Abstract
The Carpathian Basin is a lowland plain located mainly in Hungary. Due to the nature of the bedrock, alluvial deposits, and a bowl shape, many lakes and ponds of the area are characterized by high alkalinity. In this study, we characterized temporal changes in eukaryal and bacterial community dynamics with high throughput sequencing and relate the changes to environmental conditions in Lake Velence located in Fejér county, Hungary. The sampled Lake Velence microbial populations (algal and bacterial) were analyzed to identify potential correlations with other community members and environmental parameters at six timepoints over 6 weeks in the Spring of 2012. Correlations between community members suggest a positive relationship between certain algal and bacterial populations (e.g. Chlamydomondaceae with Actinobacteria and Acidobacteria), while other correlations allude to changes in these relationships over time. During the study, high nitrogen availability may have favored non-nitrogen fixing cyanobacteria, such as the toxin-producing Microcystis aeruginosa, and the eutrophic effect may have been exacerbated by high phosphorus availability as well as the high calcium and magnesium content of the Carpathian Basin bedrock, potentially fostering exopolymer production and cell aggregation. Cyanobacterial bloom formation could have a negative environmental impact on other community members and potentially affect overall water quality as well as recreational activities. To our knowledge, this is the first prediction for relationships between photoautotrophic eukaryotes and bacteria from an alkaline, Hungarian lake.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
The shallow lakes of the Carpathian Basin in Hungary are some of the most alkaline in the world (Boros et al. 2014). The basin is surrounded by slowly eroding mountains composed of limestone and carboniferous granite with high magnesium and calcium carbonate content deposited into the lakes and ponds that have formed on top of an ancient alluvial fan formed by the Danube, Drava, and Tisza Rivers (Horváth et al. 2004; Borics et al. 2016). Despite the extreme alkaline conditions, high carbon availability supports productive microbial communities in the region’s lakes and ponds (Ács et al. 1994, 2003; Vörös et al. 2006; Grant 2006; Felföldi et al. 2009; Pálffy et al. 2014; Szabó et al. 2017).
Lake Velence, located in Fejér county, is the third largest lake in Hungary (24.17 km2). Shallow, with a mean depth ranging from 0.5 to 2 m, Lake Velence is an endorheic or terminal lake supplied by two streams and catchment runoff (Horváth et al. 2004; Záray et al. 2005; Boromisza et al. 2014). Adjacent basement springs make limited contributions to the lake, evident in several recorded desiccation periods (Horváth et al. 2004). The lake has a unique limnogeology characterized by concentrated calcium carbonate inputs that reflect the region’s carbonaceous bedrock. These factors contribute to characteristically high pH (8.5–9.9), sodium (~ 300 mg L−1), bicarbonate (~ 680 mg L−1), magnesium (~ 420 mg L−1) and sulfate (~ 670 mg L−1) concentrations (Horváth et al. 2004; Borsodi et al. 2005; Haas 2012; Boromisza et al. 2014).
Submerged macrophytes differentiate the western portion of the lake from the open water of the eastern portion with close proximity to Budapest (50 km southwest), making the lake a popular holiday destination and recreational resource (Boromisza et al. 2014). Due to agricultural runoff, the lake has become eutrophic over the last century and highly susceptible to large cyanobacterial blooms (Water Management Directorate of VITUKI Environmental Protection and Water Management Research Institute on behalf of the Ministry for Environment and Water 2006).
Certain non-nitrogen fixing cyanobacteria, such as Microcystis aeruginosa isolated from Lake Velence, capitalize on high nitrogen availability and form large visible blooms capable of producing the toxin microcystin (MC) (Habermehl et al. 1997; Reskóné and Torokne 2000). MC occurs in different forms, of which MC-LR is the most toxic to lake organisms, wildlife, livestock, and humans inhibiting liver protein phosphatases leading to hemorrhaging (Runnegar et al. 1995; Gupta et al. 2003; Paerl and Otten 2013). Blooms may be augmented by the high availability of soluble calcium and magnesium, enhancing lectin activity, a key constituent of extracellular polymeric substances (EPS) important in bloom formation (Zhao et al. 2011).
Large cyanobacterial blooms threaten biodiversity by limiting light, oxygen, and nutrient availability, and although cyanobacteria release oxygen as a byproduct of photosynthesis, oxygen is consumed when these large blooms begin to decompose (Paerl and Otten 2013). Elevated global temperatures and increased eutrophication may additively increase toxic bloom frequency and extent (Davis et al. 2009; De Senerpont Domis et al. 2012; Paerl and Otten 2013; Harke et al. 2016; Wood et al. 2016; Hamilton et al. 2016). Fluctuations in community members in response to bloom frequency can reduce biodiversity, ecosystem function, and be indicative of larger global trends such as climate change (Strayer and Dudgeon 2010; Dodds et al. 2012; Paerl and Paul 2012).
Here, we investigated fluctuations in microbial community composition and diversity by observing the environmental conditions of Lake Velence during a toxic M. aeruginosa bloom. Over several weeks, increases in water temperature and nutrient availability contributed to optimal conditions for large and toxic bloom formation. By constructing a predictive inter-domain population network, our findings depict potential relationships within the microbial community. Abiotic variables, such as cations present in the bedrock, may have exacerbated bloom formation facilitating cell aggregation through EPS synthesis. By investigating these connections we can improve our understanding of toxic bloom formation and develop effective mitigation strategies.
Materials and methods
Site description
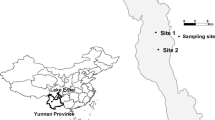
Lake Velence, located in Fejér county, Hungary, is approximately 50 km southwest of the capital, Budapest. The lake is considered endorheic, with no outlets and a shallow depth (0.5–1.6 m) that impedes seasonal nutrient exchange between the epilimnion and hypolimnion characteristic of deeper lakes. Samples were collected from a single location and are only representative of a small portion of the larger system. Figure 1 illustrates the sampling location (47°14′3.481″N, 18°37′51.456″E) on a dock located in the northwestern corner of the lake, approximately 10 m southeast of the swimming access. The northwest area of the lake is one of the most developed, providing easy access for recreation, and highly susceptible to toxic bloom formation.
Sample collection
To analyze biotic and abiotic microbial community dynamics in this portion of Lake Velence, limnological chemistry and biological samples were collected at six timepoints (1—5/18, 2—5/25, 3—6/1, 4—6/8, 5—6/13, and 6—6/21) approximately 1 week apart (every 5–8 days) at mid-day from the same location during the spring of 2012 (May 18th–June 21st). Due to the shallow depth of this portion of the lake (0.5 m), only surface water samples (0–0.25 m) were collected using sterile polypropylene bottles (Nalgene Rochester, NY, USA). Samples for chemical and community analyses (1 L) were kept at 4 °C for transport back to the laboratory for processing described in detail below. Biomass was also collected on June 8th from a large bloom that persisted for approximately 10 days. Samples were kept frozen until being lyophilized for mass spectrometer analysis described below.
