successive RR interval values (e.g., means and standard deviations). Frequency-domain methods provide information on how the power of the time series distributes as a function of frequency (Task Force of the European Society of Cardiology 1996). For example, high frequencies of HRV (HF) are well-established indices of parasympathetic control over the cardiac tone (Task Force of the European Society of Cardiology 1996). Low frequencies (LF) seem to reflect the activity of the baroreflex in response to vasomotor tone (Heathers 2014), even if they were previously considered an index of sympathetic activity (Task Force of the European Society of Cardiology 1996). Finally, the LF ratio to HF (LF/HF) has long been considered a measure of cardiac sympatho-vagal balance, even if this interpretation has been disproven (Billman 2013). A third line of analyses examine the non-linear properties of HRV. Indeed, the chaotic behavior of the cardiovascular system leads to a complexity that can be proficiently investigated through theory of non-linear dynamic systems (Acharya et al. 2006; Sassi et al. 2015). To date, a number of indices have been developed, each capable of examining different features of nonlinear dynamics of heart rate; most commonly used are Detrended Fluctuation Analysis, Correlation Dimension and Sample Entropy (for a review, see Acharya et al. 2006; Sassi et al. 2015). Despite the fact that more than 70 metrics of HRV have been published to date (Quintana, Alvares and Heathers 2016), studies focusing on short-term recordings usually report a smaller number of indices, described in Table 1. Multiple similar measures of HRV are usually employed and examined at the same time to better describe the same phenomenon (e.g., a stress response) from slightly different point of views.
Not surprisingly, in healthy individuals acute stress responses are associated with a strong parasympathetic withdrawal (Brugnera et al. 2018; Castaldo et al. 2015). A recent study from our research group showed that verbal laboratory stressors mask the reduced vagal activity through altered respiration patterns imposed by speaking, thus suggesting that non-verbal stress tasks could be better suited for psychophysiological experiments on stress (Brugnera et al. 2018). For example, the Montreal Imaging Stress Task (MIST) is one of the most advanced and methodologically sound acute stress protocols, widely adopted in fMRI (Dedovic et al. 2005; Kogler et al. 2015) and HRV studies (Brugnera et al. 2018). MIST is a mental arithmetic stressor which does not require the participant to verbalize his responses. To the best of our knowledge only a few authors investigated vagal reactivity and changes in non-linear dynamics during this specific stress task, showing mixed results (see for example Brugnera et al. 2018; Janka et al. 2015; La Marca et al. 2011; Monge et al. 2014); thus, an in-depth investigation of cardiovascular responses to MIST is necessary.
Researchers have also examined the impact of psychological factors (such as personality traits, or the presence of mental disorders) on cardiovascular activity (Allgulander 2016; Fiedorowicz 2014). Trait anxiety and depression are well-known risk factors for the development and maintenance of CVD (Allgulander 2016; Fiedorowicz 2014). Potential mechanisms linking depression/anxiety and poor cardiovascular health include (i) an autonomic nervous system dysregulation, especially in response to stress, (ii) a hyperactivity of hypothalamic–pituitary–adrenal axis, (iii) chronic pro-inflammatory states, and (iv) an increasing incidence of other risk factors (e.g., smoking and obesity; Allgulander 2016; Fiedorowicz 2014).
Interestingly, patients with psychiatric illnesses (i.e., mood, anxiety and psychotic disorders) are more likely to show reduced resting-state HRV, compared to healthy controls (Alvares et al. 2016). Previous studies have also shown that specific psychological dimensions such as hostility (Vella and Friedman 2009) or type D personality (Bibbey et al. 2015; Martin et al. 2010), as well as situational stress (Dishman et al. 2000) and state anxiety (Dimitriev et al. 2016) seem to be associated with reduced vagal activity at rest, or dysregulated autonomic responses to stressors, which may in turn contribute to an increased cardiovascular disease risk. Type D (“Distressed”) individuals, for example, are characterised by the tendency to inhibit emotions in social situations and to experience high levels of negative emotions: as such, they show greater cardiovascular stress reactions during highly social evaluative threat conditions experienced in their everyday lives (Bibbey et al. 2015). Take together, all these dimensions could be conceptualized as separate aspects of negative affectivity (NA), a personality trait which is closely related to Neuroticism and that involves the stable tendency to experience negative emotions (Denollet 2013; Watson and Clark 1984). Negative affectivity is also linked to an increased risk of affective disorder and symptoms of somatic distress (Denollet 2013). Thus, the aforementioned findings seem to suggest that NA leads to impaired physiological responses at rest or during stressful conditions. Nevertheless, the debate is still open as other studies -recently reviewed by Hamilton and Alloy (2016)—have found mixed evidence, even if the hypothesis of reduced vagal activity at rest and blunted reactivity in current depression seem to be highly supported (Hamilton and Alloy 2016). These atypical responses could be mediated by a central motivational dysregulation (Carroll et al. 2017) or by the activation of a conservation-withdrawal pattern, that is a disengaging behaviour pattern rather than a fight-flight reaction (Buerki and Adler 2005). However, no studies systematically assessed the impact of different psychological states or traits at the same time on cardiovascular responses, thus making it difficult to understand which dimension most influence the individual’s autonomic reactivity to stress.
Finally, past works suggested that linear parameters are quite unreliable in effectively discerning patients with mental disorders and healthy individuals, as opposed to non-linear measures (de la Torre-Luque et al. 2016). Indeed, emotional disorders seem to be characterized by a loss of complexity in cardiac functioning (de la Torre-Luque et al. 2016). Conversely, healthy individuals usually show complex heart dynamics, which has been thought to reflect a higher ability to adapt to a changing environment (Valenza et al. 2015). Even so, few studies have investigated linkages between psychological distress and non-linear cardiac features of HRV (for a review see de la Torre-Luque et al. 2016).
Therefore, as part of a larger study (Brugnera et al. 2017) we aimed at investigating cardiovascular responses to an acute psychosocial stressor and their relations with a number of trait and state psychological dimensions on a sample of healthy individuals, using a computerized and standardized math task (i.e., MIST). In our first study we reported exclusively the main outcomes, namely the cortical haemodynamic responses during the procedure (which were evaluated through a two-channel portable Near-Infrared Spectroscopy device; see Brugnera et al. 2017 for details). We found that the activity of the bilateral frontopolar and dorsolateral Prefrontal Cortex significantly increased from baseline to the standardized stressful math task, suggesting that a stressful condition leads to higher cognitive efforts. In addition, in the previous study we reported delta changes in Heart Rate and in High Frequencies of HRV during the procedure, and the relations among all cortical, behavioural (i.e., MIST performances), and autonomic (i.e., HR and HF) indices with depressive symptoms and anxiety. Among these results, we found that those with stronger HR reactivity experienced higher NIRS right channel activity during the stressful task; moreover, anxiety or depressive symptoms were not associated with any of the cortical indices.
