Abstract
A novel facultatively anaerobic bacterial strain, designated LAM0504T, was isolated from a pit mud of Luzhou flavour liquor alcohol fermentation in Sichuan Province, China. Cells of strain LAM0504T were observed to be Gram-stain negative, spore-forming, rod shaped and motile by means of peritrichous flagella. Strain LAM0504T was found to be able to grow at 20–48 °C (optimum: 30 °C), pH 5.0–9.0 (optimum: 7.0) and 0–3 % NaCl (w/v) (optimum: 1.0 %). The 16S rRNA gene sequence similarity analysis showed that strain LAM0504T was most closely related to Paenibacillus konsisdensis JCM 14798T, Fontibacillus phaseoli LMG 27589T and Paenibacillus motobuensis JCM 12774T, with 97.0, 96.8 and 96.7 % sequence similarity, respectively. The DNA–DNA hybridization value between strain LAM0504T and P. konsisdensis JCM 14798T was 53.3 ± 1.2 %. The genomic DNA G+C content of strain LAM0504T was 43.0 mol% as determined by the Tm method. The major fatty acids of strain LAM0504T were identified as anteiso-C15:0, C16:0 and iso-C15:0. The cell-wall peptidoglycan was found to contain meso-diaminopimelic acid. The predominant menaquinone was identified as MK-7. The major polar lipids were found to be diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, two unidentified phospholipids, two unidentified glycolipids and three unidentified lipids. On the basis of its physiological and phylogenetic characteristics, strain LAM0504T is concluded to represent a novel species of the genus Paenibacillus, for which the name Paenibacillus vini sp. nov. is proposed. The type strain is LAM0504T (=ACCC 06420T = JCM 19842T).
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The genus Paenibacillus belongs to the family Paenibacillaceae and was proposed by Ash et al. in 1993. At the time of writing, the genus Paenibacillus comprises 159 recognized species (http://www.bacterio.net/p/paenibacillus.html) and the type species is Paenibacillus polymyxa. Paenibacillus species are widely distributed throughout the biosphere such as blood (Ko et al. 2008), garbage (Iida et al. 2005), grassy sandbank (Traiwan et al. 2011) and hot spring (Zhou et al. 2012). Members of this genus have diverse physiological characteristics. Most of them are rod-shaped, facultatively anaerobic or strictly aerobic, endospore-forming and motile bacteria. Their optimal growth temperatures range from 28 to 37 °C. The optimal pH values for growth range from 6.0 to 9.0. Their DNA G+C content ranges from 39 to 54 mol%. The predominant menaquinone is MK-7 and the major fatty acids is anteiso-C15:0 (Ash et al. 1993; Shida et al. 1997; Montes et al. 2004; Osman et al. 2006; Priest 2009).
While investigating the microbial community of an alcohol fermentation pit mud used for Luzhou flavour liquor production in Sichuan Province, China, a Paenibacillus-like strain, designated LAM0504T, was isolated. By using a polyphasic taxonomic approach, we conclude that the new isolate represents a novel species of the genus Paenibacillus, for which the name Paenibacillus vini sp. nov. is proposed.
Materials and methods
Isolation and culture of bacterial strains
Strain LAM0504T was isolated from an alcohol fermentation pit mud for Luzhou flavour liquor production in Sichuan Province, China. For the isolation, 5 g of pit mud sample was added into a 150 ml Erlenmeyer flask with 50 ml sterile saline solution and incubated in the dark at 30 °C with a shaking speed of 150 rpm. After 24 h, the samples were diluted with sterilized water and spread onto TSA medium (BD/BBL 236950, Sparks, MD, USA) plates and incubated at 30 °C for 3 days. One of the isolates obtained, designated strain LAM0504T, which was purified at least twice before preservation in 25 % (v/v) glycerol at −80 °C, was selected for further study.
Biomass for chemotaxonomic and molecular studies was obtained by cultivation in shaking flasks with TSB medium (BD/Difco 211825, Sparks, MD, USA) at 30 °C for 2 days. The recommended Minimal Standards for describing new taxa of aerobic, endospore-forming bacteria as described by Logan et al. (2009) were followed. The reference type strains Paenibacillus konsisdensis JCM 14798T and Paenibacillus motobuensis JCM 12774T were obtained from the Japan Collection of Microorganisms (JCM; Japan). Fontibacillus phaseoli LMG 27589T was obtained from the Belgian Coordinated Collections of Microorganisms (BCCM/LMG; Belgian). The reference type strains were cultured under the same conditions as strain LAM0504T for comparative analyses.