MALDI-TOF MS analysis
Biomass was collected with a 50 μm mesh size plankton net, lyophilized, and assayed for microcystin with matrix-assisted desorption/ionization time-of-flight mass spectrometry (MALDI-TOF MS) according to methods described by Farkas and others (Farkas et al. 2014). Five milligrams of lyophilized biomass and 200 μL of 50% aqueous methanol were homogenized, sonicated for 5 mins, and incubated at room temperature for 1 h. Samples were analyzed utilizing a Bruker Biflex MALDI-TOF mass spectrometer (Bruker Daltonics Inc., Billerica, MA, USA) in positive-ion mode equipped with delayed-ion extraction. A nitrogen laser desorbed/ionized molecules at 337 nm. An average of > 100 spectra readings was acquired with 19-kV accelerating and 20-kV reflectron voltage. The instrument was calibrated with an external standard as per Farkas and others (Farkas et al. 2014). Compounds were identified by referencing the mass of [M 1 H]+ peak. Post-source decay (PSD) measurements were performed following the identification of mass values taken from the same microcystin and peptide template.
Chemical analyses
For chemical analyses, the guidelines given in Standard Methods for the Examination of Water and Wastewater were followed (Eaton and Franson 2005). Temperature at 0.25 m was recorded using a HI 9033 multimeter (Hanna Instruments, Woonsocket, RI, USA). Immediately following sample collection and transport to the lab, pH and conductivity were measured (Radelkis OP-264 m, Budapest, Hungary). Subsequently, aliquots from water samples were filtered through a 0.22 µm membrane filter prior to the determination of the following parameters.
Concentration of dissolved organic carbon (DOC), dissolved inorganic carbon (DIC) and total nitrogen (TN) was measured with a Multi N/C 2100S analyzer (Analytik Jena, Jena, Germany). Ammonium (ASTM 4500-NH3-D), nitrite (ASTM 4500-NO2-B), nitrate (ASTM 4500-NO3-B) and, sulfate (ASTM 4500-SO4 2-E) ion concentrations were measured by spectrometry. For ammonium ion quantification, 100 mL of each sample was acidified with 1 mL of 1 M sulfuric acid for conservation, and samples were neutralized just before the analysis. Reactive phosphate and total phosphorus (TP) were both examined with the ascorbic acid method. Before the TP measurement, persulfate oxidation was carried out (ASTM 4500-P-E). Sulfide was measured by an iodometric method (ASTM 4500-S-E). Sodium and potassium ion content was determined with a PFP-7 flame photometer (BUCK Scientific, East Norwalk, USA). Prior to analysis, samples were acidified with nitric acid to a 0.15% final concentration. Hardness was determined with the standard ethylenediamine-tetraacetic acid (EDTA) titrimetric method.
DNA extraction and sequencing
DNA extraction
Within an hour of collection, 1 L water samples were filtered in the lab on 0.22 μm polyethersulfone membrane filters (Corning Inc., Corning, NY, USA) and stored at − 80 °C for later DNA extraction. Filters were immediately stored at − 80 °C until transported on dry ice from Hungary to the USA for DNA analysis. Biomass collected on the filter was resuspended with the MO BIO PowerMax™ Soil DNA Isolation Kit PowerBead Solution (MO BIO Laboratories Inc., Carlsbad, CA, USA) followed by cycles of grinding/free-thaw with sand and liquid nitrogen to achieve cell lysis (Zhou et al. 1996; Bowen De León et al. 2012; Bell et al. 2016). DNA was then isolated using the MO BIO PowerMax™ Soil DNA Isolation Kit. Extracted DNA was cleaned and concentrated with the Wizard® SV Gel and PCR Clean-Up System (Promega Corporation, Madison, WI, USA) according to the manufacturer’s protocol.
Bar-coded pyrosequencing
Pyrosequencing was used to characterize the microbial population of the lake samples via PCR amplification of the V1–V3 region of the SSU rRNA gene sequences. Each sample was labeled with a unique 10 nucleotide-barcode for multiplexing. The SSU rRNA gene sequences for Eukarya and Bacteria were amplified via 25 cycles of PCR with the following barcoded primers; 7F (5′-ACCTGGTTGATCCTGCCAG-3′) and 591R (5′-GGAGCTGGAATTACCG-3′) for Eukarya and FD1 (5´AGAGTTTGATCCTGGCTCAG-3´) and 529R (5´-CGCGGCTGCTGGCAC-3´), which targeted the V1–V3 region of Bacteria (Bowen De León et al. 2012). An attempt to amplify archaeal sequences was made separately from Bacteria using a nested approach with non-barcoded 21F (5′-TTCYGGTTGATCCYGCCRGA-3′) and 1492R (5′-CGGTT ACCTTGTTACGACTT-3′) for 20 cycles followed by an additional 20 cycles with barcoded 751F (5′-CCGACGGTGAGRGRYGAA-3′) and 1204R (5′-TTMGGGGCATRCNKACCT-3′) primers (Baker et al. 2003; Barnhart et al. 2013). Viral dsDNA was amplified by targeting the major capsid protein (MCP), highly conserved within the Phycodnaviridae family, via touchdown PCR using a pair of degenerate primers mcpF (5′-GGYGGYCARCGYATTGA-3′) and mcpR (5′-TGIARYTGYTCRAYIAGGTA-3′) as described previously reported (Larsen et al. 2008). PCR products of the correct size were confirmed using a 1% agarose gel for all amplimers. Eukaryotic and bacterial products were cut from the gel and pooled using an Ultrafree®-DNA gel extraction column (Millipore Corporation, Bedford, MA, USA). The gel extract was cleaned and concentrated using the Wizard® SV Gel and PCR Clean-Up System, and dsDNA was quantified with a Qubit fluorometer (Invitrogen, Carlsbad, CA, USA). Adaptors for 454 sequencing were ligated to the eukaryotic and bacterial amplicons and were sequenced on a 454 GS-Junior (454 Life Sciences, Branford, CT, USA). Roche’s image analysis identified sequences by barcode. Sequences were trimmed to one standard deviation below the mean length or removed if shorter. Employing the Phred score filter, 15% of the nucleotides were allowed to be below Q27, and removed if primer errors or Ns were observed (Bowen De León et al. 2012). Sequences are publicly available through the Sequence Read Archive (SRA) as accession PRJNA480706.
Processing of pyrosequencing data and community analysis
Data analysis was performed with default settings using the Quantitative Insights into Microbial Ecology (QIIME) software package, version 1.4.0 (Caporaso et al. 2010b). Parameter settings for demultiplexing were at a default of minimum 200 base pairs and maximum 1000 base pairs in length. Metadata files were prepared according to a QIIME compatible template taking into account environmental data. The sequencing libraries were split according to barcode for each of the respective domains (Bacteria and Eukarya). Sequences were then concatenated for data normalization needed in downstream analysis. Operational taxonomic units (OTUs) were assigned using the open reference OTU picking protocol. Clusters were referenced against the Silva 108 database and pre-clustered at 97% identity using UCLUST (Edgar 2010).
Sequence reads that matched a Silva reference sequence at 97% identity were clustered within an OTU defined by a reference sequence. OTU assignment (and all subsequent steps) was performed for the combined Bacteria and Eukarya reads. The singleton OTUs were discarded. The centroid sequence in every cluster was selected to represent the cluster and aligned with the Silva core set using PyNAST (Caporaso et al. 2010a). Chimeric sequences, identified with Chimera Slayer (Haas et al. 2011) and reads that failed to align with PyNAST were excluded from subsequent analyses. PyNAST (version 1.1) was used for sequence alignment and filtering through QIIME using default parameters.
