Abstract
Marine macroalgae are emerging as an untapped source of novel microbial diversity and, therefore, of new bioactive secondary metabolites. This study was aimed at assessing the diversity and antimicrobial activity of the culturable Gram-positive bacteria associated with the surface of three co-occurring Antarctic macroalgae. Specimens of Adenocystis utricularis (brown alga), Iridaea cordata (red alga) and Monostroma hariotii (green alga) were collected from the intertidal zone of King George Island, Antarctica. Gram-positive bacteria were investigated by cultivation-based methods and 16S rRNA gene sequencing, and screened for antimicrobial activity against a panel of pathogenic microorganisms. Isolates were found to belong to 12 families, with a dominance of Microbacteriaceae and Micrococcaceae. Seventeen genera of Actinobacteria and 2 of Firmicutes were cultured from the three macroalgae, containing 29 phylotypes. Three phylotypes within Actinobacteria were regarded as potentially novel species. Sixteen isolates belonging to the genera Agrococcus, Arthrobacter, Micrococcus, Pseudarthrobacter, Pseudonocardia, Sanguibacter, Staphylococcus, Streptomyces and Tessaracoccus exhibited antibiotic activity against at least one of the indicator strains. The bacterial phylotype composition was distinct among the three macroalgae species, suggesting that these macroalgae host species-specific Gram-positive associates. The results highlight the importance of Antarctic macroalgae as a rich source of Gram-positive bacterial diversity and potentially novel species, and a reservoir of bacteria producing biologically active compounds with pharmacological potential.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The surface of marine macroalgae is an attractive habitat for many organisms and is especially prone to microbial colonization and biofilm formation. In terms of abundance and space occupation bacteria are the dominant primary colonizers of algal surfaces (Goecke et al. 2010). A number of studies have reported marked differences between the microbial populations associated with the surface of macroalgae and the surrounding seawater (Bengtsson et al. 2010; Burke et al. 2011; Lachnit et al. 2011; Staufenberger et al. 2008), from other living substrata (Longford et al. 2007) or inanimate surfaces (Dobretsov et al. 2006), and between healthy and diseased macroalgae (Zozaya-Valdés et al. 2017). In addition, different algal species from the same habitat harbour highly different bacterial communities (Lachnit et al. 2011; Nylund et al. 2009; Sneed and Pohnert 2011; Trias et al. 2012), while conspecific algae from different geographic regions show more similar epibacterial communities (Lachnit et al. 2009), suggesting that each algal host species provides a distinct ecological niche with unique biotic and abiotic characteristics.
Macroalgae-associated microbial communities are emerging as a highly diverse and rich source of novel natural products of unprecedented chemical structures, which exhibit a broad range of promising biological activities (Goecke et al. 2010; Martin et al. 2014; Singh et al. 2015). Epiphytic representatives of the genera Alteromonas, Bacillus, Pseudoalteromonas, Pseudomonas, Streptomyces and Vibrio are frequently reported as producers of antimicrobial molecules (Kanagasabhapathy et al. 2008; Rungprom et al. 2008; Wiese et al. 2009). It has been suggested that the production of antimicrobial compounds by epibiotic bacteria provides a competitive advantage against potential microbial competitors during algal surface colonization (Franks et al. 2006). Therefore, macroalgae-associated microorganisms represent an attractive source of valuable compounds with biotechnological and pharmaceutical applications.
Compared with their terrestrial relatives, little is known about the diversity, distribution and ecological significance of Gram-positive bacteria in the marine environment (Gontang et al. 2007), particularly those living in association with macroalgae. Recent culture-based and culture-independent molecular studies show that marine habitats such as sediments, seawater and invertebrates harbor a diverse community of Gram-positive taxa (Gontang et al. 2007; Li et al. 2016; Steinert et al. 2015). Bacteroidetes and Proteobacteria are dominant on the macroalgal surfaces, and the Gram-positive phyla Firmicutes and Actinobacteria have recently been identified as frequent colonizers of marine macroalgae (Goecke et al. 2013b). However, it remains unclear the natural distribution, diversity and ecological functions of Antarctic Gram-positive heterotrophic bacteria, especially of those associated with marine macroorganisms. A recent study reported that Antarctic sponges are a rich source of Gram-positive organisms, in particular actinobacteria (Xin et al. 2011).
Antarctic macroalgae are unique in several aspects of their biology and ecology. They have developed physiological strategies to prevent herbivory (Baker et al. 2008), and photosynthesize under low-light conditions and low seawater temperatures (Gómez et al. 2009). Despite the harsh environmental conditions, the Antarctic continent contains a rich diversity of macroalgae, with 117 different species recorded, almost half of them endemic to the white continent (Ramírez 2010). However, few studies to date have explored the diversity, distribution and biotechnological potential of Gram-positive bacteria associated with the surface of Antarctic macroalgae (Alvarado and Leiva 2017; Leiva et al. 2015).
This study aimed at determining the phylogenetic affiliation of the culturable Gram-positive bacterial community associated with three co-occurring intertidal macroalgae from different phyla in King George Island, Antarctica, and to examine their antimicrobial activities against clinically relevant microbes.
Materials and methods
Sample collection
Specimens of Adenocystis utricularis (Bory) Skottsberg, Monostroma hariotii Gain and Iridaea cordata (Turner) Bory were collected as attached plants from the intertidal rocky shore of Rodriguez Point (62°11′57″S, 58°56′34″W) and Artigas (62°11′17″, 58°52′16″), King George Island, Antarctica, during January and February 2014. The eulittoral and shallow sublittoral zones of both locations are colonized by a variety of macroalgal species, including the algae species under study. While co-occurrence is not the general pattern of distribution of the three macroalgae, they co-occur in pools and crevices more protected from ice scouring at the intertidal zone of both locations. Algal thalli samples of the three species were collected in their sites of co-occurrence. In total, 15 healthy individuals per algal species were sampled. Samples were put in sterile plastic bags and transported at 4 °C to the laboratory of the Antarctic research station “Base Prof. Julio Escudero”, located in Fildes Bay, King George Island. Samples were processed within 3 h of collection.
