Abstract
Mild cognitive impairment (MCI) is a syndrome characterized by a decrease in cognitive abilities, while daily function is maintained. This condition, which is associated with an increased risk for the development of Alzheimer’s disease, has no known definitive treatment at present. In this open-label pilot study we explored the possible benefits of neurofeedback for subjects with MCI. Eleven participants diagnosed with MCI were trained to increase the power of their individual upper alpha band of the electroencephalogram (EEG) signal over the central parietal region. This was achieved using an EEG-based neurofeedback training protocol. Training comprised ten 30-min sessions delivered over 5 weeks. Cognitive and electroencephalographic assessments were conducted before and after training and at 30 days following the last training session. A dose-dependent increase in peak alpha frequency was observed throughout the period of training. Memory performance also improved significantly following training, and this improvement was maintained at 30-day follow-up, while peak alpha frequency returned to baseline at this evaluation. Our findings suggest that neurofeedback may improve memory performance in subjects with mild cognitive impairment, and this benefit may be maintained beyond the training period.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Mild cognitive impairment (MCI) is a syndrome characterized by a decline in cognitive abilities without affecting daily function. The cognitive decline is more pronounced than that seen with normal aging but less pronounced than in dementia. An important feature of MCI, and more specifically amnestic MCI, is that it is associated with an increased risk of developing Alzheimer’s disease (AD) (Drago et al. 2011; Mufson et al. 2012). Longitudinal studies have found a conversion rate for MCI to AD of greater than twenty-five percent over a period of two and a half years, which represents almost seven times the rate observed in the general population (Boyle et al. 2006; Brodaty et al. 2014). The clinical diagnosis of MCI is somewhat elusive, and there is no gold standard for the definitive diagnosis of this condition (Petersen et al. 2014). The Fifth Edition of the Diagnostic and statistical manual of mental disorders (DSM-5) has redefined MCI as Mild Neurocognitive Disorder, with increasing emphasis placed on evaluating specific neurocognitive functions (American Psychiatric Association 2013).
Domains that should be assessed when evaluating cognition include executive functions, attention, language, memory, and visuospatial skills. At present, there are no approved efficacious treatments for MCI (Karakaya et al. 2013). Although MCI is a clinical diagnosis, there is increasing interest in the diagnostic use of biomarkers in the cerebrospinal fluid (CSF) and in imaging techniques such as electroencephalogram (EEG), magnetic resonance imaging (MRI) and positron emission tomography (PET) (Babiloni et al. 2011; Huang et al. 2000; Iaccarino et al. 2017; Jelic et al. 2000; Vemuri et al. 2009).
Characteristic changes in the EEG of MCI patients, seen particularly in the posterior regions of the brain (Babiloni et al. 2011; Huang et al. 2000; Jelic et al. 2000), include a general slowing of the EEG expressed by lower peak alpha frequency (PAF), lower alpha rhythm power and higher power in lower frequencies (delta and theta). These features have been linked to poor cognitive performance (Klimesch 1999), and to gray matter atrophy, and may serve as an indication that an older person will develop MCI or that a person with MCI will progress to AD (Jack et al. 1999, 2005; Karas et al. 2004). Specifically, theta power correlates negatively with neuropsychological performance in MCI (Cummins et al. 2008), while upper alpha power and PAF correlate positively with cognitive performance, as mentioned previously. Anatomically, these EEG changes correlate with atrophy of the thalamus, hippocampus and basal ganglia (Moretti et al. 2012a, b; Wolf et al. 2004).
Traditionally, the EEG has been divided into fixed “bands” or “rhythms” of frequency, the most dominant of which is the alpha rhythm. The alpha rhythm is seen most easily when the eyes are closed, and is defined as neural oscillations in the range of 8–12 Hz. However, since the alpha rhythm varies with factors such as age, cognitive ability, and brain volume, a more pragmatic approach for determining the alpha rhythm range is to define an individualized alpha rhythm rather than a fixed range (Klimesch 1999). PAF and center of gravity frequency are the two most commonly used parameters to calculate the individual alpha frequency (IAF). The PAF represents the frequency with the highest power value and its use is more suitable for calculating the alpha rhythm in resting state. PAF distinguishes two separate frequency bands: the upper alpha rhythm (representing the range from PAF to PAF + 2 Hz), and the lower alpha rhythm which rests between the transition frequency and the PAF. (The transition frequency is the frequency in which there is a transition in spectral power dominance between the alpha rhythm, which is dominant in the resting state, and the theta rhythm, which is dominant during cognitive tasks.) Anatomically, the alpha rhythm is generated by thalamic neurons (Goldman et al. 2002; Schreckenberger et al. 2004). Cognitively, the upper alpha rhythm correlates with cognitive performance and semantic memory, while the lower alpha rhythm correlates with attention (Escolano et al. 2011; Klimesch 1999). Experiments using neurofeedback have shown its potential in teaching individuals to regulate neuronal firing rate and therefore the oscillatory patterns of the EEG (Sitaram et al. 2017). Since these EEG parameters are altered in MCI (as noted previously), we hypothesized that neurofeedback may be of therapeutic value in this condition.