Taxonomic assignments were additionally made using the retrained RDP Classifier (Wang et al. 2007) on the Silva 108 database for phylogenetic resolution at the genus level. Accounting for the phylogenetic structure of the microbial community, rarefied OTU tables for Bacteria and Eukarya were analyzed with UniFrac (Lozupone and Knight 2005). Diversity was quantified by calculating the effective number of OTUs, or Hill Number, using the R package “rioja” (Hill 1973; Jost 2006; Juggins 2012). Shannon and Simspson diversity indexes were also calculated.
The co-occurrence of community members and similarity between timepoints was quantified using the Pearson correlation and illustrated in a heat-map using the R vegan package version 2.0-10 (Oksanen 2011). Some taxa had a 0% relative abundance at certain timepoints; therefore, 0.1 was added to all values to allow a log transformation. To enhance the visual distribution of taxa, log transformed values were cubed and resulting values plotted (log (relative abundance + 0.1)3). Linear regressions were utilized to evaluate statistical correlations. A SparCC analysis was subsequently used to construct community correlation networks by estimating linear correlation values between log transformed abundances based on the absolute number of sequences for an OTU rather than a relative abundance (Friedman and Alm 2012; Berry and Widder 2014). The key advantage of this analysis was that the ratio of the fractions of two OTUs was independent of the fluctuations in other OTUs included in the analysis (i.e., subcompositional coherence) (Friedman and Alm 2012).
Statistical analysis
Regression analysis and p values were calculated using Matlab Statistics Toolbox release 2012a (The MathWorks, Inc., Natick, Massachusetts, United States). Only regressions with p ≤ 0.01 were considered significant. To incorporate taxonomic data, the direct-gradient ordination technique, Canonical Correspondence Analysis (CCA) was used, which concurrently showed lake taxa (based on UniFrac distances), timepoints, and water chemistry (Hall and Smol 1992). The CCA ordination is appropriate when assessing community dynamics because it does not use Euclidean based metrics that assume linear trends in community change. Not only does the ordination show environmental factors correlating to community changes, but the results suggest potential interactions between taxa (Amaral-Zettler et al. 2010). All axes are constrained to present a linear combination of the water chemistry that maximizes the dispersion of taxa (Hall and Smol 1992).
Results and discussion
Chemical analyses
Chemical parameters (excluding calcium, magnesium, sodium, and potassium which were measured at the start of sampling) were sampled once per week over the course of 6 weeks from May 2012 to June 2012 at the same location of the lake. Over the tested timepoints, conductivity, nitrate, nitrite, DOC, DIC, and TP remained relatively constant (Table 1). The sulfate concentrations increased in the last 2 weeks, and the ammonium-N levels fluctuated between < 0.01 and 0.30 mg L−1 (Table 1). The phosphate (mg L−1) increased in weeks two and three. The temperature increased from 14 to 20 °C in week two, fluctuated between 20 and 23 °C up to week 5, and increased to almost 30 °C in the sixth week (Table 1). Concentrations of calcium, magnesium, sodium, and potassium were 30, 420, 297 and 46 mg L−1, respectively.
Biotic activity likely contributed to some chemical fluctuations. The pH increase between week three and four could have been indicative of higher photoautotrophic primary productivity. Alkaline conditions were not only the result of geochemistry but could be exacerbated by photosynthetic carbon fixation elevating pH as observed in similar systems (Imhoff et al. 1979; Wetzel 2001; López-Archilla et al. 2004). Decreases in the concentration of dissolved inorganic carbon (DIC), negatively correlated with pH (R2 = 0.75), lend support to the role of productivity in elevated pH (Hem 1985; López-Archilla et al. 2004). Subsequent increases in DIC at weeks five and six coincided with declines in pH and were further indicative of overall decreases in photoautotrophic activity during this time.
Phosphate concentrations in sampling timepoints four, five, and six were lower than that of two and three, and these results could allude to an interplay between biological storage and different rates of biotic utilization/replenishment and require further study (Hudson et al. 2000). Due to generally high nutrient concentrations in Lake Velence, especially nitrate, the lake can be characterized as eutrophic-hypertrophic suggesting that phytoplankton were not limited by nutrients during a majority of the sampling period (Wetzel 2001). Elevated nitrogen, phosphorus, and sulfate concentrations likely originated from an allochthonous source (i.e., the nutrient load provided by surrounding agriculture in addition to aquatic bird feces), as demonstrated in the case of other shallow alkaline lakes of the Carpathian Basin (Baldi and Kisbenedek 2000; Boros et al. 2008).
Sequencing and community analysis
The SSU rRNA gene sequences for Bacteria and Eukarya were amplified and sequenced for each of the samples (five samples in the case of Eukarya). Archaeal sequences were either absent or did not amplify and only Eukaryal and Bacterial amplicons were sequenced. Archaea may be insignificant members of the prokaryotic community in Lake Velence, as demonstrated recently in another soda lake in the Carpathian Basin (Szabó et al. 2017). After screening of the sequence raw data (see Methods), 118,593 quality gene sequences with a valid barcode were retrieved. Sequences with a 97% identity were clustered within an OTU totaling 7533 observed OTUs in Bacteria and Eukarya combined. Taxonomy tables are provided in the Supplementary Materials (Table IV and V).
Amplification of eukaryotic DNA resulted in low concentration and subsequently poor quality 18S reads for the fourth timepoint and were discarded (data for this 18S timepoint were removed from the statistical analysis). By conducting tandem 16S and 18S pyro-sequencing, the first to be conducted on a Hungarian lake, we were able to identify major taxa and population distributions during the spring of 2012 at six timepoints with each approximately 1 week apart.
Diversity was quantified by calculating Hill numbers, the effective number of taxa, for Bacteria and Eukarya for each timepoint. Trends in the fluctuation of bacterial and eukaryotic diversity were not correlated to one another (R2 = 0.13). Bacterial diversity was generally higher than eukaryotic diversity with the exception of the 5th timepoint (Table 2). Values for both the Simpson and Shannon indexes are provided in the supplementary materials and paralleled the Hill number trends (Supplementary Tables II and III).
Eukaryotic diversity
Eukaryotic diversity based on Hill number fluctuated over the course of the sampling period peaking during the fifth timepoint (Table 2). Diversity had a small negative correlation with the relative abundance of the cyanobacteria Microcystis (R2 = 0.17) discussed in greater detail in below. The relative abundance of Arthropoda (mainly composed of two taxa from the subphylum Crustacea) was highest during the first 3 weeks and suggestive of low suspended solids benefitting filter feeders (Vörös et al. 2006) (Fig. 2). In contrast, the relative abundances of Cryptophyta, Pavlova, and Ciliophora increased during the final 2 weeks (sampling timepoints five and six), when Microcystis abundance was the highest. Cryptophyta, which was identified via light microscopy in prior surveys of Lake Velence, demonstrated strong correlations with sulfate and DOC (R2 = 0.84, R2 = 0.53), confirming previous observations of sulfate tolerance in Cryptophyta and incorporation of available carbon into biomass (Ács et al. 1994; Camacho et al. 2001; Ács et al. 2003). Pavlova appeared to respond to carbon availability, positively correlating with DOC (R2 = 0.56). Previous research associated ciliate communities to the presence of reeds, ubiquitous on the western portion of the lake (Mieczan 2008; Boromisza et al. 2014).