The aims of this study are twofold. First, we tested the hypothesis that time, frequency, and non-linear indices of HRV will significantly change from rest to control and experimental (i.e. stressful) conditions of MIST. Based on previous studies (Brugnera et al. 2018; Castaldo et al. 2015), we expected significant decreases in SDNN, RMSSD, HF and LF absolute power, Total power, SD1, SD2 and D2 correlation dimension from rest to the stressful condition; further, we expected significant increases in HR, Stress Index and Sample Entropy from rest to stress. To date, few studies examined non-linear cardiovascular dynamics during this specific task (see for example Brugnera et al. 2018), or compared autonomic responses during the control and the experimental conditions of MIST: thus, our purpose was to provide further evidence on this topic. Second, we sought to investigate the potential associations among several psychological traits and states, resting-state HRV and task-based reactivity to stressors, in order to gain further insights on the effect of psychiatric states on autonomic control. Based on preliminary findings (Brugnera et al. 2017), we expected significant, positive correlations between all psychological questionnaires and HRV indices both at rest and during the stress task.
Methods
Participants
A total of 65 Caucasian participants (52.3% females) with a mean age of 24.7 (SD = 3.9; range 18–35) years volunteered for the experiment. Participants were university students (69.2%) recruited from undergraduate and graduate courses at University of Bergamo (Italy), or self-referred adults (30.8%) which responded to media advertisements (e.g., websites) between February 2016 and April 2016. Their mean BMI was 21.88 kg/m2 (SD = 1.99; range 17–31). Twenty of the participants (30.9%) were lighter smokers (less than 25 cigarettes per day), 65 (100%) attended at least high school, 46 (70.8%) were regular coffee drinkers, and 48 (73.8%) consumed alcohol at least once per month. Six (9.2%) regularly used oral contraceptives.
Participants were asked to refrain from consuming caffeinated drinks and smoking at least 2 h prior to testing, and to refrain from exhaustive exercise at least 24 h before the test. All of them were right-handed, as assessed with the Italian version of Edinburgh Handedness Inventory (Salmaso and Longoni 1985). None of the participants was affected by neurological, severe psychiatric (e.g., psychotic disorders or substance abuse) or other medical (e.g. cardiological) illnesses as assessed by means of an ad-hoc semi-structured interview. The study was conducted in accordance with APA (2017) ethical standards for the treatment of human experimental volunteers; each participant provided written consent in compliance with the Declaration of Helsinki (World Medical Association 2013).
Measures and Instruments
Psychological Questionnaires
We adopted the Italian version of all the following questionnaires. Means, SD, and Internal Reliability coefficients for all scales are reported in Table 2, while correlations among questionnaires are reported in Supplementary Table S3.
Depressive Symptomatology
The Beck Depression Inventory II (BDI-II; Beck et al. 1996; Ghisi et al. 2006) is a self-report 21-item measure of depressive symptoms. Each item is rated on a 4-point Likert scale (0 to 3). Total score ranges from 0 to 63, with higher scores reflecting more severe symptoms. Scores above the cut-off of 14 suggest the presence of significant levels of depressive symptoms (14–19 = mild depression; 20–29 = moderate depression; ≥ 30 = severe depression; Beck et al. 1996).
State and Trait Anxiety
The State-Trait Anxiety Inventory (STAI; Pedrabissi and Santinello 1989; Spielberger et al. 1983) is a self-report 40-item measure used to assess both the presence and severity of propensity to be anxious (Trait scale) and current symptoms of anxiety (State scale). Each item is rated on a 4-point Likert scale (1 to 4). Total scores for both scales range from 20 to 80. Higher total scores represent higher state or trait anxiety severity.
Hostility
The Cook-Medley Hostility Scale (MMPI-II Ho; Hathaway et al. 1989; Pancheri et al. 1996) is a self-report 50-item measure of hostility. Each item is rated on a dichotomic scale (yes/no). Total scores range from 0 to 50, with higher scores reflecting higher levels of hostility.
Situational Stress
The Stress Rating Questionnaire (SRQ; Edwards et al. 2015) is a self-report 5-item questionnaire that assesses stress awareness. Current stress is rated on a 7-point Likert scale (1 to 7) on five bipolar dimensions (Calm to Nervous, Fearless to Fearful, Relaxed to Anxious, Unconcerned to Worried, and Comfortable to Tense). Total scores range from 5 to 35. Higher scores indicate higher self-reported state of stress. An Italian validation is currently underway: preliminary findings suggest a unidimensional factorial structure, with strong data fit.
Type D Personality
The Type D Scale-14 (DS14; Denollet 2005; Gremigni and Sommaruga 2005) is a self-report 14-item questionnaire that evaluates two core aspects of a distressed (D) personality, Negative affectivity (NA) and social inhibition (SI). It is comprised of two subscales, that evaluate the tendency to 1) experience negative emotions (NA subscale, 7 items) and to 2) inhibit self-expression in social interactions (SI subscale, 7 items). Items are rated on a 5-points Likert scale (0 to 4), with total scores for each subscale ranging from 0 to 28. Higher total subscale scores indicate higher levels of negative affectivity or social inhibition.
Montreal Imaging Stress Task (MIST)
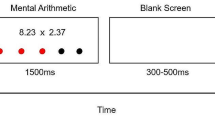
We used a computerized protocol based on the “Montreal Imaging Stress Task” (MIST; Dedovic et al. 2005) to induce psychosocial stress in participants. The protocol had four test conditions (training, control, experimental and rest). In the training session, participants were acquainted with the task, solving a series of arithmetic operations (sums, subtractions, and/or multiplications of three numbers) displayed on the computer screen. In addition, the time recorded during this training session was used to set the initial time limit for the experimental condition. During both control and experimental sessions, the same type of arithmetic operations were proposed, with the main difference between conditions being the addition of a time limit during the latter. Indeed, during the experimental condition the time limit was initially set using the mean time recorded during the training session. Afterwards, it was manipulated to be just beyond the individual’s “mental capacity”: after three correct responses the time limit decreased by 10%; on the contrary, after three incorrect responses the time limit increased by 10%, thus inducing a high failure rate (see Dedovic et al. 2005 for the details of this procedure). In addition, during the experimental condition several social evaluative threats were provided (e.g., participants were reminded that there is a required minimum performance, and that the average performance had to be close or equal to the average performance of all subjects). Finally, between each condition there was a resting phase, during which the interface of the computer program remained on the screen, but no arithmetic operations were shown. For each condition, MIST provides the mean response time (in milliseconds), the total number of correct responses and the percentage of correct over the total number of responses.