Morphological, physiological and biochemical characteristics
Cell morphological characteristics of an exponentially growing culture of strain LAM0504T were examined by light microscope (Nikon 80i, Tokyo, Japan) and transmission electron microscope (Hitachi 7500, Tokyo, Japan). Gram-staining reaction and H2S production tests were carried out according to the methods described by Smibert and Krieg (1994). Cell motility was tested in TSB medium with 0.4 % agar. Growth under anaerobic conditions was tested in TSB medium in Hungate tubes filled with oxygen-free N2 at 30 °C for 3 days. The formation of endospores was tested on TSA agar supplemented with 5 mg L−1 MnSO4 as described by Logan et al. (2009). Growth at temperatures of 4, 15, 20, 30, 37, 40, 45, 48, 50 and 55 °C was investigated in TSB medium. Growth at pH 4, 5, 6, 7, 8, 9, 10 and 11 was investigated in TSB medium. NaCl concentrations of 0, 1, 2, 3, 4 and 5 % (w/v) were produced in medium prepared according to the formula of TSB but varying the addition of NaCl. The medium was adjusted to the desired pH using the method described by Ruan et al. (2014). Catalase activity was determined by production of bubbles in 3 % (v/v) H2O2. Oxidase activity was detected by using tetramethyl-p-phenylenediamine. Methyl red and Voges–Proskauer tests were determined as described by Simbert and Krieg (1981). Hydrolysis of casein and starch were carried out on skimmed milk agar and starch agar, respectively. Egg yolk reaction was conducted on egg yolk agar. The utilisation of various substrates as the sole carbon source was conducted using Biolog GN2 MicroPlates (Biolog, Hayward, CA, USA) according to the manufacturer’s instruction. Other biochemical tests of strain LAM0504T and the three closely related reference strains, including nitrate reduction, acid production and enzyme activity, were carried out with the API 20NE, API 50CH and API ZYM systems (bioMérieux, L´Etoile, France) according to the manufacturer’s instructions.
Phylogenetic and genomic related analyses
Genomic DNA for PCR amplification was extracted and purified according to the method described by Marmur (1961). The 16S rRNA gene was amplified by PCR with the universal bacterial primers 27F and 1492R (Weisburg et al. 1991) and purified using a PCR purification kit (TianGen, Beijing) according to the manufacturer’s instructions. The purified PCR product was cloned into pMD19-T vector (TaKaRa) and sequenced by the Life Technologies Company (Shanghai, China). The 16S rRNA gene sequence similarities and multiple sequence alignment were analyzed using EzTaxon-e service (Kim et al. 2012) and CLUSTAL W software (Thompson et al. 1994). Phylogenetic trees were constructed using the MEGA 6 program package (Tamura et al. 2013) with the neighbour-joining method (Saitou and Nei 1987) and maximum-parsimony method (Fitch 1971), and evaluated by bootstrap analysis with 1000 replications as described by Felsenstein (1985).
The genomic DNA G+C content was determined by the thermal denaturation method (Marmur and Doty 1962) using a Beckman DU 800 spectrophotometer (Beckman Coulter, Brea, CA, USA). Escherichia coli K-12 was used as a reference strain. DNA–DNA hybridization between strain LAM0504T and P. konsisdensis JCM 14798T was performed according to the method described by De Ley et al. (1970) and Huss et al. (1983). The experiments were carried out in quadruplicate.