Heat map of Eukaryotic taxa. The upper dendogram shows the co-occurrence of Eukaryotic taxa (listed as genera when possible) with > 2% relative abundance based on UniFrac analysis. The dendogram on the left shows similarities in community composition and abundance between timepoints. Note that no Eukaryotic sequences were amplified for week 4
The community was also dominated by sequences closely related to unidentified Chlorophytes, an undescribed genus of Chlorella, and a Thalassiosira related diatom. The first sampling timepoint had low algal relative abundance except for Pseudoschroederia sp., in the Characiaceae family. Other members of this family have been identified within the Carpathian Basin (Hindák and Hindáková 2008). Overall, algal relative abundance (e.g., Chlorophyta) within the eukaryotes increased with time, particularly during the last 2 weeks. After the third week, Chlorophyta exhibited the highest relative abundance for all detected 18S gene amplicon sequences at which time the pH ranged from 8.26 to 9.6. Relative fluctuations in both DOC and DIC may have been partially derived from (and further promoted) the growth of green algae and some bacterial phototrophs (Bertilsson and Jones 2003).
Differences in temperature, pH, DIC, and DOC were the major drivers of statistical variation between taxa and the chemical composition of sampling timepoints in Lake Velence (Fig. 3). Eukaryotic diversity was positively correlated with DIC and DOC (R2 = 0.3 and R2 = 0.5, respectively), particularly sequences indicative of green algae and diatoms. However, a different green algae sequence group (Chlorophyceae 2) and Botryococcus sequence group appeared to not respond as strongly to DIC, DOC, and temperature. Lying almost parallel to axis 1, changes in temperature accounted for the most variation between taxa and timepoints, and negatively correlated with eukaryotic diversity (R2 = 0.56). The relative abundance of the flagellate Euglenida increased with decreases in pH suggesting the organism may have a lower pH optimum. Increases in Chlorophyta correlated best with increases in temperature, DIC, and DOC, and the combination of increased carbon availability and temperature likely contributed to increased occurrence of certain OTUs and an overall decrease in diversity. Previous studies have shown that increases in inorganic compounds essential for growth will increase overall productivity, but a tipping point exists at which further increases depress basic cellular functions leading to decreased microbial diversity (and/or evenness) (Odum et al. 1979; Fields et al. 2005, 2006).
Canonical Correspondence Analysis (CCA) plotting physiochemical variables that statistically accounted for variation in Eukaryotic genera (with > 2% relative abundance based on UniFrac analysis) and sample points. Algae generally correlated to increases in temperature and DOC, potentially contributing to the latter (CCA1 = 62%, CCA2 = 36%)
Bacterial diversity
The abundance of bacterial taxa varied during the six sampling timepoints (Figs. 4, 5) but was more consistent over the sampled time period compared to 18S sequences. However, notable changes were observed for the Cyanobacteria and Actinobacteria groups. Diversity, based on Hill number, negatively correlated with DOC (R2 = 0.75). Anaeroplasmatales, an order of Mollicutes often found in the human gut, increased with time (Stearns et al. 2011). This could be the result of the sampling location which was adjacent to swimming access utilized more frequently as spring progressed into summer. In the first 3 weeks Actinobacteria was the most abundant phylum of bacteria with a positive correlation to elevated pH. A large visible bloom, primarily containing M. aeruginosa that was previously isolated from Lake Velence, was observed on the fourth and fifth timepoints (June 8th and June 13rd) during the lowest observed bacterial diversity and suspended cell aggregates green in color were observed in all water samples from these timepoints (Kós et al. 1995). Microcystis abundance negatively correlated with bacterial diversity (Hill number) (R2 = 0.6). Specifically, a decline in the relative abundance of Actinobacteria was observed during the bloom and negatively correlated with Microcystis abundance (R2 = 0.79). This could have been the result of Microcystis outcompeting Actinobacteria taxa, such as Actinomycetales, for nitrogen and phosphorus as indicated by the CCA in Fig. 5. Previous research conducted month long incubations of Microcystis in phosphorus enriched mesocosms, enhancing nitrogen limited conditions, but failed to invoke the succession of nitrogen-fixing taxa, such as Actinobacteria (Paerl et al. 2014). This suggests that Microcystis can effectively compete for nutrients even when conditions favor nitrogen-fixers (Paerl et al. 2014; Harke et al. 2016).
Canonical Correspondence Analysis (CCA) plotting chemical variables that drive variation in Bacterial genera (with > 2% relative abundance based on UniFrac analysis) and sample points. Taxa were most influenced by increases in available nitrogen in the first weeks of sampling (CCA1 = 43%, CCA2 = 14%)
Due to the inability of Microcystis to fix nitrogen, it is often associated with eutrophication, benefitting from high nitrogen availability (Paerl et al. 2011; Gobler et al. 2016; Harke et al. 2016; Hamilton et al. 2016). Declining nitrate concentrations correlated with Microcystis abundance (R2 = 0.65). Although we did not observe a correlation between ammonium concentrations and Microcystis abundance, ammonium concentrations declined during bloom formation from 0.11 mg L−1 at the fourth timepoint to < 0.01 mg L−1 by the sixth timepoint and is generally incorporated into biomass more rapidly than nitrate (Flynn 1998). The bloom may have also benefitted from elevated pH and the availability of calcium and magnesium sourced from the bedrock possibly contributing to extracellular polysaccharide (EPS) formation facilitating M. aeruginosa cell aggregation and bloom formation (Haas 2012; Gobler et al. 2016; Harke et al. 2016).
Increased temperature could have also been an influential variable, as M. aeruginosa isolates have been shown to be limited by temperatures lower than 15 °C (Robarts and Zohary 1987; Jiang et al. 2008). Robarts and Zohary observed that growth rate increased with temperature and had an optima of 25 °C or higher (Robarts and Zohary 1987). Such blooms can pose a threat to public safety because they could release the compound, microcystin (MC), which can be lethal to humans and other biota (Paerl et al. 2001; Paerl and Otten 2013). We detected three chemical forms of microcystin in collected biomass, MC-LR, MC-YR, and MC-WR totaling 3.342 mg g−1 dry weight (DW) of MC-LR equivalent (Farkas et al. 2014). MC-LR and MC-YR was not detected in Lake Velence until 1997, but were likely responsible for widespread skin, stomach, and eye ailments related to a very large Microcystis bloom in 1992, which resulted in restricted access to Lake Velence and other infected waters (Habermehl et al. 1997). Research has demonstrated that the growth of the toxic strain, unique in containing microcystin synthesis genes (mcyA-mcyJ), was exacerbated by warmer water temperatures (25–30 °C) (Davis et al. 2009). The peak of the large bloom in Lake Velence (June 13rd) occurred at 21.9 °C, and by the following week the water temperature had risen an additional 7.8 °C providing ideal conditions for not only large Microcystis blooms, but the synthesis of microcystin. The highest relative abundance of the toxic strain also coincided with high total phosphorus availability (1.35 mg L−1) further conducive to bloom formation in Lake Velence (Davis et al. 2009).