Isolation and cultivation of bacteria
Fronds were rinsed three times with filtered sterile seawater (FSSW) to remove loosely attached microbes and cut into small pieces. Gram-positive bacteria were isolated by means of serial dilution in FSSW and plating techniques. To reduce Gram negative growth on agar plates, diluted samples were pretreated for 6 min at 55 °C (Gontang et al. 2007; Hames-Kocabas and Uzel 2012). Four media were used for the isolation of Gram-positive bacteria: Marine Agar 2216 (MA, BD), Seawater Agar (SA, 18 g agar l−1 in natural seawater), M1 and M6 (Hames-Kocabas and Uzel 2012). All media were supplemented with the antifungal agent cycloheximide (100 µg ml−1) (Calbiochem).The inoculated plates were incubated at 4 and 20 °C and inspected regularly for growth for up to 4 weeks. Distinct colony morphotypes were restreaked on fresh medium until pure cultures were obtained. Pure cultures were cryopreserved in the isolation media supplemented with 20% glycerol (v/v) − 80 °C. The Gram reaction was performed using the non-staining method (Buck 1982).
Molecular identification of isolates and phylogenetic analysis
Genomic DNA from all Gram-positive isolates was extracted using the GeneJet Genomic DNA Purification kit (Thermo Scientific). The 16S rRNA gene was amplified using the universal bacterial primers 27F (5′-AGAGTTTGATCMTGGCTCAG-3′) and 1492R (5′-TACGGYTACCTTGTTACGACTT-3′) and Platinum Taq DNA polymerase (Invitrogen). Amplifications were conducted in a Mastercycler Personal (Eppendorf) with initial denaturation step (94 °C, 3 min), followed by 30 cycles of denaturation (94 °C, 30 s), primer annealing (55 °C, 30 s), and elongation (72 °C, 2 min), with a final elongation step at 72 °C for 7 min. The products of PCR reactions were purified with a MinElute Gel Extraction kit (QIAGEN). PCR products were sequenced on a 3130XL Genetic Analyzer (Applied Biosystems®) at the Sequencing Unit, Pontificia Universidad Católica de Chile (Santiago, Chile). The near full-length 16S rRNA gene sequences were compared to those in the databases GenBank, EMBL and EzTaxon-e (Kim et al. 2012) to find closely related species. 16S rRNA sequences were grouped into phylotypes based on a ≥ 99% similarity. One representative sequence from each phylotype was selected for phylogenetic analysis. Sequences were checked for accuracy and trimmed using the software ContigExpress of Vector NTI Advance 9.1.0 (Invitrogen Corp.). A multiple alignment was created using MUSCLE (v3.7) (Edgar 2004) and then edited using Gblocks (version 0.91b) (Castresana 2000). jModeltest (Darriba et al. 2012) was run for each alignment, and the best models were used in the phylogenetic analyses. Maximum-likelihood trees were created with PhyML 3.0 (Guindon et al. 2010), using General Time Reversible plus Gamma (GTR + G) evolution model (Lanave et al. 1984), and their robustness was tested by bootstrap analysis with 100 resamplings.
The 16S rRNA gene sequences determined in this study have been deposited in GenBank under the accession numbers KR047772–KR047782, KR047784–KR047787, KR632535–KR632537, KT346365, KU925160–KU925169, KX084450–KX084451, KX130899–KX130901 and KY775490–KY775511.
Antimicrobial screening
The antimicrobial activity of epiphytic bacteria was tested using the perpendicular streaking method (Eythorsdottir et al. 2016). The test was carried out on five indicator microorganisms: Escherichia coli ATCC 8733, Mycobacterium smegmatis ATCC 14468, Pseudomonas aeruginosa PAO1, Staphylococcus aureus ATCC 25923 and Candida albicans ATCC 90029. Each epiphytic strain was inoculated as a single streak on Marine Agar 2216 (for antibacterial activity) or ISP-2 agar supplemented with Sea Salts (Sigma) at 3% w/v (for anticandidal activity) and plates were incubated at 20 °C until dense growth was obtained. Indicator strains (0.5 Mc Farland dilutions) were then streaked perpendicular to the epiphytic isolate streak, incubated at 37 °C for 24 h and examined for inhibition of test bacteria growth. The zone of inhibition was defined as an area on the indicator streak of reduced growth or complete inhibition of growth (Haber and Ilan 2014).
Results
A total of 56 Gram-positive bacterial isolates were retrieved from the three macroalgae, 30 from A. utricularis, 13 from I. cordata and 13 from M. hariotii. The analysis of the nearly full-length 16S rRNA sequences revealed that the greatest part of the Gram-positive isolates belonged to the phylum Actinobacteria (91.1%) and only 8.9% were Firmicutes. At the family level, the isolates were grouped into 12 families, and only three families (Microbacteriaceae, Micrococcaceae and Dermabacteraceae) were shared by the three macroalgae, with an overall dominance of members of Micrococcaceae and Microbacteriaceae (Fig. 1). In total, 19 genera were cultured from the three macroalgae, 17 genera of Actinobacteria and 2 of Firmicutes, and Brachybacterium was the only genus shared by the three macroalgae species. There was no dominant genus on the three macroalgae species. A. utricularis had the highest number of actinobacterial genera (11 genera), followed by I. cordata and M. hariotii (8 genera each). Firmicutes were not retrieved from the surface of M. hariotii. The Gram-positive isolates were grouped into 29 phylotypes according to a sequence similarity value ≥ 99%. Sixteen phylotypes were associated with A. utricularis, 9 phylotypes with I. cordata and 10 with M. hariotii (Figs. 2, 3 and 4). Most of the bacterial phylotypes were algae-specific and only 6 phylotypes were shared by two macroalgae species. No phylotypes were common to all three macroalgae species (Fig. 5).
