Abstract
Multidrug-resistant Escherichia coli has seriously threatened antibiotic resources and international public health. Bacteriophage lysin preparations have been widely considered as valid agents for solving multidrug resistances. Many lysins have been derived to treat diseases caused by Gram-positive bacteria, but only a few lysin preparations have been found that successively treat diseases caused by Gram-negative bacteria. The outer membrane of Gram-negative bacteria effectively blocks the interactions between peptidoglycan in the periplasmic space and bacteriophage lysins, which therefore hampers the antimicrobial effects of bacteriophage lysins. In this study, a new fusion protein (Colicin-Lysep3) was constructed by fusing the translocation and receptor binding domains of colicin A with an E. coli phage lysin, which endows Colicin-Lysep3 bactericidal activity against E. coli from outside of Gram-negative bacteria. These results show that Colicin-Lysep3 could lyse the E. coli broadly in vitro and significantly reduce the number of E. coli in an intestinal infection mouse model. Overall, our findings first demonstrated that a colicin A fragment could enable a bacteriophage lysin to lyse E. coli from the outside, promoting the application of phage lysin preparations in control of Gram-negative bacteria.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Escherichia coli is one of the main pathogens that cause human and animal digestive tract diseases (Bardhan et al. 2010; Turner et al. 2006). Because antibiotics have been widely abused, numerous strains of pathogenic E. coli exhibits multidrug resistances (Sande-Bruinsma et al. 2008; Yan and Baran 2016). Multidrug-resistant E. coli exist widely in farms and nature (Narciso-da-Rocha and Manaia 2016; Yan and Baran 2016). The drug resistance of pathogenic E. coli is a serious threat to food safety and public health (Pavlickova et al. 2017).
Bacteriophage lysin preparations have been widely considered as valid agents for solving multidrug resistance and have a good therapeutic effect (Moradpour and Ghasemian 2011; Pires et al. 2015; Qadir 2015; Young and Gill 2015). Phage lysins, which lyse bacteria mainly, function as cell wall peptidoglycan hydrolases. These lysis agents are generally applied to treat diseases caused by Gram-positive bacteria such as Staphylococcus aureus and streptococci (Cheng et al. 2005; Loeffler et al. 2001; Nelson et al. 2001). However, outer membrane proteins and impermeable lipopolysaccharides are the outermost layers of the cell envelope of E. coli and the porins among the outer membrane proteins form barrel channels which only allow small molecules to pass through (Briers and Lavigne 2015). The peptidoglycan of E. coli is located in the periplasmic space of the cell envelope. As macromolecules, bacteriophage lysins cannot pass through the outer membrane barrier and go into the periplasmic space to interact with peptidoglycan, which limits their antimicrobial effects on lysing E. coli. Lysins could also lyse Gram-negative host bacteria as long as they are able to reach the peptidoglycan. Lood et al. showed that the lysin PlyF307 from a bacteriophage genomic library could effectively lyse Acinetobacter baumannii, and rescued mice from lethal A. baumannii bacteremia (Lood et al. 2015). Briers et al. modified endolysins by protein engineering to create Artilysins that were able to pass the outer membrane and became active against Pseudomonas aeruginosa and A. baumannii, two of the most hazardous drug-resistant Gram-negative pathogens (Briers et al. 2014). Lukacik et al. designed a chimeric protein comprised of the FyuA binding domain of pesticin fused to the N-terminus of T4 lysozyme, which could kill particular Yersinia and pathogenic E. coli strains and, importantly, could evade the pesticin immunity protein (Pim), giving it a distinct advantage over pesticin (Lukacik et al. 2012a, b). Oliveira et al. have cloned, heterologously expressed and characterised a novel endolysin (ABgp46) from Acinetobacter phage vb_AbaP_CEB1 and tested its antibacterial activity against several multidrug-resistant A. baumannii strains (Oliveira et al. 2016). In addition, lysins can cause lysis of Gram-negative bacteria with the aid of EDTA, citric acid and some membrane agents, or small molecule peptides (Lim et al. 2012; Plotka et al. 2015; Thandar et al. 2016; Walmagh et al. 2013; Defraine et al. 2016).
