Abstract
Red grape musts from overripe grapes are characterised by high pH and sugar concentration. Corrections with organic acids are commonly used to secure the alcoholic fermentation and improve the organoleptic characteristics of the wine. In this study we test an alternative biological acidification method using the ability of Lactobacillus plantarum to produce high concentrations of lactic acid. The time course of sugars, organic acids and pH were measured. Available sugars were consumed by L. plantarum producing up to 8.3 g L−1 of lactic acid. Lactic acid changed the pH from 3.9 to 3.4 after 14 days post-inoculation without yielding a relevant concentration of acetic acid (0.34 g L−1).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Malolactic fermentation (MLF) is a secondary fermentation that occurs spontaneously during or after alcoholic fermentation (AF) in almost all red and some white wines, leading to a deacidification and changes in the flavour perception (Davis et al. 1985). It is carried out by different kinds of lactic acid bacteria (LAB) in which dicarboxilic l-malic acid turns into monocarboxilic l-lactic acid. Oenoccocus oeni is the bacterial species mainly involved in MLF due to its ability to be metabolically active at a low pH and in media with high concentrations of ethanol (Bartowsky 2005).
Lactobacillus plantarum is another LAB capable of performing MLF in wine and is one of the dominant species of lactobacilli present in winemaking (Davis et al. 1985). Due to the homofermentative metabolism of sugars, this LAB produces primarily lactic acid during consumption of hexoses, while no acetic acid is produced. However, some acetic acid production can occur with the consumption of pentoses. On the other hand, the heterofermentative metabolism of O. oeni does produce acetic acid from sugars (Breed et al. 1957). The ability of L. plantarum to produce high concentrations of lactic acid from sugars is used in the fermentation of products such as cucumber juice (Passos et al. 1994) or lambic beer in order to achieve low pH of the medium and decrease the concentration of sugars, but has not till now been exploited for acidification of grape musts.
A successful MLF in wine is influenced by different factors, among them the pH of the medium (Pan et al. 2011) and the interactions with other microorganisms such as yeasts (Alexandre et al. 2004). The pH of the juice to be fermented depends on the climate in which the grapes grow and the time of harvest. Usually in hot regions low acidity and a high pH are observed, which affect such wine characteristics as taste and color, and increases the risk of uncontrolled bacterial growth. These hot regions also produce musts with high sugar concentrations (Jackson and Lombard 1993), resulting in high ethanol concentrations and possible stuck fermentations (Bisson 1999). The low acidity problems can be corrected by the addition of organic acids such as L-tartaric acid, which is microbiologically stable, although it is partially lost by precipitation as potassium tartrate and the cost of such a treatment is expensive.
In order to test the use of L. plantarum as an acidification method in grape musts two experiments were designed. The objective of the first experiment was to determine the kinetics of degradation of the main organic acids in a high pH must inoculated with L. plantarum and O. oeni. The obtained observations helped to design the second experiment, in which high pH grape musts were inoculated with L. plantarum before yeasts in order to produce an acidification, lowering pH and decreasing the sugar concentration.
Materials and methods
Microorganisms
Two strains of LAB were used, VP41 (Oenococcus oeni) and V22 (Lactobacillus plantarum), and one strain of yeast LAVLIN EC1118 (Saccharomyces cerevisiae). All these microorganisms are commercially available in the Lallemand Catalogue.
Two kinds of nutritive sterile liquid media typical for wine microorganisms were used (Iland et al. 2007), YPD for yeasts and MRS (pH 5.5) for LAB (Difco, USA). The microorganisms were initially cultured in the appropriate liquid medium and then plated onto the respective solid medium, after which single colonies were selected. The selected colonies were cultured again in liquid media and counted prior to treatment inoculation using a Nebauer Improved chamber. The treatments were inoculated with 1 × 106 CFU ml−1.
Fermentation conditions
In experiment “A” grapes from the variety Carménère were used. The must was characterised by soluble solids content of 22 Brix, a pH of 3.66, and total acidity of 4.05 g L−1 as tartaric acid. Soluble solids content were corrected to 23.8 Brix with sucrose and then malic acid to 4 g L−1 using l-malic acid (Sigma Aldrich, Switzerland). Must pH was corrected to 3.9 with NaOH. The experimental design consisted in five treatments (in triplicates): the single inoculation of EC1118 (S. cerevisiae), VP41 (O. oeni), V22 (L. plantarum) and the simultaneous inoculation (same time) of EC1118 (S. cerevisiae) with VP41 (O. oeni) and EC1118 (S. cerevisiae) with V22 (L. plantarum).