Relative abundance of bacterial families associated with three Antarctic macroalgae. (
 ) Dermabacteraceae; (
) Dermabacteraceae; (
 ) Intrasporangiaceae; (
) Intrasporangiaceae; (
 ) Microbacteriaceae; (
) Microbacteriaceae; (
 ) Micrococcaceae; (
) Micrococcaceae; (
 ) Nocardiaceae; (
) Nocardiaceae; (
 ) Nocardioidaceae; (
) Nocardioidaceae; (
 ) Planococcaceae; (
) Planococcaceae; (
 ) Propionibacteriaceae; (
) Propionibacteriaceae; (
 ) Pseudonocardiaceae; (
) Pseudonocardiaceae; (
 ) Sanguibacteraceae; (
) Sanguibacteraceae; (
 ) Staphylococcaceae; (
) Staphylococcaceae; (
 ) Streptomycetaceae
) Streptomycetaceae
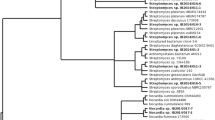
Maximum-likelihood phylogenetic tree of Gram-positive isolates (in boldface) isolated from the surface of Adenocystis utricularis. The tree was constructed using the aligned partial 16S rRNA gene sequences (1388–1432 bp). Only one representative isolate per phylotype is reported. The number of isolates within each phylotype is shown in brackets. Algae-specific phylotypes are shaded. Deinococcus radiophilus DSM 20551T was employed as outgroup. Bootstrap values (≥ 50%) based on 100 resamplings are shown at branch nodes
Maximum-likelihood phylogenetic tree of Gram-positive isolates (in boldface) isolated from the surface of Iridaea cordata. The tree was constructed using the aligned partial 16S rRNA gene sequences (1376–1434 bp). Only one representative isolate per phylotype is reported. The number of isolates within each phylotype is shown in brackets. Algae-specific phylotypes are shaded. Deinococcus radiophilus DSM 20551T was employed as outgroup. Bootstrap values (≥ 50%) based on 100 resamplings are shown at branch nodes
Maximum-likelihood phylogenetic tree of Gram-positive isolates (in boldface) isolated from the surface of Monostroma hariotii. The tree was constructed using the aligned partial 16S rRNA gene sequences (1384–1435 bp). Only one representative isolate per phylotype is reported. The number of isolates within each phylotype is shown in brackets. Algae-specific phylotypes are shaded. Deinococcus radiophilus DSM 20551T was employed as outgroup. Bootstrap values (≥ 50%) based on 100 resamplings are shown at branch nodes
Of the epiphytic actinobacteria, four isolates exhibited a 16S rRNA gene sequence similarity below the species delineation threshold value of 98.7% (Stackebrandt and Ebers 2006), suggesting that they may represent two novel species. The isolates AU C7 and AU I5 were related to the genus Tessaracoccus (family Propionibacteriaceae) and shared only 96.4% sequence similarity with the near type strain Tessaracoccus flavescens SST-39T (Lee and Lee 2008). Strains AU-G6 and AU A3.2 belonged to the genus Amycolatopsis (family Pseudonocardiaceae), sharing 97.8% sequence similarity with the close type strain, Amycolatopsis nigrescens CSC17Ta-90T (Groth et al. 2007).
Strains MH-G3, MH-G5 and MH-G8 showed 100% 16S rRNA sequence similarity with each other and fell within the genus Pseudonocardia (family Pseudonocardiaceae), sharing 98.8% sequence similarity with the close type strain, Pseudonocardia adelaidensis EUM 221T (Kaewkla and Franco 2010). Although the Pseudonocardia isolates showed a 16S rRNA similarity a little bit higher than the recommended threshold value, the strains exhibit distinct cultural and physiological characteristics (results not shown) that serve to separate them from all recognized Pseudonocardia species, which were mostly isolated from terrestrial environments. Remarkably, the Pseudonocardia isolates exhibited an obligate requirement of seawater for growth, a physiological trait reported for the marine-derived actinobacterial genus Salinispora (Maldonado et al. 2005), but not for species of Pseudonocardia.
All Gram-positive isolates obtained from macroalgae were tested in a perpendicular streaking assay against four reference bacterial strains and one reference yeast strain. In total, 16 isolates (i.e., 28.6%) showed activity against at least one of the indicator strains (Table 1). All macroalgae species provided some antimicrobial isolates, with 9 active isolates from A. utricularis, 6 from M. hariotti and one from I. cordata. The phylotypes that presented antibiotic activity were affiliated to the genera Agrococcus, Arthrobacter, Micrococcus, Pseudarthrobacter, Pseudonocardia, Sanguibacter, Staphylococcus, Streptomyces and Tessaracoccus. Three isolates exhibited activity against more than one indicator strain, with isolate MH A4 showing the broadest spectrum of antimicrobial activity, inhibiting three of the five target bacterial strains. None of the isolates showed activity against P. aeruginosa PAO1. Twelve isolates (21.4%) showed activity against the yeast indicator strain.