Neurofeedback is a method of neuromodulation using operant conditioning that has been in use for decades. Beneficial effects of neurofeedback have been described in both animal models and in human subjects, being used for conditions such as epilepsy and attention deficit and hyperactivity disorder (Egner and Sterman 2006; Strehl et al. 2006). Imaging studies suggest that structural changes may occur in the brain following neurofeedback training, including an increase in the gray and white matter (Ghaziri et al. 2013). Studies have also demonstrated the effects of neurofeedback on cognitive performance in both young and old individuals. In a study performed by Escolano and colleagues, young healthy individuals were trained to increase the power of the upper alpha band, aiming to improve working memory. The results of this study demonstrated an increase in the power of the upper alpha band, and a significant enhancement of working memory in subjects compared to controls (Escolano et al. 2011). Another study in a young healthy population found a significant improvement in a mental rotation task following training aimed at increasing the upper alpha band (Zoefel et al. 2011). In older populations, a relatively few studies have been performed using neurofeedback to enhance cognitive performance. A small (6 participants) double blind randomized controlled study trained older participants in the experimental group to increase their PAF (Angelakis et al. 2007). Results suggested that the neurofeedback training protocol had a beneficial effect on some executive functions, but had no clear effect on memory. In another study, older participants with an abnormal high theta rhythm power were trained to reduce theta power (Becerra et al. 2012). Findings showed improvement in both experimental and placebo groups, with the effects of training were more pronounced in the experimental group. A study conducted with healthy older participants who were trained to increase the power of the theta rhythm found that theta rhythms were in fact increased following training with an improvement in attention and working memory (Wang and Hsieh 2013). In a study where participants diagnosed with either AD or vascular dementia were trained using quantitative EEG-guided neurofeedback (Surmeli et al. 2015) an average improvement of six points in the Mini Mental State Examination score was observed, regardless of the dementia type. In another study, the effects of neurofeedback on executive and memory functions in subjects with AD were compared to a waiting list control (Berman and Frederick 2009). Individual abnormal feedback locations and frequencies were identified in each participant. A significant improvement in memory and executive functions was observed in the experimental group.
To the best of our knowledge the efficacy of neurofeedback training in patients with MCI has not yet been determined. Based on the potential for neurofeedback to teach individuals to regulate neuronal function and thus alter the oscillatory patterns of the EEG (Sitaram et al. 2017), we postulated that EEG-based neurofeedback may be of therapeutic value in MCI.
The main goals of this study were: (1) To expand our knowledge about the possible beneficial effects of EEG-based neurofeedback in patients with MCI. (2) To examine whether there were prolonged effects of the neurofeedback training and whether possible cognitive improvement and EEG trained parameters maintain their value even after the training has ended. This is of particular interest as there are few studies in the neurofeedback literature that have addressed this issue in general (Bink et al. 2016; Kouijzer et al. 2009), and to the best of our knowledge, this has not been previously evaluated in patients with MCI. (3) To design a study to specifically address changes in the EEG that are correlated with MCI and thus pursue a more focused approach to cognitive enhancement in this condition.
Methods
Participants
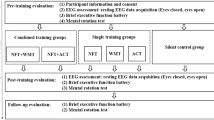
Eleven right-handed participants (six women and five men, mean age = 70 years, SD = 10), with normal, or corrected to normal, color vision participated in the study. Participants were diagnosed with MCI at the Memory Clinic of the Beer-Sheva Mental Health Center. The diagnosis was based on a clinical evaluation, cognitive assessment performed by a geriatric specialist and a neurocognitive psychologist, and the NeuroTrax™ computerized cognitive assessment battery, which has been validated for MCI (Dwolatzky et al. 2004). Inclusion criteria included age > 50 years, a diagnosis of MCI and right handedness. Exclusion criteria included traumatic brain injury, epilepsy, Parkinson’s disease, any known neurodegenerative disease, vascular disease of the Central Nervous System (CNS), CNS tumors, anxiety (short anxiety screening test (SAST) > 24), depression (geriatric depression scale (GDS) > 6), use of psychotropic drugs, and any axis 1 disorder. The study was approved by the Ethics Committee (IRB) of the Soroka University Medical Center, and all subjects provided written informed consent for their participation in the study. Repeat cognitive assessment and EEG analysis was completed on all participants at the end of the training period. At 30 days follow up, 10 completed the cognitive assessment and 8 performed the final EEG evaluation.