As a type of bacteriocin secreted by E. coli, colicin can kill closely related bacteria which do not secrete specific colicin immunity proteins (Cascales et al. 2007; Majeed et al. 2011). Most bacteriocins are 50–90 kDa and consist of three distinct domains (Braun et al. 1994; Knibiehler et al. 1989; Morlon et al. 1983a, b): a domain involved in translocation (N-terminal translocation region), a receptor binding domain (central RB region) and a pore-forming domain (C-terminal region) (Lazdunski et al. 1998, 2000; Morlon et al. 1983a, b). After colicin A interacts with BtuB (its receptor on the outer membrane of E. coli), colicin A becomes substantially unfolded compared to its previously folded state, which allows the N-terminal translocation region to access the OmpF porin. As soon as the N-terminal domain is exposed to the inner face of the outer membrane, it interacts with TolB (widely distributed over the inner face of the outer membrane), attached to Pal, and eventually displaces TolB from Pal. Then, Pal can interact with peptidoglycan. The colicin A C-terminal pore-forming domain is translocated through the outer membrane by its slow conformational change into a “molten-globule” (the tertiary structure is destroyed, whereas most of the secondary structure is preserved). Finally, the C-terminal domain may insert into the inner membrane and form a channel, causing lysis and death of the bacteria (Lazdunski et al. 1998, 2000). We hypothesised that if the C-terminal domain of colicin A was replaced by the lysin, the N-terminal translocation region and central RB region of colicin A could transport lysin to the periplasmic space to lyse the cell wall.
In a previous study, we isolated a phage lysin which could effectively lyse host E. coli. Our team has increased the positive charge at the C-terminus of E. coli bacteriophage lysin, Lysep3, which increases its bactericidal ability from outside E. coli, providing a new practical method for the development of anti-Gram-negative bacterial lysins (Ma et al. 2017). Lysep3 has 58% homology to the Pseudomonas phage PPpW-3 lysin, but no homology to E. coli phage lysin at the gene sequence level (Lv et al. 2015). In this study, the N-terminal translocation region and central RB region of colicin A were fused with Lysep3, and the fusion protein Colicin-Lysep3 had a bactericidal effect.
Materials and methods
Gene cloning and construction of expression vector
Plasmid pIC279 was used as a template for cloning the N-terminal and central domains of colicin A (GenBank: M37402.1) by using specific primers. The primers of the target genes were as follows: forward primer (f1): 5′-gggaattccatatgCCTGGATTTAATTATGGTGG-3′ (underlined region is the restriction digest sites NdeI), reverse primer (f2): 5′- cgcggatccTTCAGCCTGGCGCTGGCGCGCT-3′ (underlined region is the restriction digest sites BamHI). PCR products of the N-terminal and central region of colicin A and plasmid pET-28a were digested with NdeI and BamHI, and these linearised fragments were joined together to construct recombinant plasmid pET-Colicin.
Gene Lysep3 (accession codes: YP_009100017.1) was used as a template to clone Lysep3. The forward primer (f3) sequence was f1:5′-cgcggatccAAAATTTCATCCAATGGCCTGGC-3′ (underlined region is the restriction digest sites BamHI). The reverse primer (f4) sequence was 5′- acgcgtcgacTCATGCTGCCGCCACACCGCGTT-3′ (underlined region is the restriction digest sites SalI), and different DNA sequences were integrated at the 5′-terminal site. Lysep 3 and pET-Colicin were digested with SalI and BamHI, and these linearised fragments were joined together to construct recombinant plasmid pET-Colicin-Lysep3.