Discussion
Culture-based and culture-independent investigations have shown that marine macroalgae support phylogenetically diverse and complex epibacterial communities, dominated by Gram-negative species (Goecke et al. 2013a; Hollants et al. 2013; Lachnit et al. 2011; Martin et al. 2015; Stratil et al. 2013). In addition to knowing little about the Gram-positive microbiota colonizing the surface of marine macroalgae, even less is known about the phylogenetic diversity of the epiphytic Gram-positive organisms associated with Antarctic macroalgae. At higher taxonomic levels, the culturable bacterial community on the macroalgal surface is dominated by α-and γ-proteobacteria as well as Bacteroidetes, with Firmicutes and Actinobacteria representing a smaller component of the epiphytic bacterial community (Choi et al. 2016; Goecke et al. 2013a; Martin et al. 2015). In this study we employed a selective isolation strategy (heat pretreatment and different isolation media) to assess the phylogenetic diversity of the culturable epiphytic Gram-positive bacteria living on three co-occurring intertidal macroalgae collected at King George Island. These included one brown alga (A. utricularis), one green alga (M. hariotii) and one red alga (I. cordata).
A. utricularis is a small saccate alga found in the western Antarctic Peninsula, extending its distribution into cold temperate regions of South America, New Zealand, Tasmania and sub-Antarctic islands. This brown alga is a pioneer species commonly found in tide pools, cracks and crevices of the lower eulittoral and upper sublittoral areas. In turn, I. cordata and M. hariotii have a circum-Antarctic cold-temperate distribution. Both species are frequently found in the western Antarctic Peninsula, but also found outside this region (Ross Sea) and in sub-Antarctic islands. I. cordata is a fleshy red alga occurring in intertidal tide pools and in the shallow subtidal. M. hariotii is a pioneer species commonly found in subtidal areas (until 25 m) of recent ice scour, but also is common in the intertidal, and occasionally reported as an epiphyte on brown macroalgae (Gómez 2015; Ramírez 2010; Wiencke et al. 2014). A. utricularis and I. cordata have low temperature requirements for growth and survival (≤ 15 and ≤ 5 °C, respectively); the temperature requirement of M. hariotii has not been studied so far (Wiencke et al. 2014).
In total, 29 phylotypes were cultured from the macroalgae, based on the sharing of ≥ 99% 16S rRNA gene sequence similarity, with the great majority belonging to the phylum Actinobacteria (25 phylotypes). Although most of the studies on macroalgal bacterial communities were not specifically aimed at isolating Gram-positive bacteria, in a review of 161 macroalgal–bacterial studies, Hollants et al. (2013) reported that Bacillales and Actinomycetales were the most abundant orders within the Gram-positive bacterial community. Very few studies have explored the diversity of Gram-positive bacteria in Antarctic marine habitats, but recent reports indicate that taxonomically diverse populations of actinobacteria can be cultured from sediments (Lamilla et al. 2017) and invertebrates (Xin et al. 2011). In a study assessing the diversity of culturable Gram-positive bacteria associated with deep-sea Antarctic sponges, Xin et al. (2011) found that Actinobacteria were much more diverse than Firmicutes. In our study, Firmicutes were recovered from A. utricularis and I. cordata and were represented by species of Staphylococcus and Planomicrobium. Representatives of Staphylococcus have been previously isolated from brown, green and red macroalgae (Menezes et al. 2010; Nylund et al. 2008; Villarreal-Gomez et al. 2010). A species of the genus Planomicrobium (P. glaciei) was isolated from the brown alga Padina pavonica (Ismail et al. 2016), although isolates closely related to Planomicrobium flavidum were previously not known from the surfaces of marine macroalgae.
In this study, phylotypes of Amycolatopsis, Tessaracoccus and Pseudonocardia were identified as potentially new species. While the genus Amycolatopsis have been previously isolated from macroalgae (Wiese et al. 2009), this is the first report of Tessaracoccus and Pseudonocardia associated with marine algae. However, members of Tessaracoccus have been isolated from terrestrial Antarctic habitats (Peeters et al. 2011), and strains of Pseudonocardia have been recovered from Antarctic samples, including soils (Saul et al. 2005), moraines (Prabahar et al. 2004) and marine sponges (Xin et al. 2011).
Although the three macroalgal species occur contiguously in some areas of the intertidal zone of the sampling sites, their Gram-positive bacterial communities exhibited little overlap at the species level as no phylotype was present on the three algae species and 23 of the 29 phylotypes were unique to one host algae. The small overlap among bacterial species associated with marine macroalgae have been reported in culture-independent (Longford et al. 2007) and culture-dependent studies (Goecke et al. 2013a; Penesyan et al. 2009), although they were not specifically aimed at examining the Gram-positive component of the epiphytic community. Furbino et al. (2014) reported a low similarity of the fungal communities of two Antarctic macroalgae, M. hariotii and the red algae Pyropia endiviifolia. Interestingly, Goecke et al. (2013a) found seasonal and host-related variations in bioactivity patterns of different epiphytic phylotypes associated with two co-occurring Baltic macroalgae. It has been proposed that species-specific biological and physicochemical properties of the macroalgal thalli and specific algae-bacteria interactions play a role in shaping the structure of the associated microbial community (Beleneva and Zhukova 2006; Egan et al. 2013; Lachnit et al. 2009). Chemical studies on Antarctic macroalgae have shown significant variations in the organic content between higher taxonomic groups and between species. Gómez and Westermeier (1995) found that A. utricularis presented one of the highest values of calorific and lipid content among nine species of Antarctic and cold-temperate brown macroalgae. In a comprehensive study of the nutritional and elemental composition of 40 species of Antarctic macroalgae, Peters et al. (2005) found significant differences in most of the measured parameters between major taxonomic groups. It has been suggested that differences in the chemical composition between macroalgal taxa are related to distinct life strategies and different morphological and physiological adaptations to environmental stress in Antarctica (Gomez and Westermeier 1995; Santos et al. 2017).
Specific algal repellents or chemoattractants have an important influence on algae-bacteria interactions by selectively eliminating certain strains or promoting the growth of specific phylotypes (Lachnit et al. 2009; Sneed and Pohnert 2011). In addition, quorum sensing inhibitors (QSI) or QSI-like molecules and antimicrobial compounds produced by epiphytic bacteria work together with macroalgal secondary metabolites to inhibit the settlement and growth of herbivores, fouling organisms and pathogens (Hollants et al. 2013; Zhou et al. 2016).