Experimental Design
We performed an open-label study in which all participants participated in 16 encounters, and were trained to increase the power of the individual upper alpha (the range of PAF to PAF + 2 i.e., if a participant had a PAF value of 8 Hz, the trained frequency band would be that of 8–10 Hz) at the central parietal location. The first two pre-training encounters were dedicated to cognitive evaluation. The evaluation was performed over 2 consecutive days. On the first day, the NeuroTrax™ battery was administered, and on the second-day participants were required to perform an item-association memory task (Naveh-Benjamin et al. 2004) and an antisaccade task (Katzir et al. 2010). Subsequently, each participant was trained over ten sessions. Each session was structured to commence with the performance of a baseline resting EEG (3 min with eyes closed and 3 min with eyes open), followed by 30 min of neurofeedback training, and conclude with a resting EEG (3 min with eyes closed and 3 min with eyes open). On completion of the ten training sessions, cognitive evaluation was performed based on the same protocol as the pre-training encounters. This same assessment protocol was performed at 30 days following the last training session.
EEG Measurement
EEG recordings were obtained with a Deymed Truescan 32 acquisition device. Each participant was fitted with a 10/20 EEG cap. The EEG was recorded from 19 channels, at 128 samples per second with electrodes referenced to Fcz (according to the 10/20 international system). Impedances were kept at 5 kΩ or less. The EEG was analyzed with WinEEG software. Independent component analysis and visual examination were used to extract artifacts from the raw EEG. A rhythm pass filter was applied (0.50–30 Hz) to extract the relevant frequencies.
Neurofeedback Protocol
For each participant, an individual PAF with eyes closed was determined at the first encounter. The participant was trained to increase the power of the EEG in the range of frequencies between PAF and PAF + 2 Hz (e.g., if IAF = 8 Hz, the participant was trained to increase the power of alpha in the range of 8–10Hz). Feedback was given according to a measurement from the Pz location (according to the 10/20 international system) in the midline, above the parietal lobe. We used the Deymed Truescan 32 acquisition device with a special case of the short-time Fourier transform, the Gabor transform. Training sessions were performed with eyes open and were divided into ten 3-min trials, separated by a 10-s break. During training, participants received auditory and visual feedbacks if they had surpassed a predetermined threshold for at least 250 milliseconds. To receive a positive feedback, each participant had to surpass a level of electrical activity (measured in uV) in the Pz region within the range of frequencies formerly described. The threshold value for gaining positive feedback was determined such that each participant would surpass the threshold 8 times per minute at the beginning of training. The threshold was re-determined when a participant had achieved more than 30 feedbacks per minute. The visual feedback was continuous and composed of a 3-dimensional game in which balls approached the middle of the screen as the participant approached the threshold, while the auditory feedback was discrete and presented as a beeping sound. During each round, a counter was displayed on the screen showing the number of feedbacks a participant had gained since the beginning of the block. At the end of each block, the participant was shown a histogram of the scores for all the blocks from the beginning of the session. Participants were instructed to watch the screen and were told that they can adopt any suitable strategy to help them achieve more points.
Computerized Cognitive Testing
Computerized cognitive testing was performed using the NeuroTrax™ battery, the item-association task, and the anti-saccade task. During all tasks, participants sat approximately 70 cm away from a computer screen. For the item-association task and the anti-saccade task, stimuli were presented in black letters or black-and-white images on a white background. An experimenter accompanied the participants throughout the tasks and assisted if clarification was needed. Both the item-association task and the antisaccade task were programmed using OpenSesame (Mathôt et al. 2012).
The NeuroTrax™ battery (http://www.neurotrax.com) provides a normalized composite score for each cognitive domain tested in the battery including memory performance, executive functions, visuospatial skills and attention. The tasks included in the battery used in this study include immediate and delayed verbal and nonverbal memory, Stroop interference, catch-game, Go-No Go and visuospatial skills.
The item-association task is a task of associative and non-associative memory (Naveh-Benjamin et al. 2004). Several adjustments were made to fit the needs of the study and the specific population. We added a nonverbal version of the test using images. Four sets of 15 pairs of items were presented to the participants. The first set was a practice set. In each set, pairs were automatically replaced every 5 s. Each set was followed by a 30 s “find the differences” task. This procedure was repeated once for pairs of words (verbal memory) and once for pairs of images (non-verbal memory).
The antisaccade task (Hallett 1978) measures inhibition, an executive function that enables a distracting stimulus to be disregarded (Miyake et al. 2000). We used the version of the test described by Katzir et al. (2010). Of importance is the visual angle at which the distractor and stimuli appear (Miyake et al. 2000). The visual angle to the target was calculated to be approximately 21.4°. The antisaccade task can predict executive functions and brain structure in normal older people (Mirsky et al. 2011; Peltsch et al. 2011). The stimulus onset asynchrony (SOA) represents the time from the appearance of the distractor to the appearance of the target. The longer the time, the easier the task, and the better the precision. The expected results, which indicate correct execution of the test, is an increase in precision (percent of correct answers) as the SOA (time between distractor and target) increases.