Following the MIST’s description provided in Dedovic et al. (2005), we internally-developed a Windows version of the software using LabVIEW version 9.0.1 (National Instruments Corp., Austin, TX, USA).
ECG Measurements
Electrocardiogram (ECG) was collected continuously throughout the protocol using “Pulse”, a wearable device with a sampling rate of 256 Hz, designed by STMicroelectronics and manufactured by MRandD (Italy). The device was fixed to the centre of the person’s chest using an elastic band, which contained the electrodes. This positioning corresponds to lead 1 of a standard 12-lead ECG. The Pulse sensor filtered the signal with a bandpass filter (0.05–40 Hz). The raw ECG signal (stored in a European Data File format; EDF+) was transferred to a portable computer through a Bluetooth© connection. The EDF file was passed to Kubios HRV software 3.1 (Tarvainen 2017), which was used for all HRV analyses. Kubios HRV applied an adaptive QRS detector algorithm to extract the beat-to-beat RR intervals from the ECG data. Later, the ECG of each participant was visually inspected to detect and correct artifacts (i.e., missing or extra beats); we additionally used the automatic correction algorithm provided by Kubios HRV 3.1 on all ECG samples. All data were included in the analyses thanks to their overall good quality (< 5% artifacts). Data were pre-processed using a smoothness priors based detrending approach, thus removing very low frequency components (< 0.04 Hz) of HRV (Tarvainen et al. 2002). An ECG-derived Respiration Rate (EDR) was computed from raw-ECG throughout the procedure via a built-in algorithm of Kubios HRV software: the algorithm examined the alterations of the amplitude of the R-peak caused by chest movements during each respiratory cycle. Under stationary conditions (i.e., short-term registrations), the EDR is considered a reliable index of respiratory rates (Tarvainen 2017).
Time and Frequency Domain Analyses of HRV
Regarding the time-domain analyses, four methods were applied to the raw RR data, namely mean HR, SDNN (standard deviation of normal to normal R–R intervals), RMSSD (the square root of the mean of the squares of the successive differences between adjacent NNs), and the Baevsky’s Stress Index. The latter was computed according to the guidelines (For more information, see Tarvainen 2017). From a physiological standpoint, RMSSD reflects vagal influences in cardiac control, even if it is partly affected by lower frequency fluctuations and basal HR (Berntson et al. 2005).
Regarding frequency-domain analyses, the interbeat interval time series were interpolated with a rate of 4 Hz using a cubic spline interpolation to have equidistantly sampled data for spectral analysis. The power spectral density (PSD) was calculated by means of a Fast Fourier Transform (FFT) using the Welch’s periodogram method, with a 150 s Hanning window and an overlap of 50%. The spectral components of interest, the Low Frequency (LF) and the High Frequency (HF), were evaluated in fixed frequency bands (LF = 0.04–0.15; HF = 0.15–0.40 Hz). HF is a vagally-mediated HRV index, strongly affected by respiration (Heathers 2014). On the contrary, LF reflects the activity of the baroreflex in response to vasomotor tone (Heathers 2014). Analyses were performed on the Total Power of HRV (i.e., the integration of the entire PSD region, 0.04–0.40 Hz), on absolute power values of HF (HFpow) and LF (LFpow) calculated with FFT, on HF and LF expressed in normalized units, and on ratio between LF and HF band powers (LF/HF).
Non-linear Analyses of HRV
In addition to the time- and frequency-domain methods, four non-linear methods were applied to the raw RR data. They were computed using Kubios HRV software (formulas for all indices can be found in Tarvainen 2017). (i) Poincaré plot is a scatter plot of the correlations between successive RR intervals. According to Sassi et al. (2015), this index seems to have little connection to the nonlinear dynamical systems theory. The graph can be quantitatively analysed using two measures (i.e., SD1 and SD2) that describe the short- and the long-term variability in the signal, respectively. Finally, SD1/SD2 is the ratio between the aforementioned two measures. (ii) Sample entropy (SampEn) is a measure of the randomness (i.e., unpredictability) and irregularity of the NN interval series. Lower values suggest an increased regularity and predictability of the signal (as usually happens during a sympathetic activation), whereas higher values suggest a more random time series (Sassi et al. 2015; Tarvainen 2017). SampEn was computed setting m (embedding dimensions) to a value of m = 2, and r (tolerance) to a fraction of the standard deviation of the RR data (r = 0.2 * SDNN). (iii) Correlation dimension (D2) is a measure of the geometry of the attractor approximating the fractal dimension, and it gives information on the minimum number of dynamic variables needed to model the underlying system. Correlation Dimension decreases when the autonomous balancing shifts towards sympathetic regulation (Dimitriev et al. 2016). We computed D2 by setting m (embedding dimensions) to a value of m = 10. (iv) Detrended fluctuation analysis (DFA) measures the fractal correlation properties (i.e., the self-similarity properties of HRV; Sassi et al. 2015) within the signal at different time scales, divided into short-term fluctuations (α1; modulated by the sympathetic system) and long-term fluctuations (α2; modulated by both sympathetic and vagal activity; Dimitriev et al. 2016). The short-term fluctuations slope was estimated within the range of 4–16 beats, and long-term fluctuations within 16-64 beats correspondingly.
Procedure
Participants were asked to complete a battery of questionnaires which measured Depressive Symptoms, Trait Anxiety, Hostility and Type D Personality the day before the experiment. In addition, self-report scales on State Anxiety and Situational Stress were administered before starting the procedure, together with a semi-structured demographic interview.
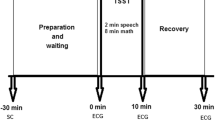
During the experiment, participants were seated in a comfortable chair, in front of a computer (approximately 60 cm from the screen), in a silent room with a comfortable temperature. They were instructed to limit movements of the body in order to reduce the amount of noise during ECG recording. After an adaptation time of 10 min, a 5 min rest period (rest) was followed by the math task (MIST) designed to induce moderate psychosocial stress: MIST’s training phase lasted approximately 3 min, whilst control and experimental conditions lasted 5 min. In addition, their order of presentation was randomized across participants. After each MIST condition, we administered questionnaires on state anxiety and situational stress as a manipulation check. An experimenter remained in the same room for the entire duration of the task. At the end of the experiment, all participants were debriefed on the purpose of the study.
ECG as well as cortical haemodynamic responses were obtained during the entire procedure (see Brugnera et al. 2017 for a discussion of the latter findings).
Statistical Analyses
According to a previous publication (Quintana 2017), 61 participants were required to detect medium effect sizes on vagally mediated HRV measures (α = .05; β = .80). Hence, our study had adequate power.