After decades of intensive screening from terrestrial sources, the discovery rate of novel antibiotics from soil-derived organisms has steadily declined since the 1970s, with the repeated identification of known molecules (Arias and Murray 2015). Given the urgent need for new classes of antibiotics due to the spread of antibiotic resistance, previously untouched and understudied habitats are now being explored, including Antarctic marine and terrestrial environments, which have proven to be an invaluable source of microbial diversity and for the discovery of new products and compounds of industrial and pharmaceutical interest (Yarzábal 2016). In our study, the antimicrobial properties of the 56 epiphytic Gram-positive strains were tested against five microbial strains of medical importance by a perpendicular streaking method. Representatives of nine bacterial genera (10 phylotypes) exhibited activity against at least one of the target strains. It is interesting to note that 75% of the antimicrobial isolates showed activity against the fungal indicator strain, a higher proportion than the observed against bacterial reference strains. A wide diversity of Gram-positive bacteria with antimicrobial activity have been isolated from various macroalgae species with different geographical origins. The epiphytic antimicrobial isolates include genera of Firmicutes (Bacillus, Paenibacillus and Staphylococcus) and Actinobacteria (Amycolatopsis, Arthrobacter, Brevibacterium, Kocuria, Leifsonia, Leucobacter, Microbacterium, Micrococcus, Micromonospora, Nocardiopsis, Salinibacterium and Streptomyces) (Ali et al. 2012; Goecke et al. 2013a; Ismail et al. 2016; Kanagasabhapathy et al. 2006, 2008; Penesyan et al. 2009; Villarreal-Gomez et al. 2010; Wiese et al. 2009; Zheng et al. 2000). The present study adds five more genera to the above-mentioned list of bioactive, macroalgae-associated Gram-positive bacteria: Agrococcus, Pseudarthrobacter, Pseudonocardia, Sanguibacter and Tessaracoccus.
An interesting outcome of our study was that isolates of the potentially new species of the genera Pseudonocardia and Tessaracoccus exhibited antimicrobial activity in the assays. A chemical characterization of the antimicrobial metabolites of these potentially novel actinobacteria could lead to the discovery of unique compounds that have not previously been described (Bull and Stach 2007).
Streptomycetes are widely distributed in the marine environment and produce a wide array of bioactive secondary metabolites with unique chemical structures that have plethora of biological activities including antitumor, cytotoxic, anti-inflammatory and antimicrobial (Manivasagan et al. 2014; ul Hassan et al. 2017). The isolate MH A4 from the green alga M. hariotii showed the broadest antimicrobial profile, exhibiting activity against three indicator strains (E. coli, S. aureus and C. albicans). An EzBioCloud search for the 16S rRNA sequence of MH A4 revealed that it was highly similar to Streptomyces pratensis ch24T. Strains of S. pratensis have been retrieved mainly from terrestrial environments (Rasuk et al. 2017; Rong et al. 2013; Zhao et al. 2016), but rarely from marine sources (Betancur et al. 2017). There is only limited information concerning the secondary metabolite profile and antimicrobial activity of S. pratensis strains, the most significant study being that of Shah et al. (2017) in which compounds of the actinomycin C complex were identified in active extracts of a soil isolated strain of S. pratensis, showing potent activity against S. aureus and Mycobacterium tuberculosis. Genome sequencing of Streptomyces have shown that each strain contain genes or gene clusters for the synthesis of a number of potential secondary metabolites, although only a fraction are expressed under conventional culture conditions (Ochi and Hosaka 2013; Ohnishi et al. 2008). Further genomic, chemical and biological characterization of this Antarctic epiphytic S. pratensis isolate may lead to the discovery of silent biosynthetic pathways and novel bioactive compounds.
Marine Antarctic macroalgae yielded a diverse assemblage of culturable Gram-positive bacteria, some of which are potentially novel species. Given the potential for bioactive natural product production that marine actinobacteria have shown, our study highlights the great potential to unveil an even larger diversity of Gram-positive epibiotic bacteria through a more systematic exploration of the intertidal and subtidal Antarctic algal flora.