Statistical Analysis
The number of participants in this study limited the use of parametric statistical analysis. Therefore, the tests of choice in this study were the Spearman rank correlation for correlation analysis and the Wilcoxon signed rank test for comparative analysis.
Results
EEG Results
A strong positive correlation between PAF and the session number was observed (p = 0.008; r = 0.779) (Fig. 1), indicating a dose–response effect of training. At follow up 30 days after the completion of training the average PAF had declined from a value of 9.32 to a value of 8.47, similar to the baseline value of 8.70 (p = 0.4; Z = − 0.84). The power of the individual upper alpha did not significantly change following training (p = 0.657; z = − 0.445).
Cognitive Results
Participants significantly improved their composite memory score on the NeuroTrax™ tests following training (p = 0.016; Z = − 2.4), from an average normalized score of 93.79–102.1 (with 100 being the average in population of MCI patients, matched for age and education level) (Fig. 2). At 30 days after training, the improved memory score did not change significantly (p = 0.441; Z = − 0.77), with an average score of 102.95. We also found that the increase in memory score was inversely correlated (p = 0.029; r = − 0.655) with the baseline memory score of the participants (Fig. 3). Other cognitive domains evaluated, such as attention (p = 0.657; Z = − 0.445), executive functions (p = 0.722; Z = − 0.356) and visuospatial ability (p = 0.953; Z = 0.059), were not significantly changed following training.
Improvement in memory performance following training. Memory score on the NeuroTrax™ Tests improved significantly after training and remained high 30 days the training has ended. (Wilcoxon signed ranks test pre to post training: p = 0.016; Z = − 2.4 and post-training to follow up: p = 0.441; Z = − 0.77)
Correlation between Baseline memory performance and absolute improvement in memory performance as measured by the NeuroTrax™ tests. A strong significant correlation was found between the baseline memory score and the ability to improve the memory performance. (Spearman rank correlation: p = 0.029; r = − 0.655)
In the NeuroTrax™ tests immediate and delayed verbal recall task, immediate memory significantly improved (p = 0.016; Z = − 2.41) from pre-training to post-training (75.25% accuracy to 87.25% accuracy sum of four repetitions) and later declined at 30-day follow up to 78%. Delayed memory showed a slight increase (81–84.4% accuracy) from pre-training to post-training, which was not statistically significant (p = 0.595; Z = − 0.531).
Similar results were found for the NeuroTrax™ tests immediate and delayed non-verbal recall task. Immediate recognition improved significantly (p = 0.049; Z = − 1.967) from pre- training to post-training (57.36% accuracy to 64.5%) and declined in the follow-up to 61.5%. In this task as well, there was an improvement only in immediate memory.
The item-association task, showed statistically non-significant changes following training for both tasks: associative verbal memory (p = 0.445; Z = − 0.764), and non-associative verbal memory (p = 0.859; Z = − 0.178). Non-significant changes were also found for associative non-verbal memory (p = 0.965; Z = − 0.04) and non-associative non-verbal memory (p = 0.623; Z = − 0.492).
Analysis of the anti-saccade task revealed a positive trend in precision as the SOA increased (p = 0.071; X2 = 7.04). The results for this test showed non-significant changes from pre-test and post-test (Z = − 0.178; p = 0.859).
Discussion
Our findings showed an improvement in both cognitive and electroencephalographical measures. The value of PAF showed a positive correlation with the number of training sessions. Cognitive measures demonstrated a significant improvement in the composite memory score, represented mainly by an improvement in both verbal and non-verbal immediate memory.
A decline in PAF is a common finding in patients with MCI. This electroencephalographic feature positively correlates with decreased thalamic neuronal activity (Moosmann et al. 2003) and decreased hippocampal volume (Babiloni et al. 2009; Moretti et al. 2011). We hypothesized that increasing the power in the range of PAF to PAF + 2 Hz would cause a shift in the EEG spectra with an increase of the PAF. In this study, an increase in the power of a trained EEG rhythm (individual upper alpha) was not observed. However, an increase in the PAF of participants was observed. Importantly, PAF increased in a dose-dependent manner throughout the training. PAF has been shown to positively correlate with good memory performance (Klimesch 1999). However, the increase in PAF parameters that was observed immediately post training, was not maintained at 30 days after training.
Cognitive evaluation of participants in this study revealed a significant improvement in the NeuroTrax composite score following training. These results were still preserved at 30 days after training, implying a lasting effect beyond the training period. While analyzing the results of the different memory tests, one can observe that the improvement was observed in immediate memory, rather than in delayed memory. A possible explanation would be that at older ages, the ability to create new memories and associations tends to be limited (Gilbert and Levee 1971), as is reflected by an impaired immediate memory with preserved delayed memory. Since delayed memory is affected to a lesser degree in age-related memory decline and MCI, a ceiling effect may have prevented a detectable improvement.