For experiment “B” Carménère grapes were also used. The must was characterised by soluble solids content of 31 Brix a pH of 4.52, a total acidity of 3.34 g L−1 as tartaric acid and a FAN content of 170 mg L−1. Soluble solids content were initially corrected to 23.1 Brix with purified water and then 1 g L−1 of l-malic acid (Sigma Aldrich, Switzerland) was added (final concentration of 3.3 g L−1) to assure a high availability of malic acid for bacteria. The must pH was corrected to 3.9 with H2SO4. The treatment (in triplicate) consisted in the inoculation of V22 (L. plantarum) alone before EC1118 (S. cerevisiae) to achieve an acidification by L. plantarum using the available sugars. After 10 days from the inoculation of L. plantarum yeasts were inoculated to start the AF.
For the two experiments 250 ml of must were transferred in 250 ml flasks and sterilized (120 °C for 15 min) to eliminate any possible effect caused by other microorganisms already present in the must. Then the flasks where inoculated with 1 × 106 CFU ml−1 of each microorganism according to the treatments.
Samples of 4 ml were taken periodically and under aseptic conditions transferred in 5 ml screw cap tubes and frozen until analyzed.
Analytical methods
Total malic and lactic acid were analyzed by high performance liquid chromatography (HPLC) using a VARIAN metacarb 67H column. Separations were carried out with a H2SO4 0.01 N mobile phase at a flow rate of 0.8 ml min−1 and a temperature of 35 °C. UV detection of organic acids was carried out at 210 nm. Samples to be analyzed by HPLC were processed to eliminate polyphenols and carbohydrates using methods previously described in literature (Kerem et al. 2004; Zotou et al. 2004). To eliminate polyphenols polyvinylpolypyrolidone PVPP (10 %) (Laffort, France) was used, then samples were centrifuged at 15,000 rpm for 6 min and the supernatant was transferred to 2 ml tubes with 1 ml of purified water and 67 µl of NaOH 1 M. The anionic exchange columns of 3 ml (Bond Elut-SAX, VARIAN) were used to eliminate carbohydrates. The columns were activated with one volume of methanol (HPLC grade, Merck) and washed with two volumes of purified water. Then columns were filled with 1 ml of sample and sugars were washed with 5 ml of purified water. The organic acids were eluted by washing with 5 ml of HCl 1 M. Finally, samples were filtered with PVDF 0.45 µm membranes (Millipore, Germany) and transferred to HPLC vials. Citric acid, acetic acid, d,l-lactic acid, glucose and fructose were analyzed with enzymatic kits following manufacturer instructions (Megazyme, Ireland; Roche, Germany).
Data representation and statistical analysis
GraphPad Prism v6.0 (GraphPad Software, La Jolla California USA) was used for data representation and JMP v7.0 (SAS Institute Inc., USA) for statistical analysis. Statistical significance of differences between averages of replicates in the two experiments were evaluated performing one-way ANOVA at a confidence level of p = 0.05 and a Tukey’s range test when necessary.
Results and discussion
Experiment A: Organic acids degradation kinetics in a high pH must
Malic and lactic acids
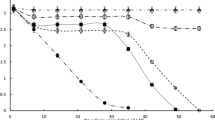
After 36 days malic acid was not completely consumed in any of the five treatments (Fig. 1), with higher concentrations of malic acid observed in treatments with yeasts alone and yeasts combined with L. plantarum.
Time course of malic and lactic acid during 36 days since inoculation of must with V22 (Lactobacillus plantarum), VP41 (Oenococcus oeni), EC1118 (Saccharomyces cerevisiae), EC1118/V22 (S. cerevisiae and L. plantarum) and EC1118/VP41 (S. cerevisiae and O. oeni). Data points represent the mean from triplicates ± SE
For treatments inoculated with L. plantarum and yeasts a final concentration of 1.29 g L−1 of malic acid was observed, while in inoculates with only L. plantarum almost all the malic acid was consumed, with a final concentration of 0.45 g L−1 (Table 1). These results could indicate that the activity of L. plantarum was inhibited by the activity of yeasts. Products released by yeasts have been previously observed to inhibit LAB activity (Alexandre et al. 2004). For these reasons a suitable yeast-bacteria combination for simultaneous inoculation is very important in order to achieve a successful AF and MLF. Previous studies show successful MLF after co-inoculation with L. plantarum and yeasts (Fumi et al. 2010). However, under the conditions of this experiment, L. plantarum is clearly more sensitive to competition with yeasts, while successful MLF occurred (final malic acid concentration of 0.12 g L−1) in inoculates which combined O. oeni with yeasts. These results are consistent with other studies, where the use of O. oeni in co-inoculation lead to satisfactory fermentations (Jussier et al. 2006; Pan et al. 2011).