In order to test hypothesis 1 of a significant change of HRV indices across the conditions, we used Linear Mixed Models on the repeated measurements of each index nested within each participant, considering the order of randomization and controlling for respiration rates. In all LMM analyses, Time and Order of Randomization task were treated as fixed effects, while the covariate (Respiration Rates) was treated as a random effect. The covariance matrix was modelled without restrictions (i.e., unstructured): parameters were estimated using Restricted Maximum Likelihood. We tested other models (e.g., different covariance matrices), which all had a worse fit of the data according to the Akaike’s Information Criterion (AIC) test. Bonferroni corrected pairwise comparisons of estimated marginal means were used as post hoc tests.
Finally, in order to investigate hypothesis 2 of significant correlations among psychological questionnaires scores and HRV indices during i) rest and ii) MIST Experimental condition, we used Pearson correlation coefficients and partial correlation coefficients (controlling for HRV indices at rest), respectively. Effect sizes were interpreted according to guidelines (small = r > 0.1; medium = r > 0.3; large = r > 0.5; Cohen 1977).
We controlled for the inflation of Type I error using the Benjamini–Hochberg Procedure for False Discovery Rate (Benjamini and Hochberg 2000) on all the resulting p values. All statistical analyses were performed using Statistical Package for the Social Sciences (SPSS) version 25.0. All statistical tests were two-sided; a p value ≤ .05 was considered significant.
Results
Preliminary Analyses
Normality was assessed for each variable by examining skewness and kurtosis values. SDNN and BDI-II were slightly positively skewed: a square root transformation corrected the non-normality. RMSSD, Stress Index, HFpow, LFpow, LF/HF ratio, SD1 and SD2 were moderately positively skewed: a natural-log transformation corrected the non-normality. The transformed variables were used for analyses (Tabachnick and Fidell 2007). Non-transformed variables can be found in Supplementary Table S1. Standardized scores and box plots were used to identify univariate outliers. One subject had few out-of-range HRV values during MIST Experimental, which were took into range as suggested by Tabachnick and Fidell (2007). In 5 cases the EDR fell outside the HF bands during one condition: therefore, we manually updated the HF bands to include the respiratory frequency. Sensitivity analyses on smoking status showed non-significant differences compared to results obtained on the complete sample. We additionally tested the effect of gender on HRV indices. Women reported significantly lower levels of HRV during the entire procedure compared to men, even if the direction of effects during the experiment resembled that of the entire sample (i.e., stress led to vagal withdrawal). Means, SD, t and p values during each condition for both groups (M and F) are reported in Supplementary Table S2. Finally, according to BDI-II cut-off scores a total of 11 participants were classified as depressed (6 with mild depression, 4 with moderate depression, and 1 with severe depression). Independent sample t-tests or ANCOVAs between those classified as depressed and those classified as not depressed showed non-significant group differences on (i) STAI and SRQ evaluated after the two MIST tasks, and on (ii) response times, number and percentage of correct responses during both control and experimental conditions of MIST (data not shown). Correlational analyses among BDI-II Total scores and the aforementioned dimensions showed similar, non-significant results (data not shown). Findings suggest that depressive symptoms did not influence task involvement or behavioural performances during the experiment.
Acute Changes in HRV Indices During the Experiment
Fixed effects of Linear Mixed Models are reported in Table 3, while estimated marginal means, SE and Bonferroni’s corrected post-hocs are reported in Table 4. Post-Hocs demonstrated that time (HR, SDNN, RMSSD and Stress Index) and frequency (Total Power, HFpow, and LFpow) domain indices were significantly different from Rest to Control and Experimental conditions, with lowest\highest point reached during the latter condition. A number of non-linear indices (SD1, SD2, D2) evidenced a similar change pattern as above, with lowest point reached during the Experimental condition. Differences among HFnu, LFnu and LF/HF ratio’s means during the 3 conditions were not statistically significant. Post-hocs on Sample Entropy index evidenced significant increases from rest to MIST Control and Experimental conditions. Post-hocs on DFA α1 showed a significant difference only between Rest and Control condition, with the lowest point reached during the latter. Finally, post-hocs on DFA α2 evidenced that the highest point was reached during Experimental condition, while no differences were found between Rest and Control conditions. Taken together, results suggest that the stressful condition induced a strong parasympathetic withdrawal.
Correlational Analyses Between Psychological Variables and HRV Indices
Correlation analyses indices are reported in Table 5. After the Benjamini–Hochberg Procedure, BDI-II was the only scale still significantly correlated with time domain, frequency domain, and non-linear indices during Rest (see Fig. 1), with small to medium effects. Partial correlations (controlling for HRV values at rest; pr) evidenced that BDI-II was also significantly and positively associated with the following HRV indices during MIST Experimental condition: SDNN (pr = .28; p = .029), TotPow (pr = .28; p = .027) and HFpow (pr = .26; p = .040), with small effects. Results suggest that those with higher self-reported levels of depressive symptoms had a higher HRV at Rest and reduced parasympathetic withdrawal during the stressful condition. Interestingly, most of the psychological variables were significantly and positively correlated between them (see Supplementary Table S3), suggesting that they referred to a latent second-order factor, which could be called negative affectivity, general psychopathology or neuroticism (Denollet 2013; Watson and Clark 1984).
Discussion
The present study investigated the impact of several state and trait psychological dimensions on cardiovascular responses in a sample of healthy adults during a psychosocial stress task.
In regards to hypothesis 1, we found that MIST induced a strong parasympathetic withdrawal, in accordance with recent findings (Brugnera et al. 2018). This controlled non-verbal protocol allowed us to evaluate if cardiovascular responses changed from a resting condition, to a mental arithmetic task (the control condition) and to a time-limited, social-pressuring stressful task (the experimental condition). It is worth noting that several previous studies adopted unstandardized, non-controlled but more ecological stress protocols (such as academic examination or speech tasks), which sometimes fail to demonstrate significant autonomic changes from rest (for a review see Castaldo et al. 2015; Schubert et al. 2009). Brugnera et al. (2018) suggested that during verbal stress tasks the vagal withdrawal is probably masked by the acute effect of speaking on HRV. In accordance with this hypothesis, the current findings evidenced that most of the linear and non-linear HRV indices changed significantly from baseline to control and experimental conditions of MIST (see Table 4) even after controlling for respiratory frequency, thus providing further support for the validity of this protocol during psychophysiological studies on stress. In addition, to the best of our knowledge this is one of the first studies to evidence significant differences in autonomic activity between a simple math task, and a properly defined stressful condition.