We further noted that the improvement in memory score was inversely correlated with the baseline memory score. This could also be due to a ceiling effect, as near maximal scores were reached by some of the participants. One other explanation could be that training could help return memory performance to normal, but not beyond. This is of interest as it may help differentiate those who have the greatest potential to benefit from training from those who might not benefit at all. Future studies should, therefore, avoid tests with a possible ceiling effect and include in the study population participants with a wider range of impaired memory performance.
The item-association task was revealed as too difficult for the study population and therefore had no discriminatory value. The antisaccade task had a positive trend but showed insignificant changes that could be attributed to the small sample size and insufficient repetitions in the test itself.
Other cognitive domains explored in this study were not significantly changed. These initially had a higher average baseline score (executive functions = 105.16, attention = 105.30, and visuospatial = 98.59) than that of memory (average = 93.79) suggesting a stronger amnestic component in the study population. It is interesting to note that at the 30-day follow up, PAF declined to its baseline values, whereas the improvement in memory remained constant. We postulate that the memory performance lags behind the EEG, and thus would be expected to decline subsequent to the decline in EEG parameters. As discussed in the introduction, studies have shown that EEG specific changes may precede and even predict cognitive decline.
Some limitations of this study include the lack of a control group and a small sample size. Controlling neurofeedback experiments is a complex and interesting topic that is beyond the scope of this study and has been discussed elsewhere (LaVaque et al. 2002; Yucha and Gilbert 2004). We clearly recognize that a placebo effect cannot be ruled out without a control group. Also, the small sample size of this pilot study limits our findings as well as the generalizability of our results. Another limitation of this study was that our program measured EEG and feedback with FCZ as the reference electrode, thus reflecting a bipolar recording. During the analysis of data however, we referenced the EEG recording to a linked ears montage, which is monopolar.
Despite these limitations, the results of this study are encouraging and should provoke further study. We suggest that future studies should include a larger sample size, a control group and preferably explore how to improve specific cognitive domains that are affected in MCI.
A notable advantage of this study is the follow up performed at 30 days. There are few studies documenting the sustained effects of neurofeedback. This has meaningful scientific and clinical implications regarding the need for continuity of training and the possible benefits of maintenance neurofeedback. It is therefore of utmost importance that a follow up evaluation be adopted by researchers in future neurofeedback studies where this is feasible. In this study, it was found that while the increase in peak alpha frequency (EEG) was not preserved at 30 days after training, the improvement in memory was still present. These results suggest that there may be a lasting effect of training. It is of interest that this discrepancy between cognitive and EEG results may indicate that the change in EEG parameters precedes the cognitive decline, thus suggesting that a subsequent cognitive decline may have occurred. If this hypothesis is confirmed in further studies, it may be possible to use EEG to guide the frequency of maintenance treatments.
Abbreviations
- MCI:
-
Mild cognitive impairment
- AD:
-
Alzheimer’s disease
- EEG:
-
Electroencephalography
- PAF:
-
Peak alpha frequency
- IAF:
-
Individual alpha frequency
- FU:
-
Follow up
- CNS:
-
Central nervous system
References
American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders, 5th Edition. Philadelphia, American Psychiatric Association. https://doi.org/10.1176/appi.books.9780890425596.
Angelakis, E., Stathopoulou, S., Frymiare, J. L., Green, D. L., Lubar, J. F., & Kounios, J. (2007). EEG neurofeedback: A brief overview and an example of peak alpha frequency training for cognitive enhancement in the elderly. The Clinical Neuropsychologist, 21(1), 110–129. https://doi.org/10.1080/13854040600744839.
Babiloni, C., Frisoni, G. B., Pievani, M., Vecchio, F., Lizio, R., Buttiglione, M., & Rossini, P. M. (2009). Hippocampal volume and cortical sources of EEG alpha rhythms in mild cognitive impairment and Alzheimer disease. NeuroImage, 44(1), 123–135. https://doi.org/10.1016/j.neuroimage.2008.08.005.
Babiloni, C., Vecchio, F., Lizio, R., Ferri, R., Rodriguez, G., Marzano, N., & Rossini, P. M. (2011). Resting state cortical rhythms in mild cognitive impairment and Alzheimer’s disease: Electroencephalographic evidence. Journal of Alzheimer’s Disease: JAD, 26(Suppl 3), 201–214. https://doi.org/10.3233/JAD-2011-0051.
Becerra, J., Fernández, T., Roca-Stappung, M., Díaz-Comas, L., Galán, L., Bosch, J., & Harmony, T. (2012). Neurofeedback in healthy elderly human subjects with electroencephalographic risk for cognitive disorder. Journal of Alzheimer’s Disease: JAD, 28(2), 357–367. https://doi.org/10.3233/JAD-2011-111055.
Berman, M. H., & Frederick, J. A. (2009). Efficacy of neurofeedback for executive and memory function in dementia. Alzheimer’s & Dementia, 5(4), e8. https://doi.org/10.1016/j.jalz.2009.07.046.