References
Ali AIB, El Bour M, Ktari L, Bolhuis H, Ahmed M, Boudabbous A, Stal L (2012) Jania rubens-associated bacteria: molecular identification and antimicrobial activity. J Appl Phycol 24:525–534
Alvarado R, Leiva S (2017) Agar-degrading bacteria isolated from Antarctic macroalgae. Folia Microbiol 62:409–416
Arias CA, Murray BE (2015) A new antibiotic and the evolution of resistance. New Engl J Med 372:1168–1170
Baker BJ, Amsler CD, McClintock JB et al (2008) Macroalgal chemical defenses in polar marine communities. In: Amsler CD (ed) Algal chemical ecology. Springer, Berlin, pp 91–103. https://doi.org/10.1007/978-3-540-74181-7_4
Beleneva IA, Zhukova NV (2006) Bacterial communities of some brown and red algae from Peter the Great Bay, the Sea of Japan. Microbiology 75:348–357
Bengtsson MM, Sjotun K, Ovreas L (2010) Seasonal dynamics of bacterial biofilms on the kelp Laminaria hyperborea. Aquat Microb Ecol 60:71–83
Betancur LA et al (2017) Marine Actinobacteria as a source of compounds for phytopathogen control: an integrative metabolic-profiling/bioactivity and taxonomical approach. PLoS ONE 12:e0170148. https://doi.org/10.1371/journal.pone.0170148
Buck JD (1982) Nonstaining (KOH) method for determination of gram reactions of marine bacteria. Appl Environ Microbiol 44:992–993
Bull AT, Stach JEM (2007) Marine actinobacteria: new opportunities for natural product search and discovery. Trends Microbiol 15:491–499
Burke C, Thomas T, Lewis M, Steinberg P, Kjelleberg S (2011) Composition, uniqueness and variability of the epiphytic bacterial community of the green alga Ulva australis. ISME J 5:590–600
Castresana J (2000) Selection of conserved blocks from multiple alignments for their use in phylogenetic analysis. Mol Biol Evol 17:540–552
Choi H-R, Park S-H, Kim D-H, Kim J-Y, Heo M-S (2016) Phylogenetic diversity and community analysis of marine bacteria associated with Ulva pertusa. J Life Sci 26:819–825
Darriba D, Taboada GL, Doallo R, Posada D (2012) jModelTest 2: more models, new heuristics and parallel computing. Nat Methods 9:772. https://doi.org/10.1038/nmeth.2109
Dobretsov S, Dahms H-U, Harder T, Qian P-Y (2006) Allelochemical defense against epibiosis in the macroalga Caulerpa racemosa var. turbinata. Mar Ecol Prog Ser 318:165–175
Edgar RC (2004) MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res 32:1792–1797. https://doi.org/10.1093/nar/gkh340
Egan S, Harder T, Burke C, Steinberg P, Kjelleberg S, Thomas T (2013) The seaweed holobiont: understanding seaweed-bacteria interactions. FEMS Microbiol Rev 37:462–476
Eythorsdottir A, Omarsdottir S, Einarsson H (2016) Antimicrobial activity of marine bacterial symbionts retrieved from shallow water hydrothermal vents. Mar Biotechnol 18:293–300. https://doi.org/10.1007/s10126-016-9695-7
Franks A, Egan S, Holmström C, James S, Lappin-Scott H, Kjelleberg S (2006) Inhibition of fungal colonization by Pseudoalteromonas tunicata provides a competitive advantage during surface colonization. Appl Environ Microbiol 72:6079–6087. https://doi.org/10.1128/aem.00559-06
Furbino LE et al (2014) Diversity patterns, ecology and biological activities of fungal communities associated with the endemic macroalgae across the Antarctic Peninsula. Microb Ecol 67:775–787. https://doi.org/10.1007/s00248-014-0374-9
Goecke F, Labes A, Wiese J, Imhoff JF (2010) Chemical interactions between marine macroalgae and bacteria. Mar Ecol Prog Ser 409:267–299
Goecke F, Labes A, Wiese J, Imhoff JF (2013a) Phylogenetic analysis and antibiotic activity of bacteria isolated from the surface of two co-occurring macroalgae from the Baltic Sea. Eur J Phycol 48:47–60
Goecke F, Thiel V, Wiese J, Labes A, Imhoff JF (2013b) Algae as an important environment for bacteria—phylogenetic relationships among new bacterial species isolated from algae. Phycologia 52:14–24. https://doi.org/10.2216/12-24.1
Gómez I (2015) Flora marina Antártica: patrimonio de biodiversidad. Ediciones El Kultrún, Valdivia
Gomez I, Westermeier R (1995) Energy contents and organic-constituents in Antarctic and south chilean marine brown-algae. Polar Biol 15:597–602
Gómez I et al (2009) Light and temperature demands of marine benthic microalgae and seaweeds in polar regions. Bot Mar 52:593–608
Gontang EA, Fenical W, Jensen PR (2007) Phylogenetic diversity of Gram-positive bacteria cultured from marine sediments. Appl Environ Microbiol 73:3272–3282. https://doi.org/10.1128/aem.02811-06
Groth I et al (2007) Amycolatopsis nigrescens sp. nov., an actinomycete isolated from a Roman catacomb. Int J Syst Evol Microbiol 57:513–519. https://doi.org/10.1099/ijs.0.64602-0
Guindon S, Dufayard JF, Lefort V, Anisimova M, Hordijk W, Gascuel O (2010) New algorithms and methods to estimate maximum-likelihood phylogenies: assessing the performance of PhyML 3.0. Syst Biol 59:307–321. https://doi.org/10.1093/sysbio/syq010
Haber M, Ilan M (2014) Diversity and antibacterial activity of bacteria cultured from Mediterranean Axinella spp. sponges. J Appl Microbiol 116:519–532. https://doi.org/10.1111/jam.12401
Hames-Kocabas EE, Uzel A (2012) Isolation strategies of marine-derived actinomycetes from sponge and sediment samples. J Microbiol Methods 88:342–347. https://doi.org/10.1016/j.mimet.2012.01.010
Hollants J, Leliaert F, De Clerck O, Willems A (2013) What we can learn from sushi: a review on seaweed-bacterial associations. FEMS Microbiol Ecol 83:1–16