Chemotaxonomic characterization
For determination of cellular fatty acid composition, strain LAM0504T, P. konsisdensis JCM 14798T, P. motobuensis JCM 12774T and F. phaseoli LMG 27589T were incubated in TSB medium at 30 °C for 48 h. Cellular fatty acids of the four strains were analysed as described by Sakamoto et al. (2002). Their identification and quantification were carried out using the Sherlock Microbial Identification System with the standard MIS Library Generation Software (VERSION 6.0 and Date 4, Microbial ID Inc., Newark, DE, USA) and a 6890 N gas chromatograph (Agilent). The respiratory quinones were analyzed with reversed-phase HPLC as described by Komagata and Suzuki (1987). The polar lipids were extracted and separated on silica gel plates (10 × 10 cm, Merck 5554) (Kates 1986) and further analysed by using the methods described by Minnikin et al. (1984) and Xu et al. (2011). Sulfuric acid was used to reveal total polar lipids. Aminolipids were determined using ninhydrin reagent and phospholipids were identified by Zinzadze reagent. The data were interpreted as described by Fang et al. (2012). The cell wall peptidoglycan structure of strain LAM0504T was tested by TLC (Komagata and Suzuki 1987) by using the methods described by Schleifer (1985).
Results and discussion
Morphological, physiological and biochemical characteristics
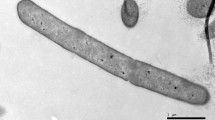
Cells of strain LAM0504T were observed to be rod shaped, with a cell size of 0.6–1.0 μm in width and 1.0–3.0 μm in length and motile by peritrichous flagella (Fig. S1). The isolate was found to be Gram-stain negative, facultatively anaerobic and spore-forming (Fig S2). Colonies of strain LAM0504T were observed to be milky, flat with regular edges after growth on TSA plates at 30 °C for 48 h. Growth was observed at 20–48 °C (optimum: 30 °C), 0–3 % (w/v) NaCl (optimum: 1.0 %) and pH 5.0–9.0 (optimum: 7.0). Strain LAM0504T was found to be catalase positive, oxidase negative and to reduce nitrate to nitrite. Cells were found to be positive for the Voges–Proskauer test, negative for the methyl red test, egg yolk reaction and H2S production. The hydrolysis of casein and starch were found to be positive. In the API 50CH system, strain LAM0504T was observed to give positive reactions for l-arabinose, d-ribose, d-xylose, d-galactose, methyl β-d-xylopyranoside, d-glucose, methyl α-d-glucoside, N-acetylglucosamine, cellobiose, amygdalin, salicin, d-lactose, melibiose, sucrose, d-raffinose and glycogen tests; negative reactions for glycerol, erythritol, d-arabinose, l-xylose, d-adonitol, d-fructose, l-rhamnose, dulcitol, inositol, d-mannitol and d-sorbitol tests; and a weak reaction with d-mannose. In the API ZYM system, activities of esterase (C4), esterase lipase (C8), leucine arylamidase, α-galactosidase, β-galactosidase, α-glucosidase and N-acetyl-β-glucosaminidase were determined to be positive; activities of α-chymotrypsin, naphthol-AS-BI-phosphohydrolase and β-glucosidase were weakly positive. In the API 20E system, the hydrolysis of O-nitrophenyl-β-d-galactopyranoside (ONPG) was found to be positive but the citrate utilization, gelatin and arginine hydrolysis tests were negative. In the API 20NE system, the hydrolysis of aesculin and urea, and the assimilation of d-glucose, l-arabinose, d-mannose, N-acetyl-glucosamine and d-maltose were found to be positive. The differences in the physiological and biochemical characteristics between strain LAM0504T and its relatives are shown in Table 1.
Molecular results
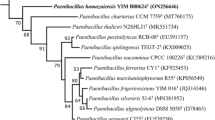
Comparative sequence analysis based on the nearly complete 16S rRNA gene sequence of strain LAM0504T (1459nt, GenBank accession number KJ005124) indicated that strain LAM0504T was most closely related to P. konsisdensis JCM 14798T, F. phaseoli LMG 27589T and P. motobuensis JCM 12774T, with 97.0, 96.8, 96.7 % sequence similarity, respectively. Although strain F. phaseoli LMG 27589T shared a higher 16S rRNA gene sequence similarity than P. motobuensis JCM 12774T, the phylogenetic analysis indicated that strain LAM0504T and F. phaseoli LMG 27589T were not in a close cluster and that strain LAM0504T presents a closer relationship with P. motobuensis JCM 12774T. Phylogenetic trees constructed with the neighbour-joining (Fig. 1) and maximum-parsimony (Fig. S3) methods showed that strain LAM0504T shares a close relationship with the members of the genus Paenibacillus. The DNA–DNA hybridization value between strain LAM0504T and P. konsisdensis JCM 14798T was 53.3 ± 1.2 %.
