Abstract
Tebuconazole is the most widely used fungicide in agriculture. Due to its long half-life, tebuconazole residues can be found in the environment media such as in soil and water bodies. Here, the metabolic pathway of tebuconazole was studied in Cunninghamella elegans (C. elegans). Approximately 98% of tebuconazole was degraded within 7 days, accompanied by the accumulation of five metabolites. The structures of the metabolites were completely or tentatively identified by gas chromatography-mass spectrometry (GC–MS) and ultra-high performance liquid chromatography-tandem mass spectrometry (UPLC-MS/MS). To identify representative oxidative enzymes that may be involved in the metabolic process, treatment with piperonyl butoxide (PB) and methimazole (MZ) was performed. PB had a strong inhibitory effect on the metabolic reactions, while MZ had a weak inhibitory effect. The results suggest that cytochrome P450 (CYP) and flavin-dependent monooxygenase are involved in the metabolism of tebuconazole. Based on the results, we propose a metabolic pathway for the fungal metabolism of tebuconazole. Data are of interest to gain insight into the toxicological effects of tebuconazole and for tebuconazole bioremediation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The fungicidal effect of triazoles is very efficient compared to other fungicides and thus they are widely used in agriculture and industry. In addition, these chemicals have been used as drugs for the treatment of a variety of fungal infections, including neonatal vaginal fungal disease and oral thrush (Cui et al. 2018). Tebuconazole belongs to the group of triazole fungicides which act by inhibiting the biosynthesis of ergosterol. It is used for the control of fungal pathogens in a range of vegetables, fruits and crops. (Azhari et al. 2018; Dong et al. 2018; Hou et al. 2017). Currently, tebuconazole has become the most widely used fungicide in agriculture. Due to its long half-life, tebuconazole resides in various environmental media such as the atmosphere, soil, environmental water samples, fruits and vegetables (You et al. 2017). This may well impact mammals. For example, they show a strong ability to interfere with CYP enzymes, leading to endocrine disrupting effects (Yang et al. 2018). Moreover, tebuconazole induces developmental disorders, immune abnormalities, reproductive dysfunction, nephrotoxicity and hepatotoxicity. Metabolic studies are important for understanding the toxicity and safety of pesticides (Madrigal-Matute and Cuervo 2016; Franco et al. 2020; Shen et al. 2019; Castro et al. 2018). Currently, studies on tebuconazole metabolism have focused on mice, birds and other mammals (Bellot et al. 2022; Hillebrands et al. 2020). In addition, the metabolism and toxicity of tebuconazole in vitro (HepG2 cells and human liver microsomes, etc.) and bacteria (Pseudomonas fluorescens and Pseudomonas putida, etc.) have also been reported (Kwon et al. 2021; Hillebrands et al. 2020). However, there are few reports on the metabolism of tebuconazole in fungi. Biodegradation by microorganisms is one of the most important steps in the dissipation and detoxification of toxic chemicals in the natural environment. Among the microbiota in the soil environment, fungal biomass is usually much higher than other species (Coombes et al. 2015; Keum et al. 2009). Therefore, their contribution to external biological dissipation is important. C. elegans is widely used as a microbial model for mammals to study the biotransformation of drugs, pesticides and the environmental decontamination of pollutants (Zhu et al. 2010). The main objective of this study was to biotransform tebuconazole and identify metabolites using C. elegans (Palmer-Brown et al. 2019). The oxidases involved in the metabolic reactions were identified by using selected inhibitors.
Materials and methods
Chemicals
Tebuconazole (98% of purity) was received from DANO (http://m.haonongzi.com). Potato dextrose agar (PDA) and potato dextrose broth (PDB) were obtained from Aladdin Reagents Ltd (https://www.aladdin-e.com). N,O-Bis(trimethylsilyl)trifluoroacetamide (BSTFA + TMCS, 99:1, sylon BFT) was purchased from Merck (https://www.merck.com). Piperonyl butoxide (PB) and methimazole (MZ) were from Macklin Corporation (http://www.macklin.cn). Ethanol, acetonitrile, acetone and dichloromethane used in the experiments were purchased from Tianjin Fuyu Chemical Co Ltd (https://www.chembk.com). Anhydrous sodium sulfate and sodium chloride were from Aladdin. All other reagents are analytical or chromatographic grade.