Expression, purification and recovery of the fusion protein
PET-Colicin-Lysep3 was transformed into BL21(DE3) to give pLysS competent cells, followed by resistance screening on kanamycin-containing LB agar plates. A single colony was cultured in LB liquid medium at 37 °C at 180 rpm. Cultures were grown until OD600 = 0.35, then IPTG was added to obtain a final concentration of 0.1 mmol/L and the culture was further incubated at 37 °C for 3 h. Following centrifugation, the bacterial pellet was lysed in 2 × SDS sample buffer by boiling for 5 min. After centrifugation at 17,500×g for 3 min, 10 μl of supernatant was subjected to SDS-PAGE to verify expression of Colicin-Lysep3. Bacterial cultures (200 mL) containing pColicin-Lysep3 were washed with phosphate buffered saline (PBS) buffer for twice, and the cell pellets were lysed by sonication. After centrifugation at 17,500×g (12,000 rpm) for 15 min to collect the inclusion bodies, they were washed for four times using sodium chloride-tris–EDTA buffer (STE, contained 10 mM Tris–Hcl, 0.1 mM NaCl, 1 mM EDTA) at 20,220×g (13,000 rpm) for 15 min. Inclusion bodies were dissolved with 20 mL denaturing lysis buffer (contained 0.1 mM PMSF, 8 M urea, 2 mM DTT). Dissolved inclusion bodies were loaded into dialysis bags and then dialyzed by incubation in a solvent gradient of 6, 5, 4, 3.5, 3, 2.5, 2, 1.5 and 1 M of 200 mL refolding buffer (contained 30 mM GSH, 0.3 mM GSSG, 0.5 M l-arginine, 10% glycerol, 1% sucrose, 0.1 mM PMSF and urea) solution at 4 °C for 3 h. Finally, the aforementioned products were dialyzed in urea-free STE buffer for three times. The purification of fusion proteins was performed using High Affinity Ni–NTA Resin by following the manufacturer’s protocol. The purity of Colicin-Lysep3 was analysed using a thin tomographic scan (Bio-RAD, USA). As controls, the N-terminal translocation region and central RB region of colicin (TBR protein) was also prepared in the same way, and Lysep3 was expressed and stored as previously described (Ma et al. 2017).
Transmission electron microscopy (TEM)
A single colony was cultured in 5 mL of LB liquid medium at 37 °C at 180 rpm until OD600 = 0.60, and then 200 μL of the culture was washed with PBS buffer three times, and 50 μL of Colicin-Lysep3 was added and incubated for 15 min. Following centrifugation at 12,000 rpm to collect the bacterial pellet, SEM fixed buffer (2.5% glutaraldehyde) was added slowly for fixing the bacterial pellet at 4 °C. After 24 h, ultra-thin sections were obtained, and TEM was performed.
Effect of pH and incubating time on the enzymatic activity of Colicin-Lysep3
When the absorbance of the E. coli culture reached OD600 = 0.6, the bacterial pellet was harvested and washed with 300 μL HCl-Tris buffer solution with different pH values (pH 3, 3.5, 4.0, 4.5, 5.0, 5.5, 6.0, 6.5, 7.0, 7.5 and 8.0), respectively. Bacterial pellets were resuspended and 0.32 μg/μL of final concentration of Colicin-Lysep3 was added and then incubated for 15 min. Then, in turn tenfold diluted bacterial dilutions were plated on LB agar culture medium and incubated at 37 °C for 18 h. Finally, the number of colonies was counted using plate colony-counting methods.
E. coli strain BL21 in logarithmic growth phase was collected, washed and diluted approximately to 5 × 105 CFU/mL with HCl-Tris buffer solution (pH 5.0). Then the bacterial suspension was added into a series tubes, 1 mL each, and a different dose of Colicin-Lysep3 (2, 4, 6, 8, 10, 12, 14, 16, 18, or 20 μg) was added into each tube in order. The mixture was then incubated for 15 min. Then, bacterial cultures were diluted and plated on LB agar culture medium at 37 °C for 18 h. Finally, the number of colonies was quantitated using a plate colony-counting method.
Different batches of bacterial suspensions and 8 μg of Colicin-Lysep3 were added and incubated for 5, 10, 15, 20, 25 and 30 min. Then, diluted bacterial liquid cultures plated on LB agar culture medium were incubated at 37 °C for 18 h. Finally, the number of colonies was quantitated using a plate colony-counting method.
Lysis spectrum of the Colicin-Lysep3
Dot assays were used to detect the effect of Colicin-Lysep3 on 32 strains of E. coli isolated from different farms. A single colony was cultured in 5 mL LB liquid medium at 37 °C at 180 rpm until the OD600 of the culture was 0.6. The culture of the bacteria was diluted to 105 CFU/mL with PBS buffer. At the same time, purified Colicin-Lysep3 was diluted to 1.5 μg/μL. Then, 50 μL of diluted bacteria and 50 μL of fusion lysin were mixed, and the mixture was incubated at 37 °C for 30 min. Subsequently, 10 μL of the aforementioned mixture was spotted on the surface of LB agar medium. After incubating for 10 h, the number of colonies was counted by using a plate colony-counting method.