Ismail A et al (2016) Antimicrobial activities of bacteria associated with the brown alga Padina pavonica. Front Microbiol 7:1072. https://doi.org/10.3389/fmicb.2016.01072
Kaewkla O, Franco CMM (2010) Pseudonocardia adelaidensis sp. nov., an endophytic actinobacterium isolated from the surface-sterilized stem of a grey box tree (Eucalyptus microcarpa). Int J Syst Evol Microbiol 60:2818–2822. https://doi.org/10.1099/ijs.0.019208-0
Kanagasabhapathy M, Sasaki H, Haldar S, Yamasaki S, Nagata S (2006) Antibacterial activities of marine epibiotic bacteria isolated from brown algae of Japan. Ann Microbiol 56:167–173
Kanagasabhapathy M, Sasaki H, Nagata S (2008) Phylogenetic identification of epibiotic bacteria possessing antimicrobial activities isolated from red algal species of Japan. World J Microbiol Biotechnol 24:2315–2321. https://doi.org/10.1007/s11274-008-9746-y
Kim OS et al (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721. https://doi.org/10.1099/ijs.0.038075-0
Lachnit T, Blümel M, Imhoff JF, Wahl M (2009) Specific epibacterial communities on macroalgae: phylogeny matters more than habitat. Aquat Biol 5:181–186
Lachnit T, Meske D, Wahl M, Harder T, Schmitz R (2011) Epibacterial community patterns on marine macroalgae are host-specific but temporally variable. Environ Microbiol 13:655–665
Lamilla C, Pavez M, Santos A, Hermosilla A, Llanquinao V, Barrientos L (2017) Bioprospecting for extracellular enzymes from culturable Actinobacteria from the South Shetland Islands, Antarctica. Polar Biol 40:719–726
Lanave C, Preparata G, Sacone C, Serio G (1984) A new method for calculating evolutionary substitution rates. J Mol Evol 20:86–93. https://doi.org/10.1007/bf02101990
Lee DW, Lee SD (2008) Tessaracoccus flavescens sp. nov., isolated from marine sediment. Int J Syst Evol Microbiol 58:785–789. https://doi.org/10.1099/ijs.0.64868-0
Leiva S, Alvarado P, Huang Y, Wang J, Garrido I (2015) Diversity of pigmented Gram-positive bacteria associated with marine macroalgae from Antarctica. FEMS Microbiol Lett. https://doi.org/10.1093/femsle/fnv206
Li Z, Xing M, Wang W, Wang D, Zhu J, Sun M (2016) Phylogenetic diversity of culturable bacteria in surface seawater from the Drake Passage, Antarctica. Chin J Oceanol Limn 34:952–963. https://doi.org/10.1007/s00343-016-5132-z
Longford SR et al (2007) Comparisons of diversity of bacterial communities associated with three sessile marine eukaryotes. Aquat Microb Ecol 48:217–229
Maldonado LA et al (2005) Salinispora arenicola gen. nov., sp. nov. and Salinispora tropica sp. nov., obligate marine actinomycetes belonging to the family Micromonosporaceae. Int J Syst Evol Microbiol 55:1759–1766. https://doi.org/10.1099/ijs.0.63625-0
Manivasagan P, Kang KH, Sivakumar K, Li-Chan ECY, Oh HM, Kim SK (2014) Marine actinobacteria: an important source of bioactive natural products. Environ Toxicol Phar 38:172–188
Martin M, Portetelle D, Michel G, Vandenbol M (2014) Microorganisms living on macroalgae: diversity, interactions, and biotechnological applications. Appl Microbiol Biotechnol 98:2917–2935. https://doi.org/10.1007/s00253-014-5557-2
Martin M, Barbeyron T, Martin R, Portetelle D, Michel G, Vandenbol M (2015) The cultivable surface microbiota of the brown alga Ascophyllum nodosum is enriched in macroalgal-polysaccharide-degrading bacteria. Front Microbiol 6:1487. https://doi.org/10.3389/fmicb.2015.01487
Menezes CBA et al (2010) Microbial diversity associated with algae, ascidians and sponges from the north coast of Sao Paulo state, Brazil. Microbiol Res 165:466–482
Nylund GM, Cervin G, Persson F, Hermansson M, Steinberg PD, Pavia H (2008) Seaweed defence against bacteria: a poly-brominated 2-heptanone from the red alga Bonnemaisonia hamifera inhibits bacterial colonisation. Mar Ecol Prog Ser 369:39–50
Nylund GM, Persson F, Lindegarth M, Cervin G, Hermansson M, Pavia H (2009) The red alga Bonnemaisonia asparagoides regulates epiphytic bacterial abundance and community composition by chemical defence. FEMS Microbiol Ecol 71:84–93. https://doi.org/10.1111/j.1574-6941.2009.00791.x
Ochi K, Hosaka T (2013) New strategies for drug discovery: activation of silent or weakly expressed microbial gene clusters. Appl Microbiol Biotechnol 97:87–98
Ohnishi Y et al (2008) Genome sequence of the streptomycin-producing microorganism Streptomyces griseus IFO 13350. J Bacteriol 190:4050–4060
Peeters K, Ertz D, Willems A (2011) Culturable bacterial diversity at the Princess Elisabeth Station (Utsteinen, Sor Rondane Mountains, East Antarctica) harbours many new taxa. Syst Appl Microbiol 34:360–367
Penesyan A, Marshall-Jones Z, Holmstrom C, Kjelleberg S, Egan S (2009) Antimicrobial activity observed among cultured marine epiphytic bacteria reflects their potential as a source of new drugs. FEMS Microbiol Ecol 69:113–124
Peters KJ, Amsler CD, Amsler MO, McClintock JB, Dunbar RB, Baker BJ (2005) A comparative analysis of the nutritional and elemental composition of macroalgae from the western Antarctic Peninsula. Phycologia 44:453–463
Prabahar V, Dube S, Reddy GSN, Shivaji S (2004) Pseudonocardia antarctica sp nov an Actinomycetes from McMurdo Dry Valleys, Antarctica. Syst Appl Microbiol 27:66–71
Ramírez ME (2010) Flora marina bentónica de la región austral de Sudamérica y la Antártica. An Inst Patagonia 38:57–71