Neighbour-joining phylogenetic tree based on a comparison of the 16S rRNA gene sequences of strain LAM0504T and its closest relatives. Genbank accession numbers are given in parentheses. The numbers at the nodes indicate the percentages of bootstrap sampling derived from 1000 replications. Bar, 0.01 nucleotide substitution per nucleotide position
The DNA G+C content of strain LAM0504T was 43.0 mol% as determined by the Tm method, a value within the range of 39–59 mol% reported for the species of the genus Paenibacillus.
Chemotaxonomic characteristics
A comparison of the whole-cell fatty acid composition of strain LAM0504T and the reference strains is shown in Table 2. The major fatty acids of strain LAM0504T were identified as anteiso-C15:0 (43.1 %), C16:0 (15.9 %) and iso-C15:0 (10.0 %). Minor differences existed in the proportions of the major fatty acids of strain LAM0504T and the reference strains. The anteiso-C15:0 content of strain LAM0504T was notably higher than that in the reference strains (from 32.4 to 35.4 %), while the C16:0 content (15.9 %) was in the range present in the reference strains (from 14.7 to 22.8 %). The diamino acid of the cell-wall peptidoglycan was determined to be meso-diaminopimelic. The predominant menaquinone was identified as MK-7. The main polar lipids of strain LAM0504T were identified as diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, two unidentified phospholipids, two unidentified glycolipids and three unidentified lipids (Fig. S4). The main polar lipids of strain LAM0504T were found to be similar to those of members of the genus Paenibacillus. The polar lipids of strain LAM0504T show significant differences when compared with the type strain F. phaseoli LMG 27589T in the absence of aminophosphoglycolipid, aminolipid and the presence of phosphatidylethanolamine.
Taxonomic conclusion
The phenotypic properties (rod-shaped, facultatively anaerobic, endospore-forming and motile bacteria), major fatty acid (anteiso-C15:0), respiratory quinone (MK-7), major polar lipids (diphosphatidylglycerol, phosphatidylglycerol and phosphatidylethanolamine), cell-wall peptidoglycan diamino acid (meso-diaminopimelic) and G+C content (43.0 mol%) are consistent with the conclusion that strain LAM0504T should be assigned to the genus Paenibacillus. However, the new isolated strain LAM504T presents significant differences in its physiological and biochemical characteristics compared with the reference strains P. konsisdensis JCM 14798T, P. motobuensis JCM 12774T and F. phaseoli LMG 27589T. Differences between strain LAM0504T and its close relatives were observed in regards to oxidase activity, nitrate reduction, Voges–Proskauer test, acid production from carbohydrates (l-arabinose, d-ribose, d-xylose, methyl β-d-xylopyranoside, d-fructose, d-mannose, l-rhamnose, amygdalin, d-turanose and d-lyxose) and enzyme activities (alkaline phosphatase, trypsin, α-chymotrypsin, acid phosphatase, α-glucosidase and N-acetyl-β-glucosaminidase) as summarised in Table 1. The cellular fatty acid composition of strain LAM0504T was mostly similar to that of the reference strains but notable differences in the proportions of some fatty acids were observed (Table 2). Differences in the polar lipid profile were also found compared to the reference strains (Fig. S4). The 16S rRNA gene sequence of strain LAM0504T showed relatively low sequence similarities to the type strains P. konsisdensis JCM 14798T, F. phaseoli LMG 27589T and P. motobuensis JCM 12774T, with 97.0, 96.8 and 96.7 % sequence similarity, respectively. The DNA–DNA hybridization value (53.3 ± 1.2 %) clearly differentiated strain LAM0504T from P. konsisdensis JCM 14798T. Based on the results of morphological, physiological, chemotaxonomic and phylogenetic characterisation, and combined with the DNA–DNA hybridization value (53.3 ± 1.2 %), strain LAM0504T is considered to represent a novel species of the genus Paenibacillus, for which the name Paenibacillus vini sp. nov. is proposed.
Description of Paenibacillus vini sp. nov.