In the case of Lake Velence, the increasing frequency and toxicity of blooming Microcystis could cause disease and possibly mortality in lake biota, potentially altering the ecosystem, its biodiversity, and severely limiting recreational activities. Microcystin has also been detected in several other Hungarian lakes and ponds suggesting the increasing probability of more toxic blooms throughout the region blooms promoted by ongoing eutrophication and temperature increases (Vasas et al. 2006; Davis et al. 2009; Vasas et al. 2010, 2013).
Algal and bacterial dynamics
The relative algal abundance declined over the course of the 6-week sampling period (May 18th–June 21st, 2012) likely due in part to competition. The simultaneous decline in phosphorus concentration with an increase in the relative abundance of cyanobacteria, particularly Microcystis, supports the efficiency of phosphorus uptake by this cyanobacterium, especially following periods of limitation (Ritchie et al. 2001; Shen and Song 2007) (Fig. 5). Phosphorus uptake could be exacerbated by a large bloom, further limiting phosphorus availability to other community members. It is possible that the aforementioned Microcystis bloom could have outcompeted algae for nitrogen as well, but there was no significant negative correlation observed between alga taxa and Microcystis. Rather, we observed positive correlations between Microcystis and an alga, Komma sp. (R2 = 0.44). Some members of the algal community may have benefitted from cyanobacteria potentially supplying important growth promoting hormones like auxins (Tarakhovskaya et al. 2007; Foster et al. 2011; Amin et al. 2012). In addition to the possible exchange of growth promoting metabolites, the Komma sp. could have been more efficient at sequestering and storing phosphorus at low concentrations, possibly giving the alga a competitive advantage over other taxa.
Viral infection could have also impacted the algae population. Several previous studies emphasized the pivotal role these viruses play in aquatic systems (Van Etten et al. 1991; Hill et al. 1998; Wilhelm and Suttle 1999; Wommack and Colwell 2000; Brussaard 2004; Martínez et al. 2007; Rhodes and Martin 2010; Short 2012; Weitz and Wilhelm 2012). We amplified the region for the major capsid protein (MCP) that is highly conserved in Phycodnaviridae, the family of double-stranded DNA (dsDNA) viruses that infect algae (Larsen et al. 2008). The results indicate the presence of dsDNA algal viruses in Lake Velence. However, due to the very limited availability of these viral genomes, we were only able to qualitatively confirm presence of the viruses via gel electrophoresis of amplified products from timepoints one, four, five, and six (See Supplementary Figure 1). Although we cannot state to what degree, if any, viral infection impacted the algal community, the potential that population dynamics and resource allocation were influenced should be acknowledged. Further work is needed to understand the potential role in algal population dynamics as well as nutrient cycling.
Early increases in the observed Crustacea populations showed correlations to α-Proteobacteria. Specifically, a significant positive correlation was observed between α-Proteobacteria and Unk. Crustacea 1 (R2 = 0.87) and Unk. Crustacea 2 (R2 = 0.63). Known members of the α-Proteobacteria can be endosymbionts and/or intracellular parasites (Newton et al. 2011). Half of the α-Proteobacteria OTUs present in the Lake Velence samples belonged to the Rickettsiales order (50.7%), a taxon that frequently has symbiotic relationships with crustaceans (Fritsche et al. 1999). These results suggest that the corresponding increase between α-Proteobacteria, Unk. Crustacea 1, and Unk. Crustacea 2 could represent linked population dynamics through cooperation or competition. The algae that could be available as potential food sources, coupled with increases in pH and sulfate, may have directly or indirectly benefitted the two observed Crustacea taxa (pH: R2 = 0.84 and sulfate: R2 = 0.86).
SparCC correlation network analysis was employed to identify possible correlations between community members (Friedman and Alm 2012). As observed in Fig. 6, the only significant alga taxa in the SparCC correlation network was an unknown Chlamydomondaceae. Members of Chlamydomondaceae have also been observed in neighboring systems, and in this case had a positive correlation (R2 = 0.99, p < 0.05) with the bacterial OTUs ACK-M1 (an Actinomycetales) and C111 (an Acidimicrobiales) (Borics et al. 2012). Previous studies have observed members of both Actinobacteria and Acidobacteria living in close, physical association with algae (e.g., the phycosphere or EPS of an alga cell) (Goecke et al. 2013; Green et al. 2015). This association is believed to supply key metabolites to the alga while providing carbon to the bacterium (Bell 1983; Sapp et al. 2007; Ramanan et al. 2015). Establishment of the phycosphere in this case could have been further encouraged by aqueous geochemistry due to the carbonaceous bedrock characteristic of the Carpathian Basin (Haas 2012). As previously discussed, the high availability of calcium and magnesium can promote EPS production, fostering cell aggregation and physical association (Kalakoutskii et al. 1990; Zhao et al. 2011). However, both of these bacterial phyla can occur in soils and the gut of domestic livestock (Lauber et al. 2009; Shepherd et al. 2011; Reti et al. 2013). Therefore, we cannot determine whether the positive correlation is due to an actual physical association with the alga, or is the byproduct of agricultural runoff and/or the aquatic bird population living in the reed islands of Lake Velence (Baldi and Kisbenedek 2000; De Jesús-Laboy et al. 2012).
In contrast, a negative correlation between Sphingobacteria, common in freshwater lakes, and two algal taxa was observed (R2 = 0.72 for Unk. Chlorophyceae 1 and R2 = 0.79 for Thalassiosira sp.) similar to a previous observation of Sphingobacteria succession during Thalassiosira rotula late stationary phase (Grossart and Simon 2007). Despite known algacidal properties for some members of this group (Sphingobacteria), results suggested that bacteria flourished on deceased algae exudates, but did not directly contribute to algal decline (Grossart and Simon 2007). Such findings indicate that a negative correlation between an alga and bacterium does not necessarily suggest predation but could also be indicative of opportunism. However, the nature of these relationships are subject to change with time and algal growth phases; i.e. in some instances bacteria can promote algal growth during early exponential phase, but at later points may transition into competitors or parasites (Seyedsayamdost et al. 2011). It is therefore critical to consider the duality of these relationships, both positive and negative, occurring in the phycosphere.
Conclusions
Characterization of the bacterial and eukaryotic microbial communities of Lake Velence indicated complex and potentially influential relationships between community composition and measured abiotic parameters during a cyanobacterial bloom. The findings reported here showed the rise of optimal conditions, specifically increases in temperature and nutrient concentration, for the formation of a toxic Microcystis bloom over 6 weeks. Eutrophic systems like Lake Velence, characterized by bedrock with high magnesium and calcium content that exacerbate cell aggregation, may be more vulnerable to large bloom formation. When put in the context of increasing lake eutrophication, supportive of bloom formation, and increasing global temperatures that ultimately favor toxin production, blooms in Lake Velence and similar system are likely to become more frequent with negative implications for biodiversity and potentially human health.