Microorganism
Cunninghamella elegans ATCC36112 was acquired from the American Type Culture Collection (Manassas, VA). Stock cultures of Cunninghamella elegans ATCC36112 were maintained on PDA plates at 27 °C. Spores and mycelia from several plates were used to inoculate on PDB medium. Fungal cultures were typically maintained on PDA at 27 °C while liquid cultures were grown on PDB at 27 °C and 170 rpm.
Metabolic response of C. elegans to tebuconazole
For the metabolic reaction system, C. elegans was cultured on PDB for 3 days. Cultured mycelium (approximately 2 g) was added to fresh PDB (500 mL, pH = 7.0), supplemented with tebuconazole (5 mg), and incubated at 27 °C (170 rpm). Negative and blank control experiments were carried out through systems using only C. elegans and only tebuconazole, and three parallel experiments were done for each level. All media throughout the experiment were sterilized before use (15 min, 121 °C).
Metabolic pathway analysis of tebuconazole by C. elegans
At each sampling time (0, 1, 2, 3, 5 and 7 days after treatment), 50 mL of the sample was transferred to a separatory funnel and then 10 g of NaCl was added and extracted twice with 50 mL of dichloromethane. The dichloromethane phases was dried through anhydrous sodium sulfate. After evaporation of the solvent, 0.5 mL pyridine was added, followed by derivatization with BSTFA + TMCS (99: 1,300 μL) at 70 °C for 35 min, and analyzed by GC–MS.
Enzyme inhibitor assay for tebuconazole by C. elegans
PB and MZ (5 and 50 mg/L, respectively) were added to the culture medium to investigate the effects of cytochrome P450 (CYP) and flavin-dependent monooxygenase (FMO) metabolism, respectively. Tebuconazole (10 mg/L) was added after 12 h of culturing C. elegans. Samples were collected at 2 h, 1, 2, 3, 5 and 7 days, respectively.
Isolation and identification of tebuconazole metabolites by large-scale culture
Four 500 mL cultures (maximal metabolite formation) were extracted after 3 days of incubation, combined and concentrated as described above. The major metabolites M1 and M2 were separated in the extracts by preparing the liquid phase. The isolated samples were analyzed by UPLC-MS/MS.
Instrumental analysis methods
Tebuconazole and its metabolites were detected by GC–MS (QP 2010, Shimadzu, Japan). The GC–MS was equipped with an RTX-5MS column (30 m, 0.25 m film thickness, 0.25 mm inner diameter; Agilent Technologies, USA). GC–MS was performed with helium as carrier gas at a flow rate of 7.0 mL/min. The programmed warming methods used in the experiments were 80 °C (1 min), 7 °C/min–240 °C (2 min), 7 °C/min–290 °C (30 min). The injection port and interface were set to 260 and 280 °C, respectively. The mass spectrometer of the GC–MS was used in electron impact (EI) mode at 70 eV during sample detection. Preparative liquid phase analysis was performed using a column equipped with a SinoChrom ODS-BP (5 μm, 10 mm × 250 mm); mobile phase A: methanol; mobile phase B: distilled water; mobile phase composition: 30–100% A and 7–0% B (gradient elution); flow rate: 3 mL/min; injection volume: 2 mL; UV detection wavelength: 230 nm; M1 eluted at 29.1 min, M2 eluted at 28.4 min. M1 eluted at 29.1 min and M2 eluted at 28.4 min.
Results
Metabolism of tebuconazole by C. elegans.