The treatments inoculated with yeasts alone consumed 18 % of the initial malic acid concentration (Fig. 1). This degradation is higher than what has been reported by Redzepovic et al. (2003), where the strain EC1118 degraded a maximum 8 % of the initial concentration of malic acid. The low malic acid consumption of S. cerevisiae in comparison with other yeasts has been explained by its passive transport system for malic acid, which enters the cells by a simple diffusion mechanism (Salmon 1987).
The course of lactic acid concentration differed largely between treatments, particularly in the treatment inoculated with L. plantarum alone (Fig. 1). In the five treatments lactic acid production began at day 4. The production of lactic acid by L. plantarum was clearly stronger than in the other treatments, with 13 g L−1 of lactic acid in day 36 (Table 1). These large amounts of lactic acid are produced mainly from sugars (du Toit et al. 2011); L. plantarum consumes hexoses via the Embden—Meyerhof pathway, producing primarily lactic acid (Breed et al. 1957). This process also explains the low concentration of acetic acid observed in this treatment on day 36, compared to the treatment with O. oeni alone (Table 1). On the other hand, when L. plantarum was combined with yeasts this large production of lactic did not take place. This could be explained by yeast rapidly degrading sugars and inhibiting L. plantarum. The production of lactic acid from sugars is an interesting phenomenon, because of its potential impact on pH of the medium, sugar concentration of the must and alcohol contents of wine (Passos et al. 1994).
The d and l lactic acid isomers were measured to identify the consumption of sugars by O oeni and L. plantarum. From the literature we know that O. oeni forms only d-lactic acid from sugars and only l-lactic acid from malic acid but L. plantarum forms d,l-lactic acid from sugars and only l-lactic acid from malic acid (Breed et al. 1957).
The treatments with O. oeni alone showed no significant production of d-lactic acid in day 7 (Table 2), which indicates that under these conditions there is a preference for consumption of malic acid prior to sugars. In day 36 d-lactic acid is observed, suggesting sugar consumption and possible acetic acid formation (Table 1). The results confirm that O. oeni degrades malic acid before consuming the available sugars, explaining the successful results for O. oeni and yeast co-inoculation without significant acetic acid production.
In treatments with L. plantarum alone, the presence of d and l-lactic acid was observed by day 7 (Table 2), indicating a simultaneous consumption of sugars and malic acid. A similar result was obtained when L. plantarum was combined with yeasts (Table 2), by day 7 from the total of 2.23 g L−1 of l-lactic acid produced, 1.65 g L−1 must derive from malic acid consumption, assuming that one mol of l-malic acid consumed forms one mol l-lactic acid. According to this observation, the remaining lactic acid (0.58 g L−1) should find its origin in the consumption of sugars. However, in this case the early degradation of sugars is safe as no acetic acid is produced by L. plantarum from hexoses (du Toit et al. 2011).
Citric and acetic acids
The treatments with L. plantarum consumed less citric acid than ones with O. oeni and lower concentrations of acetic acid were observed (Table 1). This low acetic acid formation can be explained by the homofermentative behavior of L. plantarum in relation to hexoses. Consumption of citric acid was observed by day 7 (Fig. 2), indicating the ongoing simultaneous consumption of sugars, citric and malic acid. Furthermore, when L. plantarum was combined with yeasts no consumption of citric acid was observed, showing that the possible inhibition by yeasts over L. plantarum affected not only the malic acid metabolism, but also the consumption of citric acid. This low consumption of citric acid makes L. plantarum a safer LAB than O. oeni with regard to the acetic acid production.