With regards to the HRV indices, frequency- and time-domain analyses showed a decrease in RMSSD and HRV power at both high and low frequencies, reaching the lowest point during the experimental condition. LF/HF changes over time were not significant, since absolute powers of HF and LF decreased in a similar way during the experiment (see Table 3). Results were in accordance with recent work on stress (Brugnera et al. 2018; Dimitriev et al. 2016); thus, a properly defined stress task leads to a decreased activity of the baroreflex in response to vasomotor tone (which is indexed by LF; Heathers 2014), and to a strong vagal withdrawal. Interestingly, the meta-analysis of Castaldo et al. (2015) on stress responses in healthy adults evidenced slightly different findings, with the majority of the reviewed studies reporting increases in LF/HF and LF during stress. Authors concluded that discrepancies between results were attributable to the protocols used (i.e. stress tasks requesting physical movements were associated with decreases in LF), or -as recently hypothesized by Brugnera et al. (2018)—to the act of speaking.
Non-linear analyses showed a decrease in the complexity of HRV over time. The downtrend in SD1 and SD2 values of Poincaré plot suggested a reduction in short and long-term variability of HRV signal due to stress, which is in accordance with the time-domain analyses and with previous findings (Brugnera et al. 2018). DFA α1 and α2 indices, measures of the short- and long-term correlation within the signal (Sassi et al. 2015), showed opposite trends. A1 decreased significantly only from rest to the control condition, which indicates a decrease in short-term correlations probably due to parasympathetic withdrawal (Dimitriev et al. 2016; Tarvainen 2017). On the other hand, α2 values increased only during the experimental (i.e. stressful) condition in respect to the baseline and the control condition, indicating a long-term increase in fractal-like correlation properties of the heart period (Tarvainen 2017). Few other studies investigated detrended fluctuation analyses during stress in healthy adults, showing mixed results (Brugnera et al. 2018; Dimitriev et al. 2016; Melillo et al. 2011; Vuksanovic and Gal 2007).
Correlation dimension D2 and Sample Entropy showed opposite results. D2, which refers to the number of dimensions/variables needed to reconstruct the phase space of the system under study (Sassi et al. 2015), showed a decreasing trend from baseline to the experimental condition, indicating a progressive reduction of the signal complexity from rest to stress. These results were in accordance with past literature on HRV in healthy adults during acute stress (see for example Brugnera et al. 2018; Dimitriev et al. 2016; Melillo et al. 2011; Schubert et al. 2009). Interestingly, Sample entropy (SampEn), which is a measure of irregularity of the signal commonly used to describe how chaotic the system is and its rate of information production (Sassi et al. 2015), significantly increased from baseline to both conditions, thus failing to discriminate between different states of arousal. Results were opposite to those found in previous works with verbal stress tasks (Dimitriev et al. 2016; Vuksanovic and Gal 2007), and similar to those reported by Brugnera et al. (2018) using MIST. As such, the current results strengthen the hypothesis advanced by Brugnera et al. (2018) that Sample Entropy could counter-intuitively increase during MIST due to respiratory-induced low-frequency regularities in the RR time-series induced by this specific task.
Regarding hypothesis 2, after correcting p values for the inflation of Type-I error, we found that depressive symptomatology was the only psychological dimension significantly and positively correlated with higher resting-state HRV, and with a blunted reactivity to the stress task. Interestingly, significant correlations were present among most psychological questionnaires (see Supplementary Table S3), suggesting the presence of a latent second-order factor definable as “negative affectivity”, “general psychopathology” or “neuroticism” (see for example Ormel et al. 2004). Our findings suggest that depressive symptoms could affect the cardiovascular responses of healthy adults, rather than other personality variables (i.e., Hostility, Type D Personality, Negative Affectivity, Trait Anxiety) or psychological states (Situational Stress and State Anxiety).
With regards to results at rest, we found that higher levels of depressive symptoms were positively correlated with a lower HR and LF\HF ratio, and higher RMSSD and HF; that is, in our sample of healthy individuals the depressive symptoms were associated with a parasympathetic activation before performing a stress task. In addition, BDI-II correlated positively with Poincaré plot SD1 and correlation dimension D2, and negatively with SD1\SD2 and DFA α1. That is, higher levels of depressive symptomatology were associated with (i) an increase in the dimensional complexity and in short-term variability of HRV signal, and with (ii) a decrease in the short-term correlations within the signal, before performing the task (Sassi et al. 2015). Thus, depressive symptomatology seems to be linked to a state of hypoarousal before engaging in a stress task, at least in our sample of healthy adults. These findings are of particular interest given that psychiatric patients with a current diagnosis of mood, anxious or psychotic disorders exhibit a reduced heart rate variability at rest (e.g., reduced HF and Entropy; Chalmers et al. 2014; de la Torre-Luque et al. 2016; Kemp et al. 2010; Quintana et al. 2016) compared to healthy controls.
In addition, partial correlations between depressive symptoms and HRV indices during stress showed small but significant positive associations with HF, SDNN and Total Power of HRV, thus suggesting a blunted reactivity while facing a stressor among those who reported higher levels of depressive symptomatology. The current results add further evidence to previous meta-analyses (Chida and Hamer 2008) and reviews (Carroll et al. 2017; Hamilton and Alloy 2016), which showed that individuals with high levels of depressive symptoms or anxiety may exhibit atypical patterns of cardiovascular responses to stress tasks, including blunted reactivity.
Our findings could be explained considering a central motivational dysregulation and by a reduced adaptability to environmental stimuli induced by high levels of depressive symptoms. For example, Hu et al. (2016) suggested that depressed patients experience chronic difficulties in reaching goals, which leads to a lower motivation to perform well and therefore to a blunted reactivity during cognitive tasks. One could also argue that individuals with higher levels of psychological symptoms are less motivated or have a reduced anticipatory anxiety before engaging in a stress task. Indeed, Carroll et al. (2017) advanced the hypothesis that in some individuals early childhood adversities and genetic inheritance could induce a sub-optimal functioning of specific brain areas associated with motivation and neuroendocrine\autonomic regulation (i.e., anterior cingulate cortex, medial prefrontal cortex, insula, hippocampus, and amygdala). This cerebral dysregulation is subsequently linked to both blunted physiological responses to stressors and to adverse behavioural phenotypes which includes depression (Carroll et al. 2017). Another possible interpretation for the decreased cardiovascular reactivity to the stress task takes into account a reduced task involvement in participants with higher levels of depressive symptoms: however, preliminary analyses suggested that those who exceeded the BDI-II cut-off scores had similar reaction times, number and percentage of correct responses during both conditions, and similar levels of self-perceived situational stress and state anxiety after the experimental phase. Thus, in our sample the level of involvement was unrelated to depressive symptoms. Other explanatory hypotheses consider (i) the desensitization processes and (ii) the so-called conservation–withdrawal reaction pattern of disengagement. The former hypothesis was proposed by Yu et al. (2008), which reported that healthy individuals in a state of anxiety experience a progressive desensitization of β-adrenergic receptors. High and chronic states of depression, anxiety, and stress (i.e., psychological distress) could induce a chronic hyper-activation of the sympathetic nervous system, a reduction in the sensitivity of β-adrenergic receptors and, then, an increased vagal tone and signals complexity at rest and during a stress task. Finally, the latter hypothesis suggests that individuals with high levels of an aversive coping style, called conservation–withdrawal reaction pattern of disengagement (Bosch et al. 2001), could experience a reaction pattern characterized by an enhanced parasympathetic tone during stress tasks (Kreibig et al. 2007). We suppose that depressive symptoms could be positively associated with this specific coping style, even if further studies are necessary.