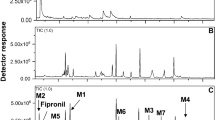
Analysis of the GC–MS results showed that tebuconazole can be rapidly converted into a variety of metabolites (Fig. 1). For example, the degradation rate of tebuconazole in the culture 72 h after treatment was about 85%. M1 and M2 were the major metabolites (Fig. 2A). M1 was detected at 24 h and reached its maximum concentration at 3 days. Another metabolite, M2 was similarly detected at 24 h and the highest accumulation of this metabolite was observed after 5 days. M1 was the most abundant metabolite throughout the experiment. Identification was performed by large scale culture. There was no substantial degradation of tebuconazole identified in sterilization control trials, and no metabolite peaks were observed. (Fig. 1A).
Effects of PB on the degradation of tebuconazole
In contrast to the control experiments, a significant amount of tebuconazole was still present in the PB-treated culture. For instance, the amount of tebuconazole that remained after 3 days was roughly 28–67% of the initial dose and after 7 days of tebuconazole was about 13–63% of the initial dose (Fig. 2). In all the incubations containing PB, minor metabolites (M3-M5) were not detected or found to accumulate in very small amounts. The concentration of M1 in PB-treated cultures (5 mg/L) was approximately 4–12-fold less than that of the control (Fig. 2A, B). Its concentration reached a maximum at 72 h. M2 concentrations found in PB-treated cultures (5 mg/L) were approximately 5–10 times lower than in the control cultures (Fig. 2B), and its concentration started to decrease after reaching a maximum at 5 days. At higher levels of PB treatment (50 mg/L), the concentrations of metabolites M1 and M2 were only found at trace levels and thereby being much lower than in the control (Fig. 2C).
Effects of MZ on the degradation of tebuconazole
Tebuconazole was rapidly degraded in both concentrations of MZ-treated cultures (5, 50 mg/L). In cultures treated with high concentration of MZ (50 mg/L), residual tebuconazole was approximately 10% of the initial dose after 7 days (Fig. 3C). The overall characteristics of the metabolites of tebuconazole and low levels of MZ (5 mg/L) were not different from the control (Fig. 3B). In PB-treated cultures, M1 was the major metabolite, and its kinetic response in MZ-treated cultures was almost similar to that of the control.
Identification of tebuconazole metabolites by C. elegans
Throughout the experiment, five tebuconazole metabolites were identified, with two major metabolites (M1–M2). The mass spectral details of these metabolites are shown in Table 1. The GC–MS results showed retention times of 35.067 min and 35.858 min for M1 and M2, respectively. The molecular ion peak of the TMS derivatives of M1 and M2 was m/z 395, which was 88 Da (-OTMS) more than tebuconazole, indicating that the metabolites M1 and M2 were monohydroxylated tebuconazole. M1 and M2 have the same fragment ion peaks (m/z 338, m/z 313, m/z 248, m/z 213). The fragment ions m/z 338, m/z 313, m/z 213 of M1 and M2 were 88 Da (-OTMS) more than the fragment ions m/z 250, m/z 225, m/z 125 of tebuconazole, respectively, and presumably the fragment ions of tebuconazole (m/z 250, m/z 225, m/z 125) all contained the structure of benzene ring, so it was concluded that M1 and M2 were hydroxylated on the benzene ring. To further determine their structures, M1 and M2 were separated using a preparative HPLC. The separated M1 and M2 were analyzed by UPLC-MS/MS, and the results showed that the molecular weight of M1 was 324.14682 ([M + H]+) (Fig. S1) and M2 was 324.14713 ([M + H]+) (Fig. S2). Their structure was deduced from the molecular weight, and the chemical formula was C16H23ClN3O2 ([M + H]+). The order of the peaks of M1 and M2 in the liquid phase enabled the determination of the polarity of both M1 and M2, and thus the position of the hydroxyl groups of the two metabolites in the benzene ring. Metabolite M2 has a greater polarity than M1. Therefore, it is presumed that M1 is 5-(3-((1H-1,2,4-triazol-1-yl)methyl)-3-hydroxy-4,4-dimethylpentyl)-2-chlorophenol and M2 is 2-(3-((1H-1,2,4-triazol-1-yl)methyl)-3-hydroxy-4,4-dimethylpentyl)-5-chlorophenol.