Bink, M., Bongers, I. L., Popma, A., Janssen, T. W. P., & van Nieuwenhuizen, C. (2016). 1-year follow-up of neurofeedback treatment in adolescents with attention-deficit hyperactivity disorder: Randomised controlled trial. British Journal of Psychiatry Open, 2(2), 107–115. https://doi.org/10.1192/bjpo.bp.115.000166.
Boyle, P. A., Wilson, R. S., Aggarwal, N. T., Tang, Y., & Bennett, D. A. (2006). Mild cognitive impairment: Risk of Alzheimer disease and rate of cognitive decline. Neurology, 67(3), 441–445. https://doi.org/10.1212/01.wnl.0000228244.10416.20.
Brodaty, H., Connors, M. H., Ames, D., & Woodward, M. (2014). Progression from mild cognitive impairment to dementia: A 3-year longitudinal study. The Australian and New Zealand Journal of Psychiatry. https://doi.org/10.1177/0004867414536237.
Cummins, T. D. R., Broughton, M., & Finnigan, S. (2008). Theta oscillations are affected by amnestic mild cognitive impairment and cognitive load. International Journal of Psychophysiology: Official Journal of the International Organization of Psychophysiology, 70(1), 75–81. https://doi.org/10.1016/j.ijpsycho.2008.06.002.
Drago, V., Babiloni, C., Bartrés-Faz, D., Caroli, A., Bosch, B., Hensch, T., & Frisoni, G. B. (2011). Disease tracking markers for Alzheimer’s disease at the prodromal (MCI) stage. Journal of Alzheimer’s Disease: JAD, 26(Suppl 3), 159–199. https://doi.org/10.3233/JAD-2011-0043.
Dwolatzky, T., Whitehead, V., Doniger, G. M., Simon, E. S., Schweiger, A., Jaffe, D., & Chertkow, H. (2004). Validity of the Mindstreams computerized cognitive battery for mild cognitive impairment. Journal of Molecular Neuroscience: MN, 24(1), 33–44. Retrieved from http://www.ncbi.nlm.nih.gov/pubmed/15314247.
Egner, T., & Sterman, M. B. (2006). Neurofeedback treatment of epilepsy: from basic rationale to practical application. Expert Review of Neurotherapeutics, 6, 247–257. https://doi.org/10.1586/14737175.6.2.247.
Escolano, C., Aguilar, M., & Minguez, J. (2011). EEG-based upper alpha neurofeedback training improves working memory performance. Conference Proceedings: Annual International Conference of the IEEE Engineering in Medicine and Biology Society. IEEE Engineering in Medicine and Biology Society. Conference, 2011, 2327–2330. https://doi.org/10.1109/IEMBS.2011.6090651.
Ghaziri, J., Tucholka, A., Larue, V., Blanchette-Sylvestre, M., Reyburn, G., Gilbert, G., & Beauregard, M. (2013). Neurofeedback training induces changes in white and gray matter. Clinical EEG and Neuroscience: Official Journal of the EEG and Clinical Neuroscience Society (ENCS), 44(4), 265–272. https://doi.org/10.1177/1550059413476031.
Gilbert, J. G., & Levee, R. F. (1971). Patterns of declining memory. Journal of Gerontology, 26(1), 70–75. https://doi.org/10.1093/geronj/26.1.70.
Goldman, R. I., Stern, J. M., Engel, J., & Cohen, M. S. (2002). Simultaneous EEG and fMRI of the alpha rhythm. Neuroreport, 13(18), 2487–2492. https://doi.org/10.1097/01.wnr.0000047685.08940.d0.
Hallett, P. E. (1978). Primary and secondary saccades to goals defined by instructions. Vision Research, 18(10), 1279–1296. https://doi.org/10.1016/0042-6989(78)90218-3.
Huang, C., Wahlund, L.-O., Dierks, T., Julin, P., Winblad, B., & Jelic, V. (2000). Discrimination of Alzheimer’s disease and mild cognitive impairment by equivalent EEG sources: A cross-sectional and longitudinal study. Clinical Neurophysiology, 111(11), 1961–1967. https://doi.org/10.1016/S1388-2457(00)00454-5.
Iaccarino, L., Chiotis, K., Alongi, P., Almkvist, O., Wall, A., Cerami, C., & Perani, D. (2017). A cross-validation of FDG- and amyloid-PET biomarkers in mild cognitive impairment for the risk prediction to dementia due to Alzheimer’s Disease in a clinical setting. Journal of Alzheimer’s Disease, 59(2), 603–614. https://doi.org/10.3233/JAD-170158.
Jack, C. R., Petersen, R. C., Xu, Y. C., O’Brien, P. C., Smith, G. E., Ivnik, R. J., & Kokmen, E. (1999). Prediction of AD with MRI-based hippocampal volume in mild cognitive impairment. Neurology, 52(7), 1397–1397. https://doi.org/10.1212/WNL.52.7.1397.