A similar assay was performed with 105 CFUs of P. aeruginosa, Salmonella and Shigella Castellani.
Construction of GFP-fluorescence labeled E. coli BL21 (DE3)
The cloned GFP gene was inserted into pET23a, which could express the exogenous gene without the presence of inducer, such as IPTG, and transformed into BL21 (DE3) to construct GFP fluorescent labeled BL21 (DE3) (BL21-GFP).
Evaluation the efficacy of Colicin-Lysep3 in a mouse intestinal infection model
Twenty Kunming mice (18–22 g/each) were randomly selected for the control group (n = 10) and the treatment group (n = 10). The mice underwent apastia and no water for 48 h. A single colony of BL21-GFP was cultured in 5 mL LB liquid medium at 37 °C at 180 rpm until OD600 was 0.45. The bacterial culture (final volume approximately 200 μL) was diluted to 105 CFU/mL with PBS buffer. 200 μL of diluted bacteria was infused into the stomach of each mouse of the treatment group and the control group. At 2 h post challenge, 200 μL 0.5 μg/μL of Colicin-Lysep3 was given to the treatment group in the same way, while 200 μL PBS buffer to the control group mice. All mice were euthanized by cervical dislocation after 8 h, then contents of the anterior, posterior and cecum segment of the small intestine were collected and filtered with a 300 mesh screen filter. Finally, the number of colonies was calculated by flow cytometry with two gating at 488 nm (BD Company, USA).
Statistical analysis
For statistical analysis, one-way ANOVA was performed using SPSS software (version 12.0). P-values <0.05 were considered significant.
Results
Construction and purification of the fusion lysin
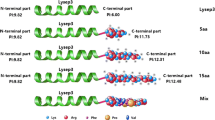
In the course of the experiments, we found that the turbidity of bacterial cultures of BL21(DE3) pLysS which contained pET-Colicin-Lysep3 was suddenly lowered within 2 h, which suggested that the expression of Colicin-Lysep3 (Fig. 1) had already lysed its host bacteria. To collect a larger number of Colicin-Lysep3 products, the optimal expression conditions for the protein were explored. It identified that Colicin-Lysep3 expressed in the form of inclusion bodies and optimal expression time was 60–90 min at 37 °C. After isolating the inclusion bodies, the optimal conditions of denaturation and renaturation were studied. Gel scanning showed that the purity of Colicin-Lysep3 reached 95%.
Observed cleavage of E. coli by Colicin-Lysep3
The morphological observation of the bacteria was performed using TEM. As shown in Fig. 2, after interacting with Colicin-Lysep3, many cells showed heterogeneous density (dark to light) indicating the extent of the damage, and very light areas in lysed cells, occasionally with leakage of cytoplasm (Fig. 2a, arrow 2) after interacting with Colicin-Lysep3, while a few cells were nearly normal (Fig. 2a, b, arrow 1). Bacterial ghosts (Fig. 2a, arrow 4) were also generated where the contents of the cell had flown out (Fig. 2a, b, arrow 3), and whole cell lysis was observed (Fig. 2b, arrow 5).
The morphological observation of the bacteria via TEM after incubation with Colicin-Lysep3 for 15 min (×4000). The morphological observation of the bacteria showed that many cells showed heterogeneous density (dark to light) indicating the extent of damage and very light areas in lysed cells, occasionally with leakage of cytoplasm (Fig. 2a, arrow 2) after interacting with Colicin-Lysep3, while a few cells were nearly normal (Fig. 2a, b, arrow 1). Bacterial ghosts (Fig. 2a, arrow 4) were also generated as the contents of the cell had flowed out of the cells (Fig. 2a, b, arrow 3), and whole cell’s lysis was observed (Fig. 2b, arrow 5)
Determination of bactericidal performance of Colicin-Lysep3 in vitro
Results showed that the pH of the environment notably affected Colicin-Lysep3 bactericidal activity (Fig. 3). Compared with the control group (no addition), colicin TBR showed no effect on the bacteria growth, while the Lysep3 group showed bactericidal activity but significantly less than Colicin-Lysep3 group. Colicin-Lysep3 showed very low bacterial kill capacity when pH = 3 while it was enhanced when pH = 5 as shown in Fig. 3. The number of bacteria decreased from 1 × 105 CFU to 900 CFU in the Colicin-Lysep3 group when pH = 5 but the bactericidal capacity decreased when the pH value changed from 5 to 8.