Additional metabolites (M3-M5) were initially identified by mass spectrometry patterns and relevant references. The TMS-derivatized molecular ion peaks for M3 and M4 were m/z 483 and m/z 409, respectively. M3 and M4 have the same characteristic ion peaks as M1 and M2 (m/z 248). The characteristic ion peaks m/z 248 of the metabolites M1 and M2 are the loss of hydroxyl and tert-butyl groups and further loss of TMS-derivatized trimethylsilane, indicating that both M3 and M4 change at the tert-butyl position. The molecular ions of M3 are 178 Da more than tebuconazole, indicating that M3 is possibly a dihydroxylated tebuconazole. In addition, M3 has the same fragment ions as M1 and M2 (m/z 248, m/z 225, m/z 163), indicating that M3 has one hydroxyl group added to the benzene ring and another hydroxyl group added to the tert-butyl group, but the position of the hydroxyl group on the benzene ring is uncertain. The molecular ion of M4 is 74 Da higher than that of tebuconazole, and the molecular ion of M4 is 14 Da larger than that of M1 and M2. The fragment ion of M4 has the same fragment ion as the metabolites M1 and M2 (m/z 313, m/z 248, m/z 213, m/z 125), indicating that M4 underwent hydroxylation on the benzene ring. M4 has the fragment ion m/z 248, indicating that on the tert-butyl change has occurred. Therefore, it is speculated that M4 is a ketone structure formed on the basis of M1 and M2. The TMS-derivatized molecular weight of M5 is m/z 395. M5 has the same characteristic fragment ion m/z 250 as tert-butyl alcohol. The fragment ion m/z 250 is formed by the loss of tert-butyl, so it is speculated that M5 is formed by the addition of hydroxyl group at the tert-butyl position. On this basis, we speculated the metabolic pathway of C. elegans to tebuconazole (Fig. 4).
Discussion
C. elegans and other strains of the same genus are capable of metabolizing a wide range of compounds. There is a considerable similarity between their xenobiotic metabolism and that of animals, which will aid us in determining the metabolic destiny of the organic compounds in mammalian hepatocytes and studying the biotransformation of medications, pesticides, and pollutants in the environment. (Schocken et al. 1997; Palmer-Brown et al. 2017; Chen et al. 2017).
The current studies on the metabolism of tebuconazole found that the main metabolic pathways are hydroxylation and hydrolysis reactions. For example, in plants, tebuconazole is first hydroxylated and then continues to undergo glycosylation at the hydroxylated site (Obanda and Shupe 2009; Stoll et al. 2006; Hillebrands et al. 2021). Mold (Trichoderma harzianum), soft rot (Chaetomium globosum), white rot (Phanerochaete chrysosporium), and brown rot (Meruliporia. incrassata) can metabolize tebuconazole, and the main metabolic modes of tebuconazole are hydroxylation and hydrolysis reactions, and the carboxylation reaction also occurred after hydroxylation (Ge et al. 2021; Teng et al. 2019; Han et al. 2021; Theron et al. 2019). In this study, only hydroxylation reactions were found, and no glycosylation reactions as in plant metabolism or hydrolysis reactions as in microbial metabolism were found. It may be due to the lack of enzymes for these metabolic reactions in C. elegans. In terms of degradation rate, C. elegans could metabolize 40% of tebuconazole within 2 days, which is 7–10 times faster than the rate of microbial metabolism in previous studies. During this rapid degradation process, it is possible that some of the metabolites were rapidly converted to other compounds or metabolites that were difficult to detect and therefore not observed in the assay system. To study the oxidases involved in the metabolism of tebuconazole, PB and MZ were added to the cultures, as PB is a well-known CYP inhibitor and MZ is also a well-known FMO inhibitor. (Greule et al. 2018). The results of this study showed that PB greatly inhibited tebuconazole dissipation and the production of the five metabolites, but MZ only produced significant inhibition at the highest dose. The research results indicated that CYP plays a major role in the transformation of tebuconazole by C. elegans and FMO may play a lesser role in tebuconazole metabolism. However, the effect of FMO on the metabolism of tebuconazole cannot be ignored (Than et al. 2008; Zhang et al. 2020). The good metabolism of tebuconazole by C. elegans can help to remove tebuconazole from the environment. In addition, the metabolic pattern of C. elegans is similar to mammalian metabolism so it can provide the toxicity study and evaluation of metabolites during the metabolism of exogenous compounds in mammals (Mueller-Eigner et al. 2022). For other metabolic function enzymes in C. elegans remains to be studied.