References
Ács É, Buczkó K, Lakatos G (1994) Changes in the mosaic-like water surfaces of the Lake Velence as reflected by reed periphyton studies. Stud Bot Hung 25:5–19
Ács É, Borsodi AK, Makk J, Molnár P, Mózes A, Rusznyák A et al (2003) Algological and bacteriological investigations on reed periphyton in Lake Velencei, Hungary. Hydrobiologia 506:549–557
Amaral-Zettler LA, Zettler ER, Theroux SM, Palacios C, Aguilera A, Amils R (2010) Microbial community structure across the tree of life in the extreme Río Tinto. ISME J 5:42–50
Amin SA, Parker MS, Armbrust EV (2012) Interactions between diatoms and bacteria. Microbiol Mol Biol Rev 76:667–684
Baker GC, Smith JJ, Cowan DA (2003) Review and re-analysis of domain-specific 16S primers. J Microbiol Methods 55:541–555
Baldi A, Kisbenedek T (2000) Bird species numbers in an archipelago of reeds at Lake Velence, Hungary. Glob Ecol Biogeogr 9:451–461
Barnhart EP, De León KB, Ramsay BD, Cunningham AB, Fields MW (2013) Investigation of coal-associated bacterial and archaeal populations from a diffusive microbial sampler (DMS). Int J Coal Geol 115:64–70
Bell WH (1983) Bacterial utilization of algal extracellular products. III: the specificity of algal-bacterial interaction. Limnol Oceanogr 28:1131–1143
Bell TAS, Prithiviraj B, Wahlen BD, Fields MW, Peyton BM (2016) A lipid-accumulating alga maintains growth in outdoor, alkaliphilic raceway pond with mixed microbial communities. Front Microbiol 6:497
Berry D, Widder S (2014) Deciphering microbial interactions and detecting keystone species with co-occurrence networks. Front Microbiol 5:219
Bertilsson S, Jones BJ (2003) Supply of dissolved organic matter to aquatic ecosystems. In: Findlay SEG, Sinsabaugh RL (eds) Aquatic ecosystems. Academic Press, New York, pp 3–24
Borics G, Tóthmérész B, Lukács BA, Várbíró G (2012) Functional groups of phytoplankton shaping diversity of shallow lake ecosystems. Hydrobiologia 698:251–262
Borics G, Acs E, Boros E, Er T, Grigorszky I, Kiss KT et al (2016) Water bodies in Hungary—an overview of their management and present state. Hung J Hydrol 96:57–67
Boromisza Z, Torok EP, Acs T (2014) Lakeshore-restoration: landscape ecology-land use: assessment of shore-sections, being suitable for restoration, by the example of Lake Velence (Hungary). Carpathian J Earth Environ Sci 9:179–188
Boros E, Nagy T, Pigniczki C, Kotymán L (2008) The effect of aquatic birds on the nutrient load and water quality of soda pans in Hungary. Acta Zool Acad Sci Hung 54:207–224
Boros E, Horváth Z, Wolfram G, Vörös L (2014) Salinity and ionic composition of the shallow astatic soda pans in the Carpathian Basin. Ann Limnol—Int J Limnol 50:59–69
Borsodi AK, Micsinai A, Rusznyák A, Vladár P, Kovács G, Tóth EM et al (2005) Diversity of alkaliphilic and alkalitolerant bacteria cultivated from decomposing reed rhizomes in a Hungarian soda lake. Microb Ecol 50:9–18
Bowen De León K, Ramsay BD, Fields MW (2012) Quality-score refinement of SSU rRNA gene pyrosequencing differs across gene region for environmental samples. Microb Ecol 64:499–508
Brussaard CPD (2004) Viral control of phytoplankton populations: a review. J Eukaryot Microbiol 51:125–138
Camacho A, Vicente E, Miracle MR (2001) Ecology of Cryptomonas at the chemocline of a karstic sulfate-rich lake. Mar Freshw Res 52:805–815
Caporaso JG, Bittinger K, Bushman FD, DeSantis TZ, Andersen GL, Knight R (2010a) PyNAST: a flexible tool for aligning sequences to a template alignment. Bioinformatics 26:266–267
Caporaso JG, Kuczynski J, Stombaugh J, Bittinger K, Bushman FD, Costello EK et al (2010b) QIIME allows analysis of high-throughput community sequencing data. Nat Methods 7:335–336
Davis TW, Berry DL, Boyer GL, Gobler CJ (2009) The effects of temperature and nutrients on the growth and dynamics of toxic and non-toxic strains of Microcystis during cyanobacteria blooms. Harmful Algae 8:715–725
De Jesús-Laboy KM, Godoy-Vitorino F, Piceno YM, Tom LM, Pantoja-Feliciano IG, Rivera-Rivera MJ et al (2012) Comparison of the fecal microbiota in feral and domestic goats. Genes 3:1–18
De Senerpont Domis LN, Elser JJ, Gsell AS, Huszar VLM, Ibelings BW, Jeppesen E et al (2012) Plankton dynamics under different climatic conditions in space and time. Freshw Biol 58:463–482
Dodds WK, Robinson CT, Gaiser EE, Hansen GJ, Powell H, Smith JM et al (2012) Surprises and insights from long-term aquatic data sets and experiments. Bioscience 62:709–721
Eaton AD, Franson MAH (2005) Standard methods for the examination of water & wastewater. Ignatius Press, San Francisco
Edgar RC (2010) Search and clustering orders of magnitude faster than BLAST. Bioinformatics 26:2460–2461
Farkas O, Gyémant G, Hajdú G, Gonda S, Parizsa P, Horgos T et al (2014) Variability of microcystins and its synthetase gene cluster in Microcystis and Planktothrix water blooms in shallow lakes of Hungary. Acta Biol Hung 65:227–239
Felföldi T, Somogyi B, Márialigeti K, Vörös L (2009) Characterization of photoautotrophic picoplankton assemblages in turbid, alkaline lakes of the Carpathian Basin (Central Europe). J Limnol 68:385–395
Fields MW, Yan T, Rhee S-K, Carroll SL, Jardine PM, Watson DB et al (2005) Impacts on microbial communities and cultivable isolates from groundwater contaminated with high levels of nitric acid-uranium waste. FEMS Microbiol Ecol 53:417–428
Fields MW, Bagwell CE, Carroll SL, Yan T, Liu X, Watson DB et al (2006) Phylogenetic and functional biomakers as indicators of bacterial community responses to mixed-waste contamination. Environ Sci Technol 40:2601–2607
Flynn KJ (1998) Estimation of kinetic parameters for the transport of nitrate and ammonium into marine phytoplankton. Mar Ecol Prog Ser 169:13–28
Foster RA, Kuypers MMM, Vagner T, Paerl RW, Musat N, Zehr JP (2011) Nitrogen fixation and transfer in open ocean diatom–cyanobacterial symbioses. ISME J 5:1484–1493
Friedman J, Alm EJ (2012) Inferring correlation networks from genomic survey data. PLoS Comput Biol 8:e1002687
Fritsche TR, Horn M, Seyedirashti S, Gautom RK, Schleifer KH, Wagner M (1999) In situ detection of novel bacterial endosymbionts of Acanthamoeba spp. phylogenetically related to members of the order Rickettsiales. Appl Environ Microbiol 65:206–212
Gobler CJ, Burkholder JM, Davis TW, Harke MJ, Johengen T, Stow CA et al (2016) The dual role of nitrogen supply in controlling the growth and toxicity of cyanobacterial blooms. Harmful Algae 54:87–97