Effect of pH on bactericidal activity of Colicin-Lysep3. Compared with the control (no addition), Colicin TBR showed no effect on the bacteria growth, Lysep3 showed limited bactericidal activity and Colicin-Lysep3 revealed significant lytic ability. The bactericidal effect of Colicin-Lysep3 is optimal at pH 5
Colicin-Lysep3 is unlike antibiotics, and it is difficult to determine its MIC value because the fusion protein is affected by many environmental conditions. Thus, we measured the bactericidal activity at pH 5 and found that a dose of 8 μg or more showed the most effective bactericidal ability (P < 0.01); however, a lower dose makes it difficult to observe a bactericidal effect (Fig. 4).
Duration of exposure between Colicin-Lysep3 and bacteria also affects its bactericidal effect. At a dose of 8 μg at pH 5, its bactericidal performance exhibited a peak at 15 min, and as the time progressed, the bactericidal effect showed no increase (Fig. 5).
Bactericidal spectrum of Colicin-Lysep3
The results showed that Colicin-Lysep3 has clear killing effects on 22 strains of E. coli among 32 E. coli strains isolated from different paddocks, i.e. an effective rate up to 69%. At the same time we found that Colicin-Lysep3 showed no bactericidal activity against P. aeruginosa, Salmonella or Shigella Castellani.
Evaluation the efficacy of Colicin-Lysep3 in a mouse intestinal infection model
Different pH values in the different intestinal regions, the presence of digestive enzymes and a variety of intestinal flora, make the intestinal environment complex, which makes it difficult to identify the role of Colicin-Lysep3 in the digestive tract. To study the bactericidal effect of Colicin-Lysep3 in the intestinal tract, we constructed a strain of E. coli BL21 (DE3) which has a GFP label and able to express GFP automatically without inducer in the intestine. Compared with the control group (without Colicin-Lysep3), the experimental group exhibited a significant change (Fig. 6). The BL21 (DE3)-GFP content in the forepart of the small intestine of mice fed Colicin-Lysep3 was 11% less than that in the control group (P < 0.01) (Fig. 6a), 3% less in the posterius segmenta of the small intestine (P < 0.01) (Fig. 6b), and 3% less in the cecum (P < 0.05) (Fig. 6c); these results suggested that Colicin-Lysep3 has bactericidal activity in the intestinal tract.
Effects of Colicin-Lysep3 on BL21 (DE3)-content of the small intestine and cecum. a BL21 (DE3)-content of the anterior segment of the small intestine of the mice fed with GFP (Colicin-Lysep3) compared with the control group (**P < 0.01). b BL21 (DE3)-content of the posterior segment of the small intestine of the mice fed with GFP (Colicin-Lysep3) compared with the control group (**P < 0.01). c BL21 (DE3)-content of the cecum of the mice fed with GFP (Colicin-Lysep3) compared with the control group (*P < 0.05)
Discussion
The drug resistance of pathogenic E. coli has become an increasingly serious problem. At present, it is considered that the application of bacteriophage or its enzyme preparations is one of the better ways to solve this problem (Young and Gill 2015; Drulis-Kawa et al. 2015). Our findings give the first demonstration of a method to enable a bacteriophage lysin to lyse E. coli from the outside via E. coli colicin, which has the potential to promote the application of phage lysin preparations in the control of Gram-negative bacteria.
Phage lysins generally act on the cell wall peptidoglycan of bacteria, causing the degradation of the cell wall, ultimately leading to lysis and death of the bacteria. Gram-positive bacterial lysis depends on direct cleavage from the outside, as the peptidoglycan is exposed to the outside. Lysin preparations have already been proven to be safe and effective in the treatment of Gram-positive bacterial diseases. In Gram-negative bacteria, the peptidoglycan is located in the periplasmic space of the cell envelope. The biggest obstacle for lysis of E. coli is the outer membrane, because of the presence of which lysin is blocked outside of the periplasmic space and is thus unable to lyse the peptidoglycan.
Much work has been done by other teams to overcome the problem that lysins cannot cross the bacterial outer membrane. Lukacik et al. designed a fusion protein comprised of the FyuA binding domain of pesticin and the N-terminus of T4 lysozyme, which killed selected Yersinia and pathogenic E. coli strains and didn’t kill the natural gut flora (Lukacik et al. 2012a, b). However, as FyuA has a more restricted distribution in STEC resistant strains isolated from animal products (Dehkordi et al. 2014), a new recombinant lysin was needed to fight multi-resistant E. coli strains. Therefore, we designed a fusion protein comprised of the N-terminal translocation region, central RB region of the colicin and the lysin (Fig. 1), whose receptor binding site is BtuB, which gives about 1000 translocation sites for colicin A on sensitive E. coli strains (Lazdunski et al. 1998). It was hoped that the fusion protein could have an improved bactericidal effect, not only for the E. coli in the intestine, but also for extra-intestinal pathogenic E. coli strains, which have become a serious problem for animals and humans (Nandanwar et al. 2014).
The C-terminus of colicin A can break through the barriers of the outer membrane into the inner membrane and kill E. coli. Structural analysis shows that phage lysin is similar to the C-terminus of colicin A in size of the structure. We designed the fusion protein, in which the C-terminus of colicin A was replaced by phage lysin, to overcome the outer membrane barrier of E. coli and kill the bacteria. As expected, Colicin-Lysep3 showed effective killing activity of E. coli. Microscopic observations showed that the morphology of E. coli after Colicin-Lysep3 treatment changed rapidly from rod-shaped to oval-shaped, and the cell walls eventually ruptured. Colicin-Lysep3 did not appear to target a specific protein of E. coli as it had broad bactericidal activity to resistant strains. We speculate that the lysin is first transported into the periplasmic space and interacts with peptidoglycan, then destroys the peptidoglycan backbone under intracellular pressure, thus leading to cytomorphosis and lysis. Electron microscopy clearly showed the cell walls rupture after Colicin-Lysep3 treatment.
Typically, each lysin has its own optimal activity conditions (Fischetti 2008). The optimal pH condition for the lysin is approximately pH 5.0. Under this condition in vitro, Colicin-Lysep3 can lyse almost all the E. coli strains tested in a liquid environment within 15 min. However, the usage dose of lysin is relatively large compared to antibiotics and we used a relatively high dose of recombinant protein. Reasons for this are two-fold. First, the protein is expected to be single use as it may remain bound to cell debris after killing. Second, the purification of the protein from inclusion bodies by denaturation and renaturation may have lead to partial inactivation of the protein.
The data indicated that Colicin-Lysep3 can only lyse 22/33 E. coli strains isolated from farms, which may be connected to the receptor binding domain (central RB region) of colicin A. The reason might be that there is a variety of bacteriocins of E. coli, each of which can only be recognised by certain E. coli. However, the fusion expression of a variety of transporter fragments of E. coli bacteriocins with the lysin, which includes mixed reagents, would be able to more extensively lyse pathogenic E. coli, and further research is in progress.
In the digestive tract, digestive enzymes and the chemical substances may inactivate the lysin. In our study, mice were fed with GFP labeled E. coli and with Colicin-Lysep3 after 2 h. The results showed that Colicin-Lysep3 could effectively reduce the proportion of the labeled bacteria among the intestinal bacteria.
According to the above analyses, the transporter segment of colicin A appears to transport E. coli phage lysin to the periplasm and kill bacteria in vitro and in vivo. Our studies therefore suggest a new method of using lysin to kill E. coli.
References
Bardhan P, Faruque AS, Naheed A, Sack DA (2010) Decrease in Shigellosis-related deaths without Shigella spp.-specific interventions. Asia. Emerg Infect Dis 16:1718–1723
Braun V, Pilsl H, Gross P (1994) Colicins: structures, modes of action, transfer through membranes, and evolution. Arch Microbiol 161:199–206
Briers Y, Lavigne R (2015) Breaking barriers: expansion of the use of endolysins as novel antibacterials against Gram-negative bacteria. Future Microbiol 10:377–390
Briers Y, Walmagh M, Van Puyenbroeck V, Cornelissen A, Cenens W, Aertsen A, Oliveira H, Azeredo J, Verween G, Pirnay JP, Miller S, Volckaert G, Lavigne R (2014) Engineered endolysin-based “Artilysins” to combat multidrug-resistant gram-negative pathogens. MBio 5:e01379-14
Cascales E, Buchanan SK, Duché D, Kleanthous C, Lloubès R, Postle K, Riley M, Slatin S, Cavard D (2007) Colicin biology. Microbiol Mol Biol Rev 71:158–229
Cheng Q, Nelson D, Zhu S, Fischetti VA (2005) Removal of group B streptococci colonizing the vagina and oropharynx of mice with a bacteriophage lytic enzyme. Antimicrob Agents Chemother 49:111–117
Defraine V, Schuermans J, Grymonprez B, Govers SK, Aertsen A, Fauvart M, Michiels J, Lavigne R, Briers Y (2016) Efficacy of Artilysin Art-175 against resistant and persistent Acinetobacter baumannii. Antimicrob Agents Chemother 60:3480–3488
Dehkordi FS, Yazdani F, Mozafari J, Valizadeh Y (2014) Virulence factors, serogroups and antimicrobial resistance properties of Escherichia coli strains in fermented dairy products. BMC Res Notes 7:217
Drulis-Kawa Z, Majkowska-Skrobek G, Maciejewska B (2015) Bacteriophages and phage-derived proteins–application approaches. Curr Med Chem 22:1757–1773
Fischetti VA (2008) Bacteriophage lysins as effective antibacterials. Curr Opin Microbiol 11:393–400
Knibiehler M, Howard SP, Baty D, Geli V, Lloubès R, Sauve P, Lazdunski C (1989) Isolation and molecular and functional properties of the amino-terminal domain of colicin A. Eur J Biochem 181:109–113
Lazdunski CJ, Bouveret E, Rigal A, Journet L, Lloubès R, Bénédetti H (1998) Colicin import into Escherichia coli cells. J Bacteriol 180:4993–5002
Lazdunski C, Bouveret E, Rigal A, Journet L, Lloubès R, Bénédetti H (2000) Colicin import into Escherichia coli cells requires the proximity of the inner and outer membranes and other factors. Int J Med Microbiol 290:337–344
Lim JA, Shin H, Kang DH, Ryu S (2012) Characterization of endolysin from a Salmonella Typhimurium-infecting bacteriophage SPN1S. Res Microbiol 163:233–241
Loeffler JM, Nelson D, Fischetti VA (2001) Rapid killing of Streptococcus pneumoniae with a bacteriophage cell wall hydrolase. Science 294:2170–2172
Lood R, Winer BY, Pelzek AJ, Diez-Martinez R, Thandar M, Euler CW, Schuch R, Fischetti VA (2015) Novel phage lysin capable of killing the multidrug-resistant gram-negative bacterium Acinetobacter baumannii in a mouse bacteremia model. Antimicrob Agents Chemother 59:1983–1991
Lukacik P, Barnard TJ, Buchanan SK (2012a) Using a bacteriocin structure to engineer a phage lysin that targets Yersinia pestis. Biochem Soc Trans 40:1503–1506
Lukacik P, Barnard TJ, Keller PW, Chaturvedi KS, Seddiki N, Fairman JW, Noinaj N, Kirby TL, Henderson JP, Steven AC, Hinnebusch BJ, Buchanan SK (2012b) Structural engineering of a phage lysin that targets gram-negative pathogens. Proc Natl Acad Sci USA 109:9857–9862
Lv M, Wang S, Yan G, Sun C, Feng X, Gu J, Han W, Lei L (2015) Genome sequencing and analysis of an Escherichia coli phage vB_EcoM-ep3 with a novel lysin, Lysep3. Virus Genes 50:487–497
Ma Q, Guo Z, Gao C, Zhu R, Wang S, Yu L, Qin W, Xia X, Gu J, Yan G, Lei L (2017) Enhancement of the direct antimicrobial activity of Lysep3 against Escherichia coli by inserting cationic peptides into its C terminus. Antonie Van Leeuwenhoek 110:347–355
Majeed H, Gillor O, Kerr B, Riley MA (2011) Competitive interactions in Escherichia coli populations: the role of bacteriocins. ISME J 5:71–81
Moradpour Z, Ghasemian A (2011) Modified phages: novel antimicrobial agents to combat infectious diseases. Biotechnol Adv 29:732–738
Morlon J, Lloubes R, Chartier M, Bonicel J, Lazdunski C (1983a) Nucleotide sequence of promoter, operator and amino-terminal region of caa, the structural gene of colicin A. EMBO J 2:787–789
Morlon J, Lloubès R, Varenne S, Chartier M, Lazdunski C (1983b) Complete nucleotide sequence of the structural gene for colicin A, a gene translated at non-uniform rate. J Mol Biol 170:271–285
Nandanwar N, Janssen T, Kühl M, Ahmed N, Ewers C, Wieler LH (2014) Extraintestinal pathogenic Escherichia coli (ExPEC) of human and avian origin belonging to sequence type complex 95 (STC95) portray indistinguishable virulence features. Int J Med Microbiol 304:835–842
Narciso-da-Rocha C, Manaia CM (2016) Multidrug resistance phenotypes are widespread over different bacterial taxonomic groups thriving in surface water. Sci Total Environ 563–564:1–9
Nelson D, Loomis L, Fischetti VA (2001) Prevention and elimination of upper respiratory colonization of mice by group A streptococci by using a bacteriophage lytic enzyme. Proc Natl Acad Sci USA 98:4107–4112
Oliveira H, Vilas Boas D, Mesnage S, Kluskens LD, Lavigne R, Sillankorva S, Secundo F, Azeredo J (2016) Structural and enzymatic characterization of ABgp46, a novel phage endolysin with broad anti-gram-negative bacterial activity. Front Microbiol 7:208
Pavlickova S, Klancnik A, Dolezalova M, Mozina SS, Holko I (2017) Antibiotic resistance, virulence factors and biofilm formation ability in Escherichia coli strains isolated from chicken meat and wildlife in the Czech Republic. J Environ Sci Health B 11:1–7
Pires DP, Vilas Boas D, Sillankorva S, Azeredo J (2015) Phage therapy: a step forward in the treatment of Pseudomonas aeruginosa infections. J Virol 89:7449–7456
Plotka M, Kaczorowska AK, Morzywolek A et al (2015) Biochemical characterization and validation of a catalytic site of a highly thermostable Ts2631 endolysin from the thermus scotoductus phage vB_Tsc2631. PLoS ONE 10:e0137374
Qadir MI (2015) Review: phage therapy: a modern tool to control bacterial infections. Pak J Pharm Sci 28:265–270
Sande-Bruinsma NVD, Grundmann H, Verloo D, Tiemersma E, Monen J (2008) Antimicrobial drug use and resistance in Europe. Emerg Infect Dis 14:1722–1730
Thandar M, Lood R, Winer BY, Deutsch DR, Euler CW, Fischetti VA (2016) Novel engineered peptides of a phage lysin as effective antimicrobials against multidrug-resistant Acinetobacter baumannii. Antimicrob Agents Chemother 60:2671–2679
Turner SM, Scott-Tucker A, Cooper LM, Henderson IR (2006) Weapons of mass destruction: virulence factors of the global killer enterotoxigenic Escherichia coli. FEMS Microbiol Lett 263:10–20
Walmagh M, Boczkowska B, Grymonprez B, Briers Y, Drulis-Kawa Z, Lavigne R (2013) Characterization of five novel endolysins from Gram-negative infecting bacteriophages. Appl Microbiol Biotechnol 97:4369–4375
Yan M, Baran PS (2016) Drug discovery: fighting evolution with chemical synthesis. Nature 533:326–327
Young R, Gill JJ (2015) MICROBIOLOGY. phage therapy redux–What is to be done? Science 350:1163–1164
Funding
The study was supported by funds from the National Key Basic Research Program of China (No. 2013CB127205).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethical approval
Mice were purchased from the Animal Experiment Center of Jilin University. All animal research was conducted according to the experimental practices and standards approved by the Animal Welfare and Research Committee at Jilin University (Approval ID: 20100926-1).
Rights and permissions
About this article
Cite this article
Yan, G., Liu, J., Ma, Q. et al. The N-terminal and central domain of colicin A enables phage lysin to lyse Escherichia coli extracellularly. Antonie van Leeuwenhoek 110, 1627–1635 (2017). https://doi.org/10.1007/s10482-017-0912-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-017-0912-9