Jack, C. R., Shiung, M. M., Weigand, S. D., O’Brien, P. C., Gunter, J. L., Boeve, B. F., & Petersen, R. C. (2005). Brain atrophy rates predict subsequent clinical conversion in normal elderly and amnestic MCI. Neurology, 65(8), 1227–1231. https://doi.org/10.1212/01.wnl.0000180958.22678.91.
Jelic, V., Johansson, S.-E., Almkvist, O., Shigeta, M., Julin, P., Nordberg, A., & Wahlund, L.-O. (2000). Quantitative electroencephalography in mild cognitive impairment: Longitudinal changes and possible prediction of Alzheimer’s disease. Neurobiology of Aging, 21(4), 533–540. https://doi.org/10.1016/S0197-4580(00)00153-6.
Karakaya, T., Fußer, F., Schroder, J., & Pantel, J. (2013). Pharmacological treatment of mild cognitive impairment as a prodromal syndrome of Alzheimer’s disease. Current Neuropharmacology, 11(1), 102–108. https://doi.org/10.2174/157015913804999487.
Karas, G. B., Scheltens, P., Rombouts, S. A. R. B., Visser, P. J., van Schijndel, R. A., Fox, N. C., & Barkhof, F. (2004). Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. NeuroImage, 23(2), 708–716. https://doi.org/10.1016/j.neuroimage.2004.07.006.
Katzir, M., Eyal, T., Meiran, N., & Kessler, Y. (2010). Imagined positive emotions and inhibitory control: The differentiated effect of pride versus happiness. Journal of Experimental Psychology. Learning, Memory, and Cognition, 36(5), 1314–1320. https://doi.org/10.1037/a0020120.
Klimesch, W. (1999). EEG alpha and theta oscillations reflect cognitive and memory performance: A review and analysis. Brain Research Reviews, 29(2–3), 169–195. https://doi.org/10.1016/S0165-0173(98)00056-3.
Kouijzer, M. E. J., de Moor, J. M. H., Gerrits, B. J. L., Buitelaar, J. K., & van Schie, H. T. (2009). Long-term effects of neurofeedback treatment in autism. Research in Autism Spectrum Disorders, 3(2), 496–501. https://doi.org/10.1016/j.rasd.2008.10.003.
LaVaque, T. J., Hammond, D. C., Trudeau, D., Monastra, V. J., Perry, J., Lehrer, P., et al. (2002). Template for developing guidelines for the evaluation of the clinical efficacy of psychophysiological interventions. Applied Psychophysiology and Biofeedback, 27, 273–281. https://doi.org/10.1023/A:1021061318355.
Mathôt, S., Schreij, D., & Theeuwes, J. (2012). OpenSesame: An open-source, graphical experiment builder for the social sciences. Behavior Research Methods, 44(2), 314–324. https://doi.org/10.3758/s13428-011-0168-7.
Mirsky, J. B., Heuer, H. W., Jafari, A., Kramer, J. H., Schenk, A. K., Viskontas, I. V., & Boxer, A. L. (2011). Anti-saccade performance predicts executive function and brain structure in normal elders. Cognitive and Behavioral Neurology: Official Journal of the Society for Behavioral and Cognitive Neurology, 24(2), 50–58. https://doi.org/10.1097/WNN.0b013e318223f6c6.
Miyake, a, Friedman, N. P., Emerson, M. J., Witzki, a H., Howerter, a, & Wager, T. D. (2000). The unity and diversity of executive functions and their contributions to complex “Frontal Lobe” tasks: A latent variable analysis. Cognitive Psychology, 41(1), 49–100. https://doi.org/10.1006/cogp.1999.0734.
Moosmann, M., Ritter, P., Krastel, I., Brink, A., Thees, S., Blankenburg, F., & Villringer, A. (2003). Correlates of alpha rhythm in functional magnetic resonance imaging and near infrared spectroscopy. NeuroImage, 20(1), 145–158. https://doi.org/10.1016/S1053-8119(03)00344-6.
Moretti, D. V., Paternicò, D., Binetti, G., Zanetti, O., & Frisoni, G. B. (2012a). EEG markers are associated to gray matter changes in thalamus and basal ganglia in subjects with mild cognitive impairment. NeuroImage, 60(1), 489–496. https://doi.org/10.1016/j.neuroimage.2011.11.086.
Moretti, D. V., Prestia, A, Fracassi, C., Binetti, G., Zanetti, O., & Frisoni, G. B. (2012b). Specific EEG changes associated with atrophy of hippocampus in subjects with mild cognitive impairment and Alzheimer’s disease. International Journal of Alzheimer’s Disease. https://doi.org/10.1155/2012/253153.