Goecke F, Thiel V, Wiese J, Labes A, Imhoff JF (2013) Algae as an important environment for bacteria-phylogenetic relationships among new bacterial species isolated from algae. Phycologia 52:14–24
Grant WD (2006) Alkaline environments and biodiversity. In: Gerday C, Glansdorff N (eds) Extremophiles. EOLSS, Developed under UNESCO. Eolss Publishers, Oxford, UK
Green DH, Echavarri-Bravo V, Brennan D, Hart MC (2015) Bacterial diversity associated with the Coccolithophorid Algae Emiliania huxleyi and Coccolithus pelagicus f. braarudii. Science 2015:194540
Grossart HP, Simon M (2007) Interactions of planktonic algae and bacteria: effects on algal growth and organic matter dynamics. Aquat Microb Ecol 47:163–176
Gupta N, Pant SC, Vijayaraghavan R, Rao PVL (2003) Comparative toxicity evaluation of cyanobacterial cyclic peptide toxin microcystin variants (LR, RR, YR) in mice. Toxicology 188:285–296
Haas J (2012) Geology of Hungary. Springer, Berlin
Haas BJ, Gevers D, Earl AM, Feldgarden M, Ward DV, Giannoukos G et al (2011) Chimeric 16S rRNA sequence formation and detection in Sanger and 454-pyrosequenced PCR amplicons. Genome Res 21:494–504
Habermehl GG, Krebs HC, Nemes P (1997) Occurrence of toxin producing cyanobacteria in Hungary. Isolation, separation and identification of microcystins. Verl Z Naturforschung 52:107–109
Hall RI, Smol JP (1992) A weighted-averaging regression and calibration model for inferring total phosphorus concentration from diatoms in British Columbia (Canada) lakes. Freshw Biol 27:417–434
Hamilton DP, Salmaso N, Paerl HW (2016) Mitigating harmful cyanobacterial blooms: strategies for control of nitrogen and phosphorus loads. Aquat Ecol 50:351–366
Harke MJ, Steffen MM, Gobler CJ, Otten TG, Wilhelm SW, Wood SA et al (2016) A review of the global ecology, genomics, and biogeography of the toxic cyanobacterium, Microcystis spp. Harmful Algae 54:4–20
Hem JD (1985) Study and interpretation of the chemical characteristics of natural water. Department of the Interior, U.S. Geological Survey, Alexandria
Hill MO (1973) Diversity and evenness: a unifying notation and its consequences. Ecology 54:427–432
Hill RW, White BA, Cottrell MT, Dacey JW (1998) Virus-mediated total release of dimethylsulfoniopropionate from marine phytoplankton: a potential climate process. Aquat Microb Ecol 14:1–6
Hindák F, Hindáková A (2008) Morphology and taxonomy of some rare chlorococcalean algae (Chlorophyta). Biologia 63:781–790
Horváth I, Daridáne M, Dudko A, Gyalog L, Ódor L (2004) A Velencei-tólimnogeológiája. In: Gyalog L, Horváth I (eds) A Velencei-hegység és a Balatonfő földtana. Geological Institute of Hungary, Budapest
Hudson JJ, Taylor WD, Schindler DW (2000) Phosphate concentrations in lakes. Nature 406:54–56
Imhoff JF, Sahl HG, Soliman G (1979) The Wadi Natrun: chemical composition and microbial mass developments in alkaline brines of eutrophic desert lakes. Geomicrobiol J 1:219–234
Jiang Y, Ji B, Wong RNS, Wong MH (2008) Statistical study on the effects of environmental factors on the growth and microcystins production of bloom-forming cyanobacterium—Microcystis aeruginosa. Harmful Algae 7:127–136
Jost L (2006) Entropy and diversity. Oikos 113:363–375
Juggins S (2012) Rioja: analysis of quaternary science data version: 0.7–3
Kalakoutskii LV, Zenova GM, Soina VS (1990) Associations of actinomycetes with algae. Actinomycetes 1:27–42
Kós P, Gorzó G, Surányi G, Borbély G (1995) Simple and efficient method for isolation and measurement of cyanobacterial hepatotoxins by plant tests (Sinapis alba L.). Anal Biochem 225:49–53
Larsen JB, Larsen A, Bratbak G, Sandaa RA (2008) Phylogenetic analysis of members of the Phycodnaviridae virus family, using amplified fragments of the major capsid protein gene. Appl Environ Microbiol 74:3048–3057
Lauber CL, Hamady M, Knight R, Fierer N (2009) Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl Environ Microbiol 75:5111–5120
López-Archilla AI, Moreira D, López-García P, Guerrero C (2004) Phytoplankton diversity and cyanobacterial dominance in a hypereutrophic shallow lake with biologically produced alkaline pH. Extremophiles 8:109–115
Lozupone C, Knight R (2005) Unifrac: a new phylogenetic method for comparing microbial communities. Appl Environ Microbiol 71:8228–8235
Martínez JM, Schroeder DC, Larsen A et al (2007) Molecular dynamics of Emiliania huxleyi and cooccurring viruses during two separate mesocosm studies. Appl Environ Microbiol 73:554–562
Mieczan T (2008) Chara and Ceratophyllum as a substrate for ciliates in two shallow lakes (Łęczna-Włodawa Lakeland, eastern Poland). Int J Oceanogr Hydrobiol 37:1–9
Newton RJ, Jones SE, Eiler A, McMahon KD, Bertilsson S (2011) A guide to the natural history of freshwater lake bacteria. Microbiol Mol Biol Rev 75:14–49
Odum EP, Finn JT, Franz EH (1979) Perturbation theory and the subsidy-stress gradient. Bioscience 29:349–352
Oksanen J (2011) Multivariate analysis of ecological communities in R: vegan tutorial. R package version, 2.0–1
Paerl HW, Otten TG (2013) Harmful cyanobacterial blooms: causes, consequences, and controls. Microb Ecol 65:995–1010
Paerl HW, Paul VJ (2012) Climate change: links to global expansion of harmful cyanobacteria. Water Res 46:1349–1363
Paerl HW, Fulton RS, Moisander PH, Dyble J (2001) Harmful freshwater algal blooms, with an emphasis on cyanobacteria. Sci World J 1:76–113
Paerl HW, Xu H, McCarthy MJ, Zhu G, Qin B, Li Y et al (2011) Controlling harmful cyanobacterial blooms ina hyper-eutrophic lake (Lake Taihu, China): the need for a dual nutrient (N & P) management strategy. Water Res 45:1973–1983
Paerl HW, Xu H, Hall NS, Zhu G, Qin B, Wu Y et al (2014) Controlling cyanobacterial blooms in hypertrophic Lake Taihu, China: will nitrogen reductions cause replacement of non-N-2 fixing by N-2 fixing taxa? PLoS ONE 9:e113123
Pálffy K, Felföldi T, Mentes A, Horváth H, Márialigeti K, Boros E et al (2014) Unique picoeukaryotic algal community under multiple environmental stress conditions in a shallow, alkaline pan. Extremophiles 18:111–119
Ramanan R, Kang Z, Kim B-H, Cho D-H, Jin L, Oh H-M et al (2015) Phycosphere bacterial diversity in green algae reveals an apparent similarity across habitats. Algal Res 8:140–144