Time course of citric acid in a sterile grape must inoculated with V22 (Lactobacillus plantarum), VP41 (Oenococcus oeni), EC1118 (Saccharomyces cerevisiae), EC1118/V22 (S. cerevisiae and L. plantarum) and EC1118/VP41 (S. cerevisiae and O. oeni). Time 0 represents the initial concentration of citric acid of the must. Data points represent the mean from duplicates ± SE
On the other hand, treatments involving O. oeni exhibited the highest citric acid degradation and acetic acid formation (Table 1). MLF was almost finished (<0.3 g L−1) by day 7 (Fig. 1) while no citric acid consumption was observed (Fig. 2). This outcome emphasizes the convenience of adding SO2 after malic acid consumption is finished, in order to avoid the formation of acetic acid.
Experiment B: Acidification of grape must by Lactobacillus plantarum
In order to obtain a consumption of sugars, malic acid and production of high concentrations of lactic acid, L. plantarum was inoculated alone before yeasts. The pH of the must changed from 3.9 to 3.4 after 14 days from inoculation with L. plantarum (Fig. 3a).
Time course of pH (a), acetic acid (b), malic and d,l-lactic acid (c) and glucose and fructose (d) in a must inoculated initially with V22 (Lactobacillus plantarum) and then with EC1118 (Saccharomyces cerevisiae). The arrow indicates the time of inoculation with EC1118 (S. cerevisiae). Values are means and SE
A similar inhibition effect to the previous experiment was observed over L. plantarum activity. Four days after the inoculation with yeasts, L. plantarum stopped producing lactic acid, which was followed by the consumption of sugars by yeasts (Fig. 3d). The limited production of acetic acid during the first 10 days by L. plantarum (0.13 g L−1) could be explained by the consumption of pentoses (Ribereau-Gayon et al. 2006) in combination with hexoses. However, the main increase of acetic acid took place with the beginning of the AF, ending with a very acceptable final concentration of 0.47 g L−1 (Fig. 3b).
On day 14 the maximum concentration of lactic acid was observed (8.3 g L−1) (Fig. 3c), deriving from the consumption of malic acid and sugars by L. plantarum. Estimating that 1.95 g L−1 of lactic acid originated from the consumption of malic acid (1 mol of malic acid consumed forms 1 mol of lactic acid), it was possible to approximately determine that 6.35 g L−1 of sugars were consumed by L. plantarum. Considering that 17 g of sugar produces 1 % alcohol in volume, sugar degradation corresponding to a potential reduction of 0.37 % alcohol in volume was estimated.
This method could be used not only as a biological acidification technique, but also as a potential way to reduce alcohol in wines. Dequin et al. (1999) used genetically modified strains of S. cerevisiae with the LDH gene of Lactobacillus cassei to acidify grape must, producing high concentrations of lactic acid from sugars and obtaining the same effects in grape musts as in this experiment. Nevertheless, use of genetically modified yeasts is not yet accepted in winemaking, while the use of bacteria is a viable option for this application.
This experiment was designed to test the capability of L. plantarum as a biological acidification method under sterile grape juice conditions and in small volumes of 250 ml, which is not representative of real winemaking conditions. Therefor if this method was to be up-scaled to real winemaking conditions, the must would be loaded with microorganisms, mainly yeasts that would strongly affect the activity of L. plantarum. Possible solutions to this problem should rely mostly on the partial sterilization of the must prior to inoculation with L. plantarum using the available winery equipment such as cross flow filtration, centrifugation or the addition of dymethildicarbonate (DMDC). The inoculation with high concentrations of L. plantarum may also provide more favorable conditions for the biological acidification.
Conclusions
Even though in our first experiment inoculation with L. plantarum brought the benefit of a low acetic acid production, this LAB was found to be highly sensitive to the interaction with yeasts. The outcomes of the second experiment support the possibility of using L. plantarum for acidification, providing a potential solution for musts with high pH and high sugar concentration. However, the interactions of L. plantarum with other microorganisms present in grape musts need to be investigated by conducting experiments under real conditions of winemaking. It is important to understand whether L. plantarum would be able to lower the pH before being inhibited by the interaction with the indigenous microbes, and to assure the quality of the wines obtained after the acidification with lactic acid deriving from the fermentation of sugars in the grape must.
In conclusion, it is clear that more research with L. plantarum is needed before it can be used as a starter culture for biological acidification in standard winemaking. This alternative tool should be seen as a potential solution for some recurrent problems of winemaking and, therefore, further investigated by the wine research community.