Conclusions
In this study, the metabolism of tebuconazole by C. elegans is reported. Five metabolites were totally or tentatively identified with GC–MS and UPLC-MS/MS. The metabolism of tebuconazole by C. elegans was demonstrated to involve CYP and FMO. The experimental results revealed that the degradation of tebuconazole by C. elegans was superior to that of previously reported microorganisms. These findings will aid in the understanding of tebuconazole fungal degradation and its possible application in tebuconazole bioremediation.
Data availability
The datasets used and analyzed during the current study are available from the corresponding author on reasonable request.
References
Azhari EN, Dermou E, Barnard RL, Storck V et al (2018) The dissipation and microbial ecotoxicity of tebuconazole and its transformation products in soil under standard laboratory and simulated winter conditions. Sci Total Environ 638:892–906. https://doi.org/10.1016/j.scitotenv.2018.05.088
Bellot P, Dupont SM, Brischoux F, Budzinski H, Chastel O, Fritsch C, Lourdais O, Prouteau L, Rocchi S, Angelier F (2022) Experimental exposure to tebuconazole affects metabolism and body condition in a passerine bird, the house sparrow (Passer domesticus). Environ Toxicol Chem 41:2500–2511. https://doi.org/10.1002/etc.5446
Castro TFD, Da Silva Souza JG, de Carvalho AFS, de Lima AI, Palmieri MJ, Vieira LFA, Marcussi S, Machado MRF, Murgas LDS (2018) Anxiety-associated behavior and genotoxicity found in adult danio rerio exposed to tebuconazole-based commercial product. Environ Toxicol Pharm 62:140–146. https://doi.org/10.1016/j.etap.2018.06.011
Chen Y, Tian JL, Wu JS, Sun TM, Zhou LN, Song SJ, You S (2017) Biotransfomation of cyperenoic acid by Cunninghamella elegans as 3.2028 and the potent anti-angiogenic activities of its metabolites. Fitoterapia 118:32–37. https://doi.org/10.1016/j.fitote.2017.02.004
Coombes MA, Marca EL, Naylor LA, Piccini L, Waele JD, Sauro F (2015) The influence of light attenuation on the biogeomorphology of a marine karst cave: a case study of Puerto Princesa underground river, Palawan, The Philippines. Geomorphology 229:125–133. https://doi.org/10.1016/j.geomorph.2014.10.007
Cui N, Xu H, Yao S, He Y, Zhang H, Yu Y (2018) Chiral triazole fungicide tebuconazole: enantioselective bioaccumulation, bioactivity, acute toxicity, and dissipation in soils. Environ Sci Pollut Res Int 25:25468–25475. https://doi.org/10.1007/s11356-018-2587-9
Dong BZ, Yang YP, Pang NN, Hu JY (2018) Residue dissipation and risk assessment of tebuconazole, thiophanate-methyl and its metabolite in table grape by liquid chromatography-tandem mass spectrometry. Food Chem 260:66–72. https://doi.org/10.1016/j.foodchem.2018.03.062
Franco ME, Sutherland GE, Fernandez-Luna MT, Lavado R (2020) Altered expression and activity of phase I and II biotransformation enzymes in human liver cells by perfluorooctanoate (PFOA) and perfluorooctane sulfonate (PFOS). Toxicology 430:152339. https://doi.org/10.1016/j.tox.2019.152339
Ge JZ, Fu WH, Bai MW, Zhang L, Guo BL, Qiao QL, Tao RY, Kou JC (2021) The degradation of residual pesticides and the quality of white clover silage are related to the types and initial concentrations of pesticides. J Pestic Sci 46(4):342–351. https://doi.org/10.1584/jpestics.D21-017
Greule A, Stok JE, De Voss JJ, Cryle MJ (2018) Unrivalled diversity: the many roles and reactions of bacterial cytochromes P450 in secondary metabolism. Nat Prod Rep 35:751–797. https://doi.org/10.1039/C7NP00063D
Han LX, Kong XB, Xu M, Nie JY (2021) Repeated exposure to fungicide tebuconazole alters the degradation characteristics, soil microbial community and functional profiles. Environ Pollut 287:117660. https://doi.org/10.1016/j.envpol.2021.117660
Hillebrands L, Lamshoeft M, Lagojda A, Stork A, Kayser O (2020) Evaluation of callus cultures to elucidate the metabolism of tebuconazole, flurtamone, fenhexamid, and metalaxyl-m inbrassica napusl. Glycine max(l.) merr. zea maysl. andtriticum aestivuml. J Agric Food Chem 68:14123–14134. https://doi.org/10.1021/acs.jafc.0c05277
Hillebrands L, Lamshoeft M, Lagojda A, Stork A, Kayser O (2021) Metabolism of fenhexamid, metalaxyl-m, tebuconazole, flurtamone, and spirodiclofen in Cannabis sativa l. (hemp) plants. ACS Agric Sci Technol 1(3):192–201. https://doi.org/10.1021/acsagscitech.1c00010
Hou F, Teng PP, Liu FM, Wang WZ (2017) Tebuconazole and azoxystrobin residue behaviors and distribution in field and cooked peanut. J Agric Food Chem 65:4484–4492. https://doi.org/10.1021/acs.jafc.7b01316
Keum YS, Lee YH, Kim JH (2009) Metabolism of methoxychlor by Cunninghamella elegans ATCC36112. J Agric Food Chem 57:7931–7937. https://doi.org/10.1021/jf902132j
Kwon HC, Kim DH, Jeong CH, Kim YJ, Han JH, Lim SJ, Shin DM, Kim DW, Han SG (2021) Tebuconazole fungicide induces lipid accumulation and oxidative stress in HepG2 Cells. Foods 10:2242. https://doi.org/10.3390/foods10102242
Madrigal-Matute J, Cuervo AM (2016) Regulation of liver metabolism by autophagy. Gastroenterology 150:328–339. https://doi.org/10.1053/j.gastro.2015.09.042
Mueller-Eigner A, Berry B, Vodickova A, Wojtovich A, Peleg S, Kaeberlein M (2022) Optogenetic increase in mitochondrial protonmotive force causes increased lifespan in C. elegans. Free Radical Biol Med 192:22–23. https://doi.org/10.1016/j.freeradbiomed.2022.10.021
Obanda DN, Shupe TF (2009) Biotransformation of tebuconazole by microorganisms: evidence of a common mechanism. Wood Fiber Sci 41:157–167
Palmer-Brown W, Dunne B, Ortin Y, Fox MA, Murphy CD (2017) Biotransformation of fluorophenyl pyridine carboxylic acids by the model fungus Cunninghamella elegans. Xenobiotica 47(9):763–770. https://doi.org/10.1080/00498254.2016.1227109
Palmer-Brown W, de Melo Souza PL, Murphy CD (2019) Cyhalothrin biodegradation in Cunninghamella elegans. Environ Sci Pollut Res 26(2):1414–1421. https://doi.org/10.1007/s11356-018-3689-0
Schocken MJ, Mao J, Schabacker DJ (1997) Microbial transformations of the fungicide cyprodinil (CGA-219417). J Agric Food Chem 45:3647–3651. https://doi.org/10.1021/jf970298l
Shen XM, Chen YJ, Zhang J, Yan X, Liu W, Guo YF, Shan QL, Liu SJ (2019) Low-dose PCB126 compromises circadian rhythms associated with disordered glucose and lipid metabolism in mice. Environ Int 128:146–157. https://doi.org/10.1016/j.envint.2019.04.058
Stoll N, Schmidt E, Thurow K (2006) Isotope pattern evaluation for the reduction of elemental compositions assigned to high-resolution mass spectral data from electrospray ionization Fourier transform ion cyclotron resonance mass spectrometry. J Am Soc Mass Spectrom 17(12):1692–1699. https://doi.org/10.1016/j.jasms.2006.07.022
Teng MM, Zhao F, Zhou YM, Yan S, Tian SN, Yan J, Meng ZY, Bi S, Wang CJ (2019) Effect of propiconazole on the lipid metabolism of zebrafish embryos (Danio rerio). J Agric Food Chem 67:4623–4631. https://doi.org/10.1021/acs.jafc.9b00449
Than PP, Jeewon R, Hyde KD, Pongsupasamit S, Mongkolporn O, Taylor PWJ (2008) Characterization and pathogenicity of Colletotrichum species associated with anthracnose on chilli (Capsicum spp.) in Thailand. Plant Pathol 57(3):562–572. https://doi.org/10.1111/j.1365-3059.2007.01782.x
Theron CW, Labuschagne M, Albertyn J, Smit MS (2019) Heterologous coexpression of the benzoate-para-hydroxylase CYP53B1 with different cytochrome P450 reductases in various yeasts. Microb Biotechnol 12:1226–1138. https://doi.org/10.1111/1751-7915.13321
Yang JD, Liu SH, Liao MH, Chen RM, Ueng TH (2018) Effects of tebuconazole on cytochrome P450 enzymes, oxidative stress, and endocrine disruption in male rats. Environ Toxicol 33:899–907. https://doi.org/10.1002/tox.22575
You XW, Li YQ, Wang XG, Xu JL, Zheng X, Sui CC (2017) Residue analysis and risk assessment of tebuconazole in jujube (Ziziphus jujuba mill). Biomed Chromatogr 31:e3917. https://doi.org/10.1002/bmc.3917
Zhang LQ, Song LL, Xu XM, Zou XH, Duan K, Gao QH (2020) Characterization and fungicide sensitivity of Colletotrichum species causing strawberry anthracnose in eastern China. Plant Dis 104(7):1960–1968. https://doi.org/10.1094/PDIS-10-19-2241-RE
Zhu YZ, Keum YS, Yang L, Lee H, Park H, Kim JH (2010) Metabolism of a fungicide mepanipyrim by soil fungus Cunninghamella elegans ATCC36112. J Agric Food Chem 58:12379–12384. https://doi.org/10.1021/jf102980y
Funding
This study was carried out with the support of the “Research Program for Agricultural Science & Technology Development (Project PJ0140182018)”, National Institute of Agricultural Sciences, and Rural Development Administration, Republic of Korea.
Author information
Authors and Affiliations
Contributions
YZ contributed to the study conception and design. MM, ZZ and ZZ performed the experiments and analysis. Material preparation, data collection, and analysis were performed by MM, ZZ and ZZ. The manuscript was written by ZZ, and edited by MM. All authors read and approved the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Meng, M., Zhai, Z., Zhang, Z. et al. Metabolic pathway of tebuconazole by soil fungus Cunninghamella elegans ATCC36112. Antonie van Leeuwenhoek 116, 1385–1393 (2023). https://doi.org/10.1007/s10482-023-01894-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-023-01894-1