Reskóné MN, Torokne AK (2000) Toxic Microcystis aeruginosa in Lake Velencei. Environ Toxicol 15:554–557
Reti KL, Thomas MC, Yanke LJ, Selinger LB, Inglis GD (2013) Effect of antimicrobial growth promoter administration on the intestinal microbiota of beef cattle. Gut Pathog 5:8
Rhodes CJ, Martin AP (2010) The influence of viral infection on a plankton ecosystem undergoing nutrient enrichment. J Theor Biol 265:225–237
Ritchie RJ, Trautman DA, Larkum A (2001) Phosphate limited cultures of the cyanobacterium Synechococcus are capable of very rapid, opportunistic uptake of phosphate. New Phytol 152:189–201
Robarts RD, Zohary T (1987) Temperature effects on photosynthetic capacity, respiration, and growth rates of bloom-forming cyanobacteria. N Z J Mar Freshwat Res 21:391–399
Runnegar M, Berndt N, Kaplowitz N (1995) Microcystin uptake and inhibition of protein phosphatases: effects of chemoprotectants and self-inhibition in relation to known hepatic transporters. Toxicol Appl Pharmacol 134:264–272
Sapp M, Schwaderer AS, Wiltshire KH, Hoppe H-G, Gerdts G, Wichels A (2007) Species-specific bacterial communities in the phycosphere of microalgae? Microb Ecol 53:683–699
Seyedsayamdost MR, Case RJ, Kolter R, Clardy J (2011) The Jekyll-and-Hyde chemistry of Phaeobacter gallaeciensis. Nat Chem 3:331–335
Shen H, Song L (2007) Comparative studies on physiological responses to phosphorus in two phenotypes of bloom-forming Microcystis. Hydrobiologia 592:475–486
Shepherd ML, Swecker WS Jr, Jensen RV, Ponder MA (2011) Characterization of the fecal bacteria communities of forage-fed horses by pyrosequencing of 16S rRNA V4 gene amplicons. FEMS Microbiol Lett 326:62–68
Short SM (2012) The ecology of viruses that infect eukaryotic algae. Environ Microbiol 14:2253–2271
Stearns JC, Lynch MDJ, Senadheera DB, Tenenbaum HC, Goldberg MB, Cvitkovitch DG et al (2011) Bacterial biogeography of the human digestive tract. Sci Rep 1:1–9
Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. J N Am Benthol Soc 29:344–358
Szabó A, Korponai K, Kerepesi C, Somogyi B, Vörös L, Bartha D et al (2017) Soda pans of the Pannonian steppe harbor unique bacterial communities adapted to multiple extreme conditions. Extremophiles 21:639–649
Tarakhovskaya ER, Maslov YI, Shishova MF (2007) Phytohormones in algae. Russ J Plant Physiol 54:163–170
Van Etten JL, Lane LC, Meints RH (1991) Viruses and viruslike particles of eukaryotic algae. Microbiol Rev 55:586–620
Vasas G, Szydlowska D, Gáspár A, Welker M, Trojanowicz M, Borbély G (2006) Determination of microcystins in environmental samples using capillary electrophoresis. J Biochem Biophys Methods 66:87–97
Vasas G, Bácsi I, Surányi G, Hamvas MM, Máthé C, Nagy SA et al (2010) Isolation of viable cell mass from frozen Microcystis viridis bloom containing microcystin-RR. Hydrobiologia 639:147–151
Vasas G, Farkas O, Borics G, Felföldi T, Sramkó G, Batta G et al (2013) Appearance of Planktothrix rubescens bloom with [D-Asp3, Mdha7]MC–RR in gravel pit pond of a shallow lake-dominated area. Toxins 5:2434–2455
Vörös L, Gulyás P, Németh J (2006) Occurrence, dynamics and production of picoplankton in Hungarian shallow lakes. Int Rev Gesamten Hydrobiol Hydrogr 76:617–629
Wang Q, Garrity GM, Tiedje JM, Cole JR (2007) Naïve bayesian classifier for rapid assignment of rRNA sequences into the new bacterial taxonomy. Appl Environ Microbiol 73:5261–5267
Water Management Directorate of VITUKI Environmental Protection and Water Management Research Institute on behalf of the Ministry for Environment and Water (2006) Groundwaters in Hungary II. In: H.M.F. Environment Water (eds)
Weitz J, Wilhelm S (2012) Ocean viruses and their effects on microbial communities and biogeochemical cycles. F1000 Biol Rep 4:1–8
Wetzel RG (2001) Limnology, 3rd edn. Academic Press, London
Wilhelm SW, Suttle CA (1999) Viruses and nutrient cycles in the sea: viruses play critical roles in the structure and function of aquatic food webs. Bioscience 49:781–788
Wommack KE, Colwell RR (2000) Virioplankton: viruses in aquatic ecosystems. Microbiol Mol Biol Rev 64:69–114
Wood SA, Borges H, Puddick J, Biessy L, Atalah J, Hawes I et al (2016) Contrasting cyanobacterial communities and microcystin concentrations in summers with extreme weather events: insights into potential effects of climate change. Hydrobiologia 785:71–89
Záray G, Kröpfl K, Szabó K, Taba G, Acs E, Berlinger B et al (2005) Comparison of freshwater biofilms grown on polycarbonate substrata in Lake Velence (Hungary) and Lake Mogan (Turkey). Microchem J 79:145–148
Zhao L, Lu L, Li M, Xu Z, Zhu W (2011) Effects of Ca and Mg levels on colony formation and EPS content of cultured M. aeruginosa. Procedia Environ Sci 10:1452–1458
Zhou J, Bruns MA, Tiedje JM (1996) DNA recovery from soils of diverse composition. Appl Environ Microbiol 62:316–322
Acknowledgements
The authors wish to thank Katalin Barkács and Laura Jurecska (Cooperative Research Centre for Environmental Sciences, Budapest, Hungary) for the chemical analysis of water samples. We would also like to thank Frank W. Schneider Agriculture Specialist, for U.S. Customs and Border Protection for his assistance and Dr. Bharath Prithiviraj with the Plant Biology Division at the Samuel Roberts Noble Foundation Inc. for his bioinformatics expertise. Thanks are also due to Devon Andor Bell, Susi Szeremy, Ajna Akantisz, Hugh MacMurray, and Shane Mortensen for sampling assistance.
Funding
Support was provided by the US Department of Energy-Advancements in Sustainable Algal Production (ASAP) program under contract DE-EE0005993. T.S.A. Bell was also partially supported by the NSF IGERT Program in Geobiological Systems (DGE 0654336). T. Felföldi was supported by the János Bolyai Research Scholarship of the Hungarian Academy of Sciences. Additional support was provided by the National Research, Development, and Innovation Office of Hungary (Grant Number K 116275). G. Vasas was supported by the National Research, Development, and Innovation Office of Hungary (NKFIH-119647).
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Bell, T.A.S., Sen-Kilic, E., Felföldi, T. et al. Microbial community changes during a toxic cyanobacterial bloom in an alkaline Hungarian lake. Antonie van Leeuwenhoek 111, 2425–2440 (2018). https://doi.org/10.1007/s10482-018-1132-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1132-7