Change history
01 August 2017
An erratum to this article has been published.
References
Alexandre H, Costello PJ, Remize F, Guzzo J, Guilloux-Benatier M (2004) Saccharomyces cerevisiae-Oenococcus oeni interactions in wine: current knowledge and perspectives. Int J Food Microbiol 93:141–154
Bartowsky EJ (2005) Oenococcus oeni and malolactic fermentation—moving into the molecular arena. Aust J Grape Wine Res 11:174–187
Bisson LF (1999) Stuck and Sluggish Fermentations. Am J Enol Vitic 50:107–119
Breed R, Murray EGD, Smith NR (1957) Bergey’s manual of determinative bacteriology, 7th edn. Williams & Wilkins Co, Baltimore
Davis CR, Wibowo D, Eschenbruch R, Lee TH, Fleet GH (1985) Practical implications of malolactic fermentation: a review. Am J Enol Vitic 36:290–301
Dequin S, Baptista E, Barre P (1999) Acidification of grape musts by Saccharomyces cerevisiae wine yeast strains genetically engineered to produce lactic acid. Am J Enol Vitic 50:45–50
du Toit M, Engelbrecht L, Lerm E, Krieger-Weber S (2011) Lactobacillus: the next generation of malolactic fermentation starter cultures-an overview. Food Bioprocess Technol 4:876–906
Fumi MD, Krieger-Weber S, Déléris-Bou M, Silva A, du Toit M (2010) Una nueva generación de bacterias malolácticas para vinos con pH elevado. Enoviticultura 6:34–38
Iland P, Grbin P, Grinbergs M, Schmidtke L, Soden A (2007) Microbiological analysis of grapes and wine: techniques and concepts. (Patrick Iland Wine Promotions: Adelaide) ISBN 978-0-9581605-3-7
Jackson DI, Lombard PB (1993) Environmental and management practices affecting grape composition and wine quality—a review. Am J Enol Vitic 44:409–430
Jussier D, Dube Morneau A, Mira de Orduna R (2006) Effect of simultaneous inoculation with yeast and bacteria on fermentation kinetics and key wine parameters of cool-climate chardonnay. Appl Environ Microbiol 72:221–227
Kerem Z, Bravdo B, Shoseyov O, Tugendhaft Y (2004) Rapid liquid chromatography-ultraviolet determination of organic acids and phenolic compounds in red wine and must. J Chromatogr A 1052:211–215
Pan W, Jussier D, Terrade N, Yada RY, Mira de Orduña R (2011) Kinetics of sugars, organic acids and acetaldehyde during simultaneous yeast-bacterial fermentations of white wine at different pH values. Food Res Int 44:660–666
Passos FV, Fleming HP, Ollis DF, Felder RM, McFeeters RF (1994) Kinetics and modeling of lactic acid production by Lactobacillus plantarum. Appl Environ Microbiol 60:2627–2636
Redzepovic S, Orlic S, Majdak A, Kozina B, Volschenk H, Viljoen-Bloom M (2003) Differential malic acid degradation by selected strains of Saccharomyces during alcoholic fermentation. Int J Food Microbiol 83:49–61
Ribereau-Gayon P, Dubourdieu D, Doneche B, Lonvaud A (2006) Handbook of enology—the microbiology of wine and vinifications, vol 1, 2nd edn. Wiley\Blackwell, West Sussex\England, p 512
Salmon JM (1987) l-Malic-acid permeation in resting cells of anaerobically grown Saccharomyces cerevisiae. Biochim Biophys Acta Biomembr 901:30–34
Zotou A, Loukou Z, Karava O (2004) Method development for the determination of seven organic acids in wines by reversed-phase high performance liquid chromatography. Chromatographia 60:39–44
Acknowledgments
This work was supported by the Pontificia Universidad Catolica de Chile University.
Author information
Authors and Affiliations
Corresponding author
Additional information
An erratum to this article is available at https://doi.org/10.1007/s10482-017-0909-4.
Rights and permissions
About this article
Cite this article
Onetto, C.A., Bordeu, E. Pre-alcoholic fermentation acidification of red grape must using Lactobacillus plantarum . Antonie van Leeuwenhoek 108, 1469–1475 (2015). https://doi.org/10.1007/s10482-015-0602-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0602-4