Paenibacillus vini (vi’ni. L. neut. gen. n. vini of wine, referring to the isolation of the type strain from pit mud of a Luzhou flavour liquor alcohol fermentationin Sichuan Province, China).
Cells are Gram-stain negative, facultatively anaerobic, spore-forming, catalase positive, oxidase negative and rod-shaped with a cell size of 0.6–1.0 μm in width and 1.0–3.0 μm in length. Endospores are spherical or ellipsoidal and lie at subterminal positions in slightly swollen sporangia. The temperature and pH ranges for growth are 20–48 °C (optimum: 30 °C) and pH 5.0–9.0 (optimum: 7.0). Does not require NaCl for growth but tolerates up to 3 % (w/v) NaCl (optimum: 1.0 %). Cells are positive for the Voges–Proskauer reaction but negative for methyl red reaction, H2S and indole production. Nitrate is reduced to nitrite. The hydrolysis of ONPG, aesculin, casein, starch and urea is positive but that of the gelatin is negative. Positive for the utilisation of the following carbon sources: α-cyclodextrin, dextrin, glycogen, N-acetyl-d-glucosamine, l-arabinose, d-cellobiose, d-galactose, gentiobiose, α-d-glucose, α-d-lactose, maltose, d-mannose, d-melibiose, β-methyl-d-glucoside, d-raffinose, sucrose, d-trehalose, turanose, pyruvic acid methyl ester, α-ketobutyric acid, inosine, uridine and thymidine but negative for utilisation of Tween 40 and 80, l-rhamnose, d-sorbitol, citric acid, malonic acid, propionic acid, ebacic acid, succinic acid, d-alanine, l-alanine, l-aspartic acid, l-glutamic acid, l-leucine and l-ornithine. The major fatty acids are anteiso-C15:0, C16:0 and iso-C15:0. The cell-wall peptidoglycan contains meso-diaminopimelic acid. The predominant menaquinone is MK-7. The main polar lipids are diphosphatidylglycerol, phosphatidylglycerol, phosphatidylethanolamine, two unidentified phospholipids, two unidentified glycolipids and three unidentified lipids. The DNA G+C content of the type strain is 43.0 mol% as determined by the Tm method.
The type strain is LAM0504T (=ACCC 06420T = JCM 19842T), which was isolated from pit mud of a Luzhou flavour liquor alcohol fermentation in Sichuan Province, China.
References
Ash C, Priest FG, Collins MD (1993) Molecular identification of rRNA group 3 bacilli (Ash, Farrow, Wallbanks and Collins) using a PCR probe test. Proposal for the creation of a new genus Paenibacillus. Antonie Van Leeuwenhoek 64:253–260
De Ley J, Cattoir H, Reynaerts A (1970) The quantitative measurement of DNA hybridization from renaturation rates. Eur J Biochem 12:133–142
Fang M-X, Zhang W-W, Zhang Y-Z, Tan H-Q, Zhang X-Q, Wu M, Zhu X-F (2012) Brassicibacter mesophilus gen. nov., sp. nov., a strictly anaerobic bacterium isolated from food industry wastewater. Int J Syst Evol Microbiol 62:3018–3023
Felsenstein J (1985) Confidence limits on phylogenies: an approach using the bootstrap. Evolution 39:783–791
Fitch WM (1971) Toward defining the course of evolution: minimum change for a specific tree topology. Syst Zool 20:406–416
Flores-Félix JD, Mulas R, Ramírez-Bahena M-H, Cuesta MJ, Rivas R, Barañas J, Mulas D, González-Andrés F, Peix A, Velázquez E (2014) Fontibacillus phaseoli sp. nov. isolated from Phaseolus vulgaris nodules. Antonie Van Leeuwenhoek 105:23–28
Huss VAR, Festl H, Schleifer KH (1983) Studies on the spectrophotometric determination of DNA hybridization from renaturation rates. Syst Appl Microbiol 4:184–192
Iida K, Ueda Y, Kawamura Y, Ezaki T, Takade A, Yoshida S, Amako K (2005) Paenibacillus motobuensis sp. nov., isolated from a composting machine utilizing soil from Motobu-town, Okinawa, Japan. Int J Syst Evol Microbiol 55:1811–1816
Kates M (1986) Techniques of lipidology, 2nd edn. Elsevier, Amsterdam
Kim OS, Cho YJ, Lee K, Yoon SH, Kim M, Na H, Park SC, Jeon YS, Lee JH, Yi H, Won S, Chun J (2012) Introducing EzTaxon-e: a prokaryotic 16S rRNA Gene sequence database with phylotypes that represent uncultured species. Int J Syst Evol Microbiol 62:716–721
Ko KS, Kim YS, Lee MY, Shin SY, Jung DS, Peck KR, Song JH (2008) Paenibacillus konsidensis sp. nov., isolated from a patient. Int J Syst Evol Microbiol 58:2164–2168
Komagata K, Suzuki K (1987) Lipid and cell-wall analysis in bacterial systematics. Methods Microbiol 19:161–207
Logan NA, Berge O, Bishop AH, Busse H-L, Vos PD, Fritze D, Heyndrickx M, Kämper P, Rabinovitch L, Salkinoja-Salonen MS, Seldin L, Ventosa A (2009) Proposed minimal standards for describing new taxa of aerobic, endospore-forming bacteria. Int J Syst Evol Microbiol 59:2114–2121
Marmur J (1961) A procedure for the isolation of deoxyribonucleic acid from microorganisms. J Mol Biol 3:208–218
Marmur J, Doty P (1962) Determination of the base composition of deoxyribonucleic acid from its thermal denaturation temperature. J Mol Biol 5:109–118
Minnikin DE, Odonnell AG, Goodfellow M, Alderson G, Athalye M, Schaal A, Parlett JH (1984) An integrated procedure for the extraction of bacterial isoprenoid quinones and polar lipids. J Microbiol Methods 2:233–241
Montes MJ, Mercade E, Bozal N, Guinea J (2004) Paenibacillus antarcticus sp. nov., a novel psychrotolerant organism from the Antarctic environment. Int J Syst Evol Microbiol 54:1521–1526
Osman S, Satomi M, Venkateswaran K (2006) Paenibacillus pasadenensis sp. nov. and Paenibacillus barengoltzii sp. nov., isolated from a spacecraft assembly facility. Int J Syst Evol Microbiol 56:1509–1514
Priest FG (2009) Genus I. Paenibacillus Ash, Priest and Collins 1994, 852VP. In: De Vos P, Garrity GM, Jones D, Krieg NR, Ludwig W, Rainey FA, Schleifer K-H, Whitman WB (eds) Bergey’s manual of systematic bacteriology, vol 3, 2nd edn. Springer, New York, pp 269–295
Ruan Z, Wang Y, Song J, Jiang S, Wang H, Li Y, Zhao B, Jiang R, Zhao B (2014) Kurthia huakuii sp. nov., isolated from biogas slurry, and emended description of the genus Kurthia. Int J Syst Evol Microbiol 64:518–521
Saitou N, Nei M (1987) The neighbor-joining method: a new method for reconstructing phylogenetic trees. Mol Biol Evol 4:406–425
Sakamoto M, Suzuki M, Umeda M, Ishikawa I, Benno Y (2002) Reclassification of bacteroides forsythus (Tanner et al. 1986) as Tannerella forsythensis corrig., gen. nov., comb. Nov. Int J Syst Evol Microbiol 52:841–849
Schleifer KH (1985) Analysis of the chemical composition and primary structure of murein. Methods Microbiol 18:123–156
Shida O, Takagi H, Kadowaki K, Nakamura LK, Komagata K (1997) Transfer of Bacillus alginolyticus, Bacillus chondroitinus, Bacillus curdlanolyticus, Bacillus glucanolyticus, Bacillus kobensis and Bacillus thiaminolyticus to the genus Paenibacillus and emended description of the genus Paenibacillus. Int J Syst Bacteriol 47:289–298
Simbert RM, Krieg NR (1981) General characterization. In: Gerhardt P, Murray RGE, Costilow RN, Nester EW, Wood WA, Krieg NR, Philips GB (eds) Manual of methods for general bacteriology. American Society for Microbiology, Washington, DC, pp 409–443
Simbert RM, Krieg NR (1994) Phenotypic characterization. In: Gerhare P, Murray RGE, Wood WA, Krieg NR (eds) Methods for general and molecular bