Abstract
A new species, Globisporangium oryzicola, was isolated from directly seeded rice seedlings, and from soils of paddy fields and an uncultivated field. Despite their different origins, five of the seven isolates studied caused poor seedling establishment of rice in a laboratory inoculation experiment. The species is characterized by oogonia with smooth-walled or sometimes one projection, with one to two antheridia, and aplerotic oospores. Hyphal swellings were rarely observed. Phylogenetic analyses based on the internal transcribed spacer region of the ribosomal RNA gene and mitochondrial cytochrome c oxidase subunit 1 and 2 genes confirmed that the species differed from other Globisporangium species. This novel species is described and illustrated in detail.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Pythium Pringsh. was originally described by Pringsheim (1858), and subsequently there have been attempts to split the genus into several genera or subgenera based on morphological characteristics (Fischer 1892; Schröter 1893; Edson 1915). As molecular phylogenetic studies have advanced, new classifications of the genus Pythium have been proposed. Bala et al. (2010) established a new genus Phytopythium Abad, de Cock, Bala, Robideau and Lévesque comprising the Pythium species from clade K (Lévesque and de Cock 2004), which appear to be morphologically and phylogenetically distinct between Pythium and Phytophthora. In the same year, Uzuhashi et al. (2010) split the genus Pythium into five genera including four new genera, Ovatisporangium, Globisporangium, Elongisporangium, and Pilasporangium, based on sporangial morphology and the phylogeny of the D1–D2 region of the large subunit ribosomal RNA gene (LSU) and mitochondrial cytochrome oxidase II (Cox2) gene. The genus Ovatisporangium is now a synonym of the genus Phytopythium. The genus Globisporangium is characterized by globose hyphal swellings or sporangia, and it corresponds with the species of clades E–G, I and J presented by Lévesque and de Cock (2004).
Many species of the genus Pythium s. lat. (Pythium Pringsh.), which includes the genus Globisporangium, are known as pathogens of various plants, and they incite a wide range of disease symptoms such as pre- and post- emergence damping-off and seed and/or root rot. Some Pythium s. lat. species are known as causal agents of disease in rice (Oryza sativa) seedlings in many countries, especially P. arrhenomanes, which is one of the most important pathogens of rice (Van Buyten and Höfte 2013; Toda et al. 2015). On the other hand, many Pythium s. lat. species are also known as nonpathogenic saprophytes that colonize soil and water (van der Plaats-Niterink 1981; Uzuhashi et al. 2015).
A novel species of the genus Globisporangium was isolated from rice seedlings, and from soils of paddy fields and an uncultivated field. The species showed unique pathogenicity to the host plant and phylogeny of multi-gene sequence data. Here, we have characterized this new species.
Materials and methods
Isolation
Globisporangium oryzicola isolates were obtained from seedlings of directly seeded rice showing poor growth and from soil of a paddy field in Hiroshima Pref., Japan, and also from soil of an uncultivated field in Nagano Pref., Japan (Table 1). From the seedlings and soil of a paddy field, isolation was performed on Pythium selective nystatin-ampicillin-rifampicin-miconazole (NARM) medium (Morita and Tojo 2007). Discolored roots and shoots of rice seedlings (cv. Koshihikari) were washed in tap water to remove soil particles, placed on NARM, and incubated at 25 °C in darkness for 3 days. From the uncultivated field, Globisporangium isolate was obtained by a baiting technique using sterile green pepper seeds as described previously (Uzuhashi et al. 2009). A dried type-specimen was deposited at the Department of Botany, National Museum of Nature and Science, Tokyo (TNS), and living cultures were deposited at the Centraalbureau voor Schimmelcultures (CBS), Utrecht, The Netherlands, the NARO Genebank-Microorganisms Section (MAFF), Genetic Resources Center, National Agriculture and Food Research Organization (NARO), Tsukuba, Ibaraki, Japan, the NITE, Biological Resource Center (NBRC), Kisarazu, Chiba, Japan, and Osaka Prefecture University (OPU), Sakai, Osaka, Japan.
Morphology and growth temperature
Colony patterns of all seven isolates were examined after incubation for 3 days at 25 °C on potato dextrose agar (PDA), potato carrot agar (PCA) prepared in accordance with the report by van der Plaats-Niterink (1981), and V8 juice agar (V8A) plates prepared as previously reported (Miller 1955). The morphology of the Globisporangium isolates was examined in grass blade water culture (van der Plaats-Niterink 1981). At least thirty hyphal swellings, oogonia, and oospores were measured for each isolate. To determine hyphal growth rates, the isolates were incubated on PCA at 0, 3, 7, 10, 13, 16, 19, 22, 25, 28, 31, 34, 37, and 40 °C for 1–3 days. Hyphal growth was evaluated by visual measurement of the average increase in the colony along its longest diameter. The experiment was repeated two times by using a single plate per repetition.
DNA extraction and amplification
Genomic DNA of seven Globisporangium isolates was extracted in accordance with the protocol of Möller et al. (1992) with a modification to the mycelia grinding step. A piece of mycelium on PDA was placed in a 2 ml mastertube hard (BMS, Tokyo, Japan) with two metal beads and incubated at −30 °C until frozen. The frozen mycelia were crushed using a Shake master Neo (BMS) for 90 s at 15,000 rpm.
The internal transcribed spacer (ITS) region of the ribosomal RNA gene and mitochondrial cytochrome c oxidase 1 (Cox1) and 2 (Cox2) regions were amplified by PCR using the following primers; ITS5 and ITS4 (White et al. 1990) for ITS, OomCoxI-Levup and OomCoxI-Levlo (Robideau et al. 2011) for Cox1, and FM66 and FM58 (Martin 2000) for Cox2. PCR reaction volume was 25 μl, containing 2.5 μl 10 × ExTaq buffer (20 mM Mg2+), 2 μl dNTP mixture (2.5 mM each), 0.2 μM of each primer, 0.625 units Taq DNA polymerase (Takara Bio, Shiga, Japan), and 1 μl template DNA. The thermocycler program for amplification of the ITS region was 95 °C for 3 min followed by 35 cycles of 95 °C for 30 s, 55 °C for 1 min, 72 °C for 1 min, and a final extension was made at 72 °C for 10 min. The program for Cox1 and Cox2 were identical to that for ITS, except for a shorter annealing time of 30 s at 55 °C. All PCR products were purified using a MiniElute PCR Purification Kit (QIAGEN, Tokyo, Japan) in accordance with the manufacturer’s instructions.
DNA sequencing and phylogenetic analyses
DNA sequencing reactions were performed using a BigDye Terminator V3.1 Cycle Sequencing Kit (Applied Biosystems, Tokyo, Japan) with the same primers as in the initial PCR step. The products of the sequencing reactions were analyzed using an ABI Prism 3130×l Genetic analyzer (Applied Biosystems). The sequences were aligned with relevant Pythium sequences obtained from the GenBank database using the ClustalW program included in MEGA version 7 (Kumar et al. 2016). The complete alignments were deposited in TreeBase as 19650 (http://treebase.org/treebase-web/). Phylogenetic analyses were conducted using MEGA 7 with Neighbor-Joining (NJ) method of a distance matrix with Kimura 2-parameter model and minimal Maximum Likelihood (ML) method using Tamura-Nei model. The sequences of the ITS and Cox1 regions were analyzed separately. Bootstrap values were obtained from 500 replicates.
Pathogenicity
The seven isolates for G. oryzicola were used for pathogenicity analysis (Table 1). A CMA plug containing mycelium of each isolate was transferred to a 300 ml Erlenmeyer flask containing 1 g of autoclaved seeds of highland bent grass (Agrostis castellana Boiss. & Reut.), and 3 ml of distilled water. After 4 days of incubation at 25 °C in darkness, 10 ml of sterile distilled water was added. The water-soaked culture were further incubated for 7 days at 25 °C in darkness. The mycelium with the autoclaved seeds was macerated with approximately 5000 rpm for 1 min in sterile water using a juicer mixer (SML-G25, Sun Co Ltd., Osaka, Japan). The concentrations of propagules, mainly consisted of oospores, were determined using a plankton counting chamber (Matsunami Glass Industrial, Osaka, Japan) and adjusted to 107 propagules/ml of the water. The 100 ml propagule suspension was mixed with 1 kg of a commercial rice nursery soil (Yanmar Sukoyakabaido, Yanmar Co. Ltd., Osaka, Japan) and adjusted to 106 propagules/g of dry soil. Then, 160 g of the G. oryzicola-infested soil was placed in a plastic pot (inner diameter 90 mm, inner depth 80 mm), and 20 germinated seeds of rice (Oryza sativa, cv. Koshihikari) were sown 5 mm deep in the soil. The soil was saturated with tap water, covered with a plastic bag, and kept in an incubator at 30 °C (day 12 h)/25 °C (night 12 h) with a light intensity of 73 mol/m2/s (measured at the plant level). The soil was irrigated daily with tap water. Plants with emerged second leaves was regarded as the seedling stand according to the anatomical monograph of rice seedlings (Hoshikawa 1989). The percentage emergence was recorded at 5 days after sowing. The infection of isolates was confirmed by reisolation on Pythium selective NARM medium. The experiments were repeated six times with one pot per treatment. Analysis of variance was conducted for the percentage emergence data of different treatments using JMP software (version 8; SAS Institute, Cary, NC, USA). Means of the data were compared using the least significant difference based on a Tukey–Kramer honestly significant different (HSD) test (P < 0.05).
Results
Morphology and growth temperature
All seven strains of G. oryzicola showed similar colony patterns and growth temperature results. Colony patterns comprised cottony aerial mycelium with no special pattern on PDA, and some aerial mycelium with no special pattern on PCA and V8A (Fig. 1). To more clearly define the growth temperature, additional incubations were conducted at 4, 5, 6, 32, and 33 °C for just the HT2-5 strain. The strain was also able to grow at 32 °C, but no growth was observed at any of other temperatures mentioned.
The morphology of asexual and sexual structures were also similar among the 7 strains, although oogonia and oospores of HT42-1 were slightly smaller than the others. Hyphal swellings were rarely observed, although they were sometimes difficult to distinguish from abortive oogonia. Zoospores were not observed in any of the strains. The sexual structures were abundantly produced in the water culture as well as in agar culture such as PCA. Oogonia were mostly smooth-walled, but sometimes had one projection (Fig. 2e, h). Antheridia were monoclinous or diclinous, produced one or two per oogonium, and sessile or with stalks, which sometimes bifurcated near the oogonium (Fig. 2i). Oospores were mostly one, but sometimes two per oogonium (Fig. 2g).
Morphology of G. oryzicola. a Globose, terminal hyphal swellings. b Terminal hyphal swelling. c Oogonium with an aplerotic oospore and a diclinous antheridium. d Oogonium with an aplerotic oospore and an antheridium. e Oogonium with a projection, and an aplerotic oospore, and a monoculinous antheridium. f Terminal oogonium with an aplerotic oospore, and two monoclinous antheridia. g Terminal oogonium with two oospores. h Terminal oogonium with an aplerotic oospore, and a hypogynous antheridium. i Terminal oogonium with an aplerotic oospore, and two antheridia arising on stalks bifurcate near the oogonium. Scale bar 10 µm
Phylogenetic analyses
The sequences of ITS, Cox1, and Cox2 genes were identical among the isolated strains of G. oryzicola. The ITS and Cox1 sequences of G. oryzicola had 97% similarity with those of G. paroecandrum, and 98–99% similarity with those of G. spinosum as the highest one, respectively. Based on comparison with previous studies (e.g. Robideau et al. 2011; Ellis et al. 2012), the sequences of G. oryzicola differ sufficiently from those of any other described Pythium s. lat. species. The Cox2 sequence of G. oryzicola showed 99% similarity with P. spinosum as the highest similarity. However, only a few Cox2 sequences of clade F species have been deposited in the GenBank database. Therefore, a phylogenetic tree was not constructed based on this region.
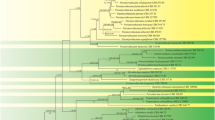
In phylogenetic analyses based on the ITS and Cox1 sequences, G. oryzicola belonged to clade F, as described by Lévesque and de Cock (2004), in both of the phylogenetic trees (data not shown). Phylogenetic trees were constructed based on ITS and Cox1 sequences separately with G. oryzicola and species of clade F (Lévesque and de Cock 2004; Robideau et al. 2011), and G. splendens as the outgroup (Figs. 3, 4). Based either on the analysis of the ITS or Cox1 sequences, the topologies of the trees were quite similar between ML and NJ analyses (Table 2).
Maximum-Likelihood (ML) tree based on the ITS sequence, showing the relationship between G. oryzicola and other species in clade F (Lévesque and de Cock 2004). G. splendens from clade I was used as an outgroup. Numbers along the nodes indicate bootstrap support values above 80% for ML/Neighbor-Joining, respectively
Maximum-Likelihood (ML) tree based on the Cox1 sequence, showing the relationship between G. oryzicola and other species in clade F (Lévesque and de Cock 2004). G. splendens from clade I was used as an outgroup. Numbers along the nodes indicate bootstrap support values above 80% for ML/Neighbor-Joining, respectively
Pathogenicity
Five G. oryzicola strains (HT2-5, HT42-1, HT42-2, HT45-2, and UZ382) significantly (P < 0.05) reduced seedling emergence of rice comparing with an uninoculated control (Table 3; Fig. 5). The other two strains (HT44-1 and HT44-2) had no significant effect on the seedling stands. G. oryzicola was isolated from all the diseased plants.
Taxonomy
Globisporangium oryzicola Uzuhashi and Tojo, sp. nov. (Figs 1, 2) MycoBank MB817839.
Etymology oryzicola refers to the host from which it was isolated.
Colonies forming cottony aerial mycelium on PDA and some aerial mycelium with no special pattern on PCA and V8A. Daily growth at 25 °C on PCA 26.5 mm. Cardinal temperatures minimum 7 °C, optimum 25–28 °C, maximum 32 °C. Main hyphae up to 6 µm wide. Hyphal swellings rarely observed, terminal, globose or sub-globose, 13.1–21.8 µm (av. 17.8 µm) in diameter. Zoospores not observed. Oogonia produced in single culture, globose, smooth-walled of sometimes one finger-like projection, terminal, occasionally intercalary, 16.6–24.7 µm (av. 20.5 µm) in diameter. Antheridia sac-like, club-shaped, diclinous or monoclinous, one or two per oogonium, antheridial stalks sometimes branched. Oospores aplerotic, one or rarely two per oogonium, 13.0–18.9 µm (av. 15.8 µm) in diameter, thin-walled up to 1.0 µm.
Holotype Japan, Hiroshima, Higashihiroshima, in a rice seedling, June 2014, M. Tojo (Holotype, TNS-F-66690; ex-type strain, HT2-5 = CBS 142206 = MAFF 245646 = NBRC 112448 = OPU 861).
Other material examined Japan, Hiroshima, Kure, from soil of a paddy field, July 2014, M. Tojo (HT42-1 = MAFF 245721 = NBRC 112449 = OPU 862, HT42-2 = MAFF 245722 = OPU 863, HT44-1 = MAFF 245723 = OPU 864, HT44-2 = MAFF 245724 = OPU 865, HT45-2 = MAFF 245725 = OPU 866) and Japan, Nagano, from uncultivated soil, S. Uzuhashi (UZ382 = MAFF 241143 = NBRC 112450 = OPU 867).
Discussion
Globisporangium oryzicola is clearly distinct from other Globisporangium spp. by its phylogenetic relationship. On the other hand, there are no unique morphological characters that clearly distinguish G. oryzicola from other species. G. oryzicola belongs to clade F (Lévesque and de Cock 2004) in the phylogenetic analyses. Some species of this clade form ornamented oogonia, although the length, numbers, shapes, or frequency of projections are diverse among these species. G. oryzicola mainly produced smooth-walled oogonia, but it also sometimes produced oogonia with a projection. This feature is similar to those of Globisporangium irregulare and G. cryptoirregulare in this clade. However, both of these species sometimes produce oogonia with more than one projection (van der Plaats-Niterink 1981; Garzón et al. 2007), which has not been observed in G. oryzicola. G. irregulare also differs from G. oryzicola by producing plerotic oospores occasionally (Table 2). In any cases, G. oryzicola has quite similar morphological characters with G. irregulare and G. cryptoirregulare. Molecular phylogenetic analyses would be the easiest way to distinguish G. oryzicola from G. irregulare and G. cryptoirregulare (Figs. 3, 4). G. oryzicola also morphologically resembles G. ultimum var. ultimum of clade I. For example, none of these species produce zoospores, and form smooth surface oogonia, aplerotic oospores, and sac-like antheridia. G. oryzicola can be distinguished from G. ultimum var. ultimum by the smaller hyphal swellings, oogonia, and oospores (Table 2). In the BLAST searches, G. oryzicola showed the closest homologies with G. paroecandrum and G. spinosum in the ITS and Cox1 sequences, respectively. However, G. oryzicola differs from G. paroecandrum by the smaller hyphal swellings, oogonia with a projection and stalked antheridia (Table 2). G. spinosum occasionally produces the hyphal swellings with 1–2 digitate protuberances and ornamented oogonia, and its antheridia vanish after fertilization (Table 2), so G. oryzicola is easily distinguished from G. spinosum by these features.
G. oryzicola isolates were not only found in Hiroshima Pref., but also in Nagano Pref. Because the two areas are separated geographically by about 550 km, G. oryzicola is considered to have a wide distribution throughout Japan.
The pathogenicity test demonstrated that G. oryzicola is a potential rice pathogen, with varying levels of aggressiveness among the strains. The pathogenicity was not only found in strains from rice plants or rice paddy fields, but also in the strain UZ382, which was from an uncultivated field. Because of the various origins and pathogenicities, G. oryzicola may act as an opportunistic pathogen of rice seedlings as well as being a saprophyte in nature. Its economic impact should be tested in further studies in actual farming conditions.
References
Bala K, Robideau GP, Lévesque A, de Cock AWAM, Abad ZG, Lodhi AM, Shahzad S, Ghaffar A, Coffey MD (2010) Phytopythium Abad, de Cock, Bala, Robideau, Lodhi and Lévesque, gen. nov. and Phytopythium sindhum Lodhi, Shahzad & Lévesque, sp. nov. Persoonia 24:136–137
Edson HA (1915) Rheosporangium aphanidermatum, a new genus and species of fungus parasitic on sugar beets and radishes. J Agric Res 4:279–292
Ellis ML, Paul PA, Dorrance AE, Broders KD (2012) Two new species of Pythium, P. schmitthenneri and P. selbyi pathogens of corn and soybean in Ohio. Mycologia 104:477–487. doi:10.3852/11-162
Fischer A (1892) Phycomyctes. Rabenhorst, Kryptogamenflora 1:505
Garzón CD, Yánez JM, Moorman GW (2007) Pythium cryptoirregulare, a new species within the P. irregulare complex. Mycologia 99:291–301. doi:10.3852/mycologia.99.2.291
Hoshikawa K (1989) The growing rice plant: an anatomical monograph. Nobunkyo, Tokyo, pp 1–310
Kumar S, Stecher G, Tamura K (2016) MEGA7: molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol Biol Evol 33:1870–1874. doi:10.1093/molbev/msw054
Lévesque CA, de Cock AWAM (2004) Molecular phylogeny and taxonomy of the genus Pythium. Mycol Res 108:1363–1383. doi:10.1017/S0953756204001431
Martin FN (2000) Phylogenetic relationships among some Pythium species inferred from sequence analysis of the mitochondrially encoded cytochrome oxidase II gene. Mycologia 95:269–284. doi:10.2307/3761428
Miller PM (1955) V-8 juice agar as a general purpose medium for fungi and bacteria. Phytopathology 45:461–462
Möller EM, Bahnweg G, Sandermann H, Geiger HH (1992) A simple and efficient protocol for isolation of high molecular weight DNA from Filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res 20:6115–6116. doi:10.1093/nar/20.22.6115
Morita Y, Tojo M (2007) Modifications of PARP medium using fluazinam, miconazole, and nystatin for detection of Pythium spp. in soil. Plant Dis 91:1591–1599. doi:10.1094/PDIS-91-12-1591
Pringsheim N (1858) Beiträge zur Morphology and Systematik der Algen. 2. Die Saprolegníeen. Jb Wíss Bot 1:284–306
Robideau GP, De Cock AWAM, Coffey MD, Voglmayr H, Brouwer H, Bala K, Chitty DW, Desaulniers N, Eggertson QA, Gachon CMM, Hu CH, Kupper FC, Rintoul TL, Sarhan E, Verstappen ECP, Zhang Y, Bonants PJM, Ristaino JB, Lévesque CA (2011) DNA barcoding of oomycetes with cytochrome c oxidase subunit I and internal transcribed spacer. Mol Ecol Resour 11:1002–1011. doi:10.1111/j.1755-0998.2011.03041.x
Schröter J (1893) Pythiaceae. Engler Prantl Nat Pfl Fam 1:104–105
Toda T, Iwase A, Fuji S, Furuya H (2015) Widespread occurrence of Pythium arrhenomanes pathogenic to rice seedlings around Japanese rice fields. Plant Dis 99:1823–1831. doi:10.1094/PDIS-01-15-0124-RE
Uzuhashi S, Tojo M, Kobayashi S, Tokura K, Kakishima M (2009) Pythium apinafurcum sp. nov.: its morphology, molecular phylogeny, and infectivity for plants. Mycoscience 50:281–290. doi:10.1007/s10267-009-0486-0
Uzuhashi S, Tojo M, Kakishima M (2010) Phylogeny of the genus Pythium and description of new genera. Mycoscience 51:337–365. doi:10.1007/s10267-010-0046-7
Uzuhashi S, Okada G, Ohkuma M (2015) Four new Pythium species from aquatic environments in Japan. Anton Leeuw J Microb 107:375–391. doi:10.1007/s10482-014-0336-8
Van Buyten E, Höfte M (2013) Pythium species from rice roots differ in virulence, host colonization and nutritional profile. BMC Plant Biol 13:203. doi:10.1186/1471-2229-13-203
van der Plaats-Niterink AJ (1981) Monograph of the genus Pythium. Stud Mycol 21:1–242
White TJ, Bruns T, Lee SB, Taylor J (1990) Amplification and direct sequencing of fungal ribosomal RNA genes for phylogenetics. In: Innis MA, Gelfand DH, Sninsky JJ, White T (eds) PCR protocols: a guide to methods and applications. Academic Press, San Diego, pp 315–322
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Uzuhashi, S., Hata, K., Matsuura, S. et al. Globisporangium oryzicola sp. nov., causing poor seedling establishment of directly seeded rice. Antonie van Leeuwenhoek 110, 543–552 (2017). https://doi.org/10.1007/s10482-016-0822-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-016-0822-2