This study has some limitations. First, we did not implement a recovery condition after stress, due to the fact that we were mainly interested in psychophysiological responses at rest and in response to stressors, and to their relations with psychological traits and states. Recovery measures the capacity of the cardiovascular system to reverse the vagal withdrawal that occurs during a task, and it seems to be impaired in individuals with emotional disorders (Taylor 2010). Second, we included only one type of stressor (mental arithmetic task), even if the addition of physiological ones (i.e. pain induced by a cold pressure task) could have provided a better insight into participants’ stress responses (Kogler et al. 2015). Third, our sample was composed only by educated Italian participants of Caucasian ethnicity, so our findings may not be generalized to individuals with a lower socio-economic status or from other countries, cultures and ethnicities. Because of the correlational nature of our data no causal inferences on negative affectivity and impaired physiological regulation could be drawn, especially considering that most of the participants had a relatively low depressive symptomatology. Finally, during our experiment we only investigated heart rate variability, without examining specific indices of sympathetic activity, such as left ventricular ejection fraction, or electrodermal activity.
Concluding, it is well known that exaggerated cardiovascular reactivity to acute psychological stressors are implicated in cardiovascular diseases (Carroll et al. 2017; Lagraauw et al. 2015). However, an increasing body of literature suggests that blunted autonomic responses to stress might also be associated with obesity and other negative health or psychological outcomes (Carroll et al. 2017). Our results add to the literature that healthy individuals with high levels of depressive symptoms could also experience a higher resting-state HRV, in addition to blunted responses to stress. Carroll and colleagues (2017) provided an interesting explanatory framework, suggesting that a specific fronto-limbic network normally involved in motivational regulation, adaptation to environmental stimuli and autonomic regulation, is hypoactivated in some individuals (e.g., those with depression), leading to suboptimal functioning during acute stress exposures. Due to the paucity of findings in this area, future research is needed to better understand the individual psychophysiological differences in resting-state and task-induced changes in HRV and if these atypical cardiovascular responses are associated with specific health-related outcomes among individuals with high levels of depressive symptoms.
References
Acharya, R. U., Joseph, P. K., Kannathal, N., Lim, C. M., & Suri, J. S. (2006). Heart rate variability: A review. Medical & Biological Engineering & Computing, 44(12), 1031–1051. https://doi.org/10.1007/s11517-006-0119-0.
Allgulander, C. (2016). Anxiety as a risk factor in cardiovascular disease. Current Opinion in Psychiatry, 29(1), 13–17. https://doi.org/10.1097/YCO.0000000000000217.
Alvares, G. A., Quintana, D. S., Hickie, I. B., & Guastella, A. J. (2016). Autonomic nervous system dysfunction in psychiatric disorders and the impact of psychotropic medications: A systematic review and meta-analysis. Journal of Psychiatry and Neuroscience, 41(2), 89–104.
American Psychological Association. (2017). Ethical principles of psychologists and code of conduct. (2002, Amended June 1, 2010 and January 1, 2017). Retrieved from http://www.apa.org/ethics/code/index.aspx.
Beck, A. T., Steer, R. A., & Brown, G. K. (1996). BDI-II: Beck depression inventory manual. San Antonio: Psychological Corporation.
Benjamini, Y., & Hochberg, Y. (2000). On the adaptive control of the false discovery rate in multiple testing with independent statistics. Journal of Educational and Behavioral Statistics, 25(1), 60–83. https://doi.org/10.3102/10769986025001060.
Berntson, G. G., Lozano, D. L., & Chen, Y.-J. (2005). Filter properties of root mean square successive difference (RMSSD) for heart rate. Psychophysiology, 42(2), 246–252. https://doi.org/10.1111/j.1469-8986.2005.00277.x.
Berntson, G. G., Norman, G. J., Hawkley, L. C., & Cacioppo, J. T. (2008). Cardiac autonomic balance versus cardiac regulatory capacity. Psychophysiology, 45(4), 643–652. https://doi.org/10.1111/j.1469-8986.2008.00652.x.
Bibbey, A., Carroll, D., Ginty, A. T., & Phillips, A. C. (2015). Cardiovascular and cortisol reactions to acute psychological stress under conditions of high versus low social evaluative threat: Associations with the Type D personality construct. Psychosomatic Medicine, 77(5), 599–608. https://doi.org/10.1097/psy.0000000000000194.
Billman, G. (2013). The LF/HF ratio does not accurately measure cardiac sympatho-vagal balance. Frontiers in Physiology, 4, 26. https://doi.org/10.3389/fphys.2013.00026.
Bosch, J. A., de Geus, E. J., Kelder, A., Veerman, E. C., Hoogstraten, J., & Amerongen, A. V. (2001). Differential effects of active versus passive coping on secretory immunity. Psychophysiology, 38(5), 836–846.
Brugnera, A., Zarbo, C., Adorni, R., Tasca, G. A., Rabboni, M., Bondi, E., et al. (2017). Cortical and cardiovascular responses to acute stressors and their relations with psychological distress. International Journal of Psychophysiology, 114, 38–46. https://doi.org/10.1016/j.ijpsycho.2017.02.002.
Brugnera, A., Zarbo, C., Tarvainen, M. P., Marchettini, P., Adorni, R., & Compare, A. (2018). Heart rate variability during acute psychosocial stress: A randomized cross-over trial of verbal and non-verbal laboratory stressors. International Journal of Psychophysiology, 127, 17–25.
Buerki, S., & Adler, R. H. (2005). Negative affect states and cardiovascular disorders: A review and the proposal of a unifying biopsychosocial concept. General Hospital Psychiatry, 27(3), 180–188. https://doi.org/10.1016/j.genhosppsych.2004.12.003.
Carroll, D., Ginty, A. T., Der, G., Hunt, K., Benzeval, M., & Phillips, A. C. (2012). Increased blood pressure reactions to acute mental stress are associated with 16-year cardiovascular disease mortality. Psychophysiology, 49(10), 1444–1448. https://doi.org/10.1111/j.1469-8986.2012.01463.x.
Carroll, D., Ginty, A. T., Whittaker, A. C., Lovallo, W. R., & de Rooij, S. R. (2017). The behavioural, cognitive, and neural corollaries of blunted cardiovascular and cortisol reactions to acute psychological stress. Neuroscience and Biobehavioral Reviews, 77, 74–86. https://doi.org/10.1016/j.neubiorev.2017.02.025.
Castaldo, R., Melillo, P., Bracale, U., Caserta, M., Triassi, M., & Pecchia, L. (2015). Acute mental stress assessment via short term HRV analysis in healthy adults: A systematic review with meta-analysis. Biomedical Signal Processing and Control, 18, 370–377. https://doi.org/10.1016/j.bspc.2015.02.012.
Chalmers, J. A., Quintana, D. S., Abbott, M. J., & Kemp, A. H. (2014). Anxiety disorders are associated with reduced heart rate variability: A meta-analysis. Frontiers in Psychiatry, 5, 80. https://doi.org/10.3389/fpsyt.2014.00080.
Chida, Y., & Hamer, M. (2008). Chronic psychosocial factors and acute physiological responses to laboratory-induced stress in healthy populations: A quantitative review of 30 years of investigations. Psychological Bulletin, 134(6), 829–885. https://doi.org/10.1037/a0013342.
Cohen, J. (1977). Statistical power analysis for the behavioral sciences (revised ed.), New York: Academic Press.
de la Torre-Luque, A., Bornas, X., Balle, M., & Fiol-Veny, A. (2016). Complexity and nonlinear biomarkers in emotional disorders: A meta-analytic study. Neuroscience and Biobehavioral Reviews, 68, 410–422. https://doi.org/10.1016/j.neubiorev.2016.05.023.
Dedovic, K., Renwick, R., Mahani, N. K., Engert, V., Lupien, S. J., & Pruessner, J. C. (2005). The Montreal Imaging Stress Task: Using functional imaging to investigate the effects of perceiving and processing psychosocial stress in the human brain. Journal of Psychiatry and Neuroscience, 30(5), 319–325.
Denollet, J. (2005). DS14: Standard assessment of negative affectivity, social inhibition, and Type D personality. Psychosomatic Medicine, 67(1), 89–97.
Denollet, J. (2013). Negative Affectivity. In M. D. Gellman & J. R. Turner (Eds.), Encyclopedia of behavioral medicine (pp. 1304–1305). New York, NY: Springer.
Dimitriev, D. A., Saperova, E. V., & Dimitriev, A. D. (2016). State anxiety and nonlinear dynamics of heart rate variability in students. PLoS ONE, 11(1), e0146131. https://doi.org/10.1371/journal.pone.0146131.
Dishman, R. K., Nakamura, Y., Garcia, M. E., Thompson, R. W., Dunn, A. L., & Blair, S. N. (2000). Heart rate variability, trait anxiety, and perceived stress among physically fit men and women. International Journal of Psychophysiology, 37(2), 121–133. https://doi.org/10.1016/S0167-8760(00)00085-4.
Edwards, E. J., Edwards, M. S., & Lyvers, M. (2015). Cognitive trait anxiety, situational stress, and mental effort predict shifting efficiency: Implications for attentional control theory. Emotion, 15(3), 350–359. https://doi.org/10.1037/emo0000051.
Fiedorowicz, J. G. (2014). Depression and cardiovascular disease: An update on how course of illness may influence risk. Current Psychiatry Reports, 16(10), 492. https://doi.org/10.1007/s11920-014-0492-6.
Ghisi, M., Flebus, G. B., Montano, A., Sanavio, E., & Sica, C. (2006). Manuale del BDI-II Beck Depression Inventory-II, Adattamento italiano [BDI-II, Beck Depression Inventory-II Manual, Italian adaptation]. Firenze, Italy: Giunti OS.
Gremigni, P., & Sommaruga, M. (2005). Personalità di tipo D, un costrutto rilevante in cardiologia. Studio preliminare di validazione del questionario italiano. Psicoterapia Cognitiva e Comportamentale, 11(1), 7–18.
Hamilton, J. L., & Alloy, L. B. (2016). Atypical reactivity of heart rate variability to stress and depression across development: Systematic review of the literature and directions for future research. Clinical Psychology Review, 50, 67–79. https://doi.org/10.1016/j.cpr.2016.09.003.
Hathaway, S. R., McKinley, J. C., & Committee, M. R. (1989). MMPI-2: Minnesota multiphasic personality inventory-2: Manual for administration and scoring. Minneapolis: University of Minnesota Press.
Heathers, J. A. (2014). Everything Hertz: Methodological issues in short-term frequency-domain HRV. Frontiers in Physiology, 5, 177. https://doi.org/10.3389/fphys.2014.00177.
Hu, M. X., Lamers, F., De Geus, E. J. C., & Penninx, B. W. J. H. (2016). Differential autonomic nervous system reactivity in depression and anxiety during stress depending on type of stressor. Psychosomatic Medicine, 78(5), 562–572. https://doi.org/10.1097/PSY.0000000000000313.
Janka, A., Adler, C., Fischer, L., Perakakis, P., Guerra, P., & Duschek, S. (2015). Stress in crisis managers: evidence from self-report and psychophysiological assessments. Journal of Behavioral Medicine, 38(6), 970–983. https://doi.org/10.1007/s10865-015-9654-1.
Kemp, A. H., Quintana, D. S., Gray, M. A., Felmingham, K. L., Brown, K., & Gatt, J. M. (2010). Impact of depression and antidepressant treatment on heart rate variability: A review and meta-analysis. Biological Psychiatry, 67(11), 1067–1074. https://doi.org/10.1016/j.biopsych.2009.12.012.
Kogler, L., Muller, V. I., Chang, A., Eickhoff, S. B., Fox, P. T., Gur, R. C., et al. (2015). Psychosocial versus physiological stress—Meta-analyses on deactivations and activations of the neural correlates of stress reactions. Neuroimage, 119, 235–251. https://doi.org/10.1016/j.neuroimage.2015.06.059.
Kreibig, S. D., Wilhelm, F. H., Roth, W. T., & Gross, J. J. (2007). Cardiovascular, electrodermal, and respiratory response patterns to fear- and sadness-inducing films. Psychophysiology, 44(5), 787–806. https://doi.org/10.1111/j.1469-8986.2007.00550.x.
La Marca, R., Waldvogel, P., Thörn, H., Tripod, M., Wirtz, P. H., Pruessner, J. C., et al. (2011). Association between cold face test-induced vagal inhibition and cortisol response to acute stress. Psychophysiology, 48(3), 420–429.
Lagraauw, H. M., Kuiper, J., & Bot, I. (2015). Acute and chronic psychological stress as risk factors for cardiovascular disease: Insights gained from epidemiological, clinical and experimental studies. Brain, Behavior, and Immunity, 50, 18–30. https://doi.org/10.1016/j.bbi.2015.08.007.
Martin, L. A., Doster, J. A., Critelli, J. W., Lambert, P. L., Purdum, M., Powers, C., et al. (2010). Ethnicity and Type D personality as predictors of heart rate variability. International Journal of Psychophysiology, 76(2), 118–121. https://doi.org/10.1016/j.ijpsycho.2010.03.001.
Melillo, P., Bracale, M., & Pecchia, L. (2011). Nonlinear Heart Rate Variability features for real-life stress detection. Case study: Students under stress due to university examination. Biomedical Engineering Online, 10, 96. https://doi.org/10.1186/1475-925x-10-96.
Monge, M. M., Gomez, I., & Molina, A. (2014). Robustness of parameters from heart rate for mental stress detection. Experimental & Clinical Cardiology, 20(8), 3509–3517.
Ormel, J., Rosmalen, J., & Farmer, A. (2004). Neuroticism: A non-informative marker of vulnerability to psychopathology. Social Psychiatry and Psychiatric Epidemiology, 39(11), 906–912. https://doi.org/10.1007/s00127-004-0873-y.
Pancheri, P., Sirigatti, S., & Biondi, M. (1996). Adaptation of the MMPI-2 in Italy. In J. N. Butcher (Ed.), International adaptations of the MMPI-2: Research and clinical applications (pp. 416–441). Minneapolis, MN: University of Minnesota Press.
Pedrabissi, L., & Santinello, M. (1989). Inventario per l’ansia di « Stato » e di « Tratto » : nuova versione italiana dello STAI Forma Y: Manuale. Firenze, Italy: Giunti OS.
Quintana, D. S. (2017). Statistical considerations for reporting and planning heart rate variability case-control studies. Psychophysiology, 54(3), 344–349. https://doi.org/10.1111/psyp.12798.
Quintana, D. S., Alvares, G. A., & Heathers, J. A. J. (2016a). Guidelines for reporting articles on psychiatry and heart rate variability (GRAPH): Recommendations to advance research communication. Translational Psychiatry, 6, e803. https://doi.org/10.1038/tp.2016.73.
Quintana, D. S., Westlye, L. T., Kaufmann, T., Rustan, Ø., Brandt, C. L., Haatveit, B., et al. (2016b). Reduced heart rate variability in schizophrenia and bipolar disorder compared to healthy controls. Acta Psychiatrica Scandinavica, 133(1), 44–52. https://doi.org/10.1111/acps.12498.
Salmaso, D., & Longoni, A. M. (1985). Problems in the assessment of hand preference. Cortex, 21(4), 533–549.
Sassi, R., Cerutti, S., Lombardi, F., Malik, M., Huikuri, H. V., Peng, C.-K., et al. (2015). Advances in heart rate variability signal analysis: joint position statement by the e-Cardiology ESC Working Group and the European Heart Rhythm Association co-endorsed by the Asia Pacific Heart Rhythm Society. EP Europace, 17(9), 1341–1353. https://doi.org/10.1093/europace/euv015.
Schubert, C., Lambertz, M., Nelesen, R. A., Bardwell, W., Choi, J. B., & Dimsdale, J. E. (2009). Effects of stress on heart rate complexity–A comparison between short-term and chronic stress. Biological Psychology, 80(3), 325–332. https://doi.org/10.1016/j.biopsycho.2008.11.005.
Spielberger, C. D., Gorsuch, R. L., Lushene, R., Vagg, P. R., & Jacobs, G. A. (1983). Stait-Trait Anxiety Inventory (Form Y). CA: Palo Alto.
Tabachnick, B. G., & Fidell, L. S. (2007). Using multivariate statistics (5th (ed ed.). Boston, MA: Pearson Education Inc.
Tarvainen, M. P. (2017). Kubios HRV User’s Guide. Kubios Ltd, Kuopio, Finland. Retrieved from http://www.kubios.com/support.
Tarvainen, M. P., Ranta-Aho, P. O., & Karjalainen, P. A. (2002). An advanced detrending method with application to HRV analysis. IEEE Transactions on Biomedical Engineering, 49(2), 172–175. https://doi.org/10.1109/10.979357.
Task Force of the European Society of Cardiology. (1996). Heart rate variability standards of measurement, physiological interpretation, and clinical use. European Heart Journal, 17, 354–381.
Taylor, C. B. (2010). Depression, heart rate related variables and cardiovascular disease. International Journal of Psychophysiology, 78(1), 80–88. https://doi.org/10.1016/j.ijpsycho.2010.04.006.
Valenza, G., Garcia, R. G., Citi, L., Scilingo, E. P., Tomaz, C. A., & Barbieri, R. (2015). Nonlinear digital signal processing in mental health: Characterization of major depression using instantaneous entropy measures of heartbeat dynamics. Front Physiol, 6, 74. https://doi.org/10.3389/fphys.2015.00074.
Vella, E. J., & Friedman, B. H. (2009). Hostility and anger In: Cardiovascular reactivity and recovery to mental arithmetic stress. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 72(3), 253–259. https://doi.org/10.1016/j.ijpsycho.2009.01.003.
Vuksanovic, V., & Gal, V. (2007). Heart rate variability in mental stress aloud. Medical Engineering & Physics, 29(3), 344–349. https://doi.org/10.1016/j.medengphy.2006.05.011.
Watson, D., & Clark, L. A. (1984). Negative affectivity: The disposition to experience aversive emotional states. Psychological Bulletin, 96(3), 465–490.
World Medical Association. (2013). World medical association declaration of helsinki: Ethical principles for medical research involving human subjects. JAMA, 310(20), 2191–2194. https://doi.org/10.1001/jama.2013.281053.
Yu, B. H., Kang, E. H., Ziegler, M. G., Mills, P. J., & Dimsdale, J. E. (2008). Mood states, sympathetic activity, and in vivo beta-adrenergic receptor function in a normal population. Depress Anxiety, 25(7), 559–564. https://doi.org/10.1002/da.20338.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Brugnera, A., Zarbo, C., Tarvainen, M.P. et al. Higher levels of Depressive Symptoms are Associated with Increased Resting-State Heart Rate Variability and Blunted Reactivity to a Laboratory Stress Task among Healthy Adults. Appl Psychophysiol Biofeedback 44, 221–234 (2019). https://doi.org/10.1007/s10484-019-09437-z
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-019-09437-z