Moretti, D. V., Prestia, A, Fracassi, C., Geroldi, C., Binetti, G., Rossini, P. M., & Frisoni, G. B. (2011). Volumetric differences in mapped hippocampal regions correlate with increase of high alpha rhythm in Alzheimer’s disease. International Journal of Alzheimer’s Disease. https://doi.org/10.4061/2011/208218.
Mufson, E. J., Binder, L., Counts, S. E., DeKosky, S. T., de Toledo-Morrell, L., Ginsberg, S. D., & Scheff, S. W. (2012). Mild cognitive impairment: Pathology and mechanisms. Acta Neuropathologica, 123(1), 13–30. https://doi.org/10.1007/s00401-011-0884-1.
Naveh-Benjamin, M., Guez, J., & Shulman, S. (2004). Older adults’ associative deficit in episodic memory: Assessing the role of decline in attentional resources. Psychonomic Bulletin & Review, 11(6), 1067–1073. https://doi.org/10.3758/BF03196738.
Peltsch, A., Hemraj, A., Garcia, A., & Munoz, D. P. (2011). Age-related trends in saccade characteristics among the elderly. Neurobiology of Aging, 32(4), 669–679. https://doi.org/10.1016/j.neurobiolaging.2009.04.001.
Petersen, R. C., Caracciolo, B., Brayne, C., Gauthier, S., Jelic, V., & Fratiglioni, L. (2014). Mild cognitive impairment: A concept in evolution. Journal of Internal Medicine, 275(3), 214–228. https://doi.org/10.1111/joim.12190.
Schreckenberger, M., Lange-Asschenfeldt, C., Lange-Asschenfeld, C., Lochmann, M., Mann, K., Siessmeier, T., & Gründer, G. (2004). The thalamus as the generator and modulator of EEG alpha rhythm: A combined PET/EEG study with lorazepam challenge in humans. NeuroImage, 22(2), 637–644. https://doi.org/10.1016/j.neuroimage.2004.01.047.
Sitaram, R., Ros, T., Stoeckel, L., Haller, S., Scharnowski, F., Lewis-Peacock, J., & Sulzer, J. (2017). Closed-loop brain training: The science of neurofeedback. Nature Reviews Neuroscience, 18(2), 86–100. https://doi.org/10.1038/nrn.2016.164.
Strehl, U., Leins, U., Goth, G., Klinger, C., Hinterberger, T., & Birbaumer, N. (2006). Self-regulation of slow cortical potentials: A new treatment for children with attention-deficit/hyperactivity disorder. Pediatrics, 118, e1530–e1540. https://doi.org/10.1111/j.1469-7610.2008.02033.x.
Surmeli, T., Eralp, E., Mustafazade, I., Kos, H., Özer, G. E., & Surmeli, O. H. (2015). Quantitative EEG neurometric analysis—guided neurofeedback treatment in dementia 20 cases. How neurometric analysis is important for the treatment of dementia and as a biomarker? Clinical EEG and Neuroscience. https://doi.org/10.1177/1550059415590750.
Vemuri, P., Wiste, H. J., Weigand, S. D., Shaw, L. M., Trojanowski, J. Q., Weiner, M. W., & Jack, C. R. (2009). MRI and CSF biomarkers in normal, MCI, and AD subjects: Diagnostic discrimination and cognitive correlations. Neurology, 73(4), 287–293. https://doi.org/10.1212/WNL.0b013e3181af79e5.
Wang, J.-R., & Hsieh, S. (2013). Neurofeedback training improves attention and working memory performance. Clinical Neurophysiology: Official Journal of the International Federation of Clinical Neurophysiology, 124(12), 2406–2420. https://doi.org/10.1016/j.clinph.2013.05.020.
Wolf, H., Hensel, A., Kruggel, F., Riedel-Heller, S. G., Arendt, T., Wahlund, L.-O., & Gertz, H.-J. (2004). Structural correlates of mild cognitive impairment. Neurobiology of Aging, 25(7), 913–924. https://doi.org/10.1016/j.neurobiolaging.2003.08.006.
Yucha, C., & Gilbert, C. (2004). Evidence-based practice in biofeedback and neurofeedback. Retrieved May 4, 2016, from https://www.aapb.org/files/public/Yucha-Gilbert_EvidenceBased2004.pdf.
Zoefel, B., Huster, R. J., & Herrmann, C. S. (2011). Neurofeedback training of the upper alpha frequency band in EEG improves cognitive performance. NeuroImage, 54(2), 1427–1431. https://doi.org/10.1016/j.neuroimage.2010.08.078.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflicts of interest.
Rights and permissions
About this article
Cite this article
Lavy, Y., Dwolatzky, T., Kaplan, Z. et al. Neurofeedback Improves Memory and Peak Alpha Frequency in Individuals with Mild Cognitive Impairment. Appl Psychophysiol Biofeedback 44, 41–49 (2019). https://doi.org/10.1007/s10484-018-9418-0
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10484-018-9418-0