Rasuk MC, Ferrer GM, Kurth D, Portero LR, Farias ME, Albarracin VH (2017) UV-resistant actinobacteria from high-altitude Andean lakes: isolation, characterization and antagonistic activities. Photochem Photobiol 93:865–880
Rong XY, Doroghazi JR, Cheng K, Zhang LM, Buckley DH, Huang Y (2013) Classification of Streptomyces phylogroup pratensis (Doroghazi and Buckley, 2010) based on genetic and phenotypic evidence, and proposal of Streptomyces pratensis sp nov. Syst Appl Microbiol 36:401–407
Rungprom W et al (2008) Cyclic tetrapeptides from marine bacteria associated with the seaweed Diginea sp. and the sponge Halisarca ectofibrosa. Tetrahedron 64:3147–3152
Santos MAZ, Colepicolo P, Pupo D, Fujii MT, de Pereira CMP, Mesko MF (2017) Antarctic red macroalgae: a source of polyunsaturated fatty acids. J Appl Phycol 29:759–767
Saul DJ, Aislabie JM, Brown CE, Harris L, Foght JM (2005) Hydrocarbon contamination changes the bacterial diversity of soil from around Scott Base, Antarctica. FEMS Microbiol Ecol 53:141–155
Shah AM et al (2017) Antimicrobial investigation of selected soil actinomycetes isolated from unexplored regions of Kashmir Himalayas, India. Microb Pathogenesis 110:93–99
Singh RP, Kumari P, Reddy CRK (2015) Antimicrobial compounds from seaweeds-associated bacteria and fungi. Appl Microbiol Biotechnol 99:1571–1586. https://doi.org/10.1007/s00253-014-6334-y
Sneed JM, Pohnert G (2011) The green alga Dicytosphaeria ocellata and its organic extracts alter natural bacterial biofilm communities. Biofouling 27:347–356. https://doi.org/10.1080/08927014.2011.576317
Stackebrandt E, Ebers J (2006) Taxonomic parameters revisited: tarnished gold standards. Microbiol Today 8:6–9
Staufenberger T, Thiel V, Wiese J, Imhoff JF (2008) Phylogenetic analysis of bacteria associated with Laminaria saccharina. FEMS Microbiol Ecol 64:65–77
Steinert G, Taylor MW, Schupp PJ (2015) Diversity of actinobacteria associated with the marine ascidian Eudistoma toealensis. Mar Biotechnol 17:377–385. https://doi.org/10.1007/s10126-015-9622-3
Stratil SB, Neulinger SC, Knecht H, Friedrichs AK, Wahl M (2013) Temperature-driven shifts in the epibiotic bacterial community composition of the brown macroalga Fucus vesiculosus. Microbiology Open 2:338–349
Trias R, García-Lledó A, Sánchez N, López-Jurado JL, Hallin S, Bañeras L (2012) Abundance and composition of epiphytic bacterial and archaeal ammonia oxidizers of marine red and brown macroalgae. Appl Environ Microbiol 78:318–325. https://doi.org/10.1128/aem.05904-11
ul Hassan SS, Anjum K, Abbas SQ, Akhter N, Shagufta BI, Shah SAA, Tasneem U (2017) Emerging biopharmaceuticals from marine actinobacteria. Environ Toxicol Phar 49:34–47
Villarreal-Gomez LJ, Soria-Mercado IE, Guerra-Rivas G, Ayala-Sanchez NE (2010) Antibacterial and anticancer activity of seaweeds and bacteria associated with their surface. Rev Biol Mar Oceanog 45:267–275
Wiencke C, Amsler C, Clayton M (2014) Macroalgae. In: Biogeographic Atlas of the Southern Ocean. Scientific Committee on Antarctic Research, pp 66-73
Wiese J, Thiel V, Nagel K, Staufenberger T, Imhoff JF (2009) Diversity of antibiotic-active bacteria associated with the brown alga Laminaria saccharina from the Baltic Sea. Mar Biotechnol 11:287–300. https://doi.org/10.1007/s10126-008-9143-4
Xin Y, Kanagasabhapathy M, Janussen D, Xue S, Zhang W (2011) Phylogenetic diversity of Gram-positive bacteria cultured from Antarctic deep-sea sponges. Polar Biol 34:1501–1512. https://doi.org/10.1007/s00300-011-1009-y
Yarzábal LA (2016) Antarctic psychrophilic microorganisms and biotechnology: History, current trends, applications, and challenges. In: Castro-Sowinski S (ed) Microbial models: From environmental to industrial sustainability. Springer, Singapore, pp 83–118. https://doi.org/10.1007/978-981-10-2555-6_5
Zhao F et al (2016) Biogeography and adaptive evolution of Streptomyces strains from saline environments. Sci Rep 6:32718. https://doi.org/10.1038/srep32718
Zheng ZH, Zeng W, Huang YJ, Yang ZY, Li J, Cai HR, Su WJ (2000) Detection of antitumor and antimicrobial activities in marine organism associated actinomycetes isolated from the Taiwan Strait, China. FEMS Microbiol Lett 188:87–91
Zhou J, Lyu YH, Richlen ML, Anderson DM, Cai ZH (2016) Quorum sensing is a language of chemical signals and plays an ecological role in algal-bacterial interactions. Crit Rev Plant Sci 35:81–105
Zozaya-Valdés E, Roth-Schulze AJ, Egan S, Thomas T (2017) Microbial community function in the bleaching disease of the marine macroalgae Delisea pulchra. Environ Microbiol 19:3012–3024. https://doi.org/10.1111/1462-2920.13758
Acknowledgements
This work was supported by a grant of Instituto Antartico Chileno (INACH, Grant RT_06-13) and Grant 31470142 from the National Natural Science Foundation of China (NSFC). We also thank the INACH staff at Station Prof. Julio Escudero for logistic support. We are also grateful to Dr Ivan Gómez and his group (Project Anillo ART1101) for its valuable support during the fieldwork.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Rights and permissions
About this article
Cite this article
Alvarado, P., Huang, Y., Wang, J. et al. Phylogeny and bioactivity of epiphytic Gram-positive bacteria isolated from three co-occurring antarctic macroalgae. Antonie van Leeuwenhoek 111, 1543–1555 (2018). https://doi.org/10.1007/s10482-018-1044-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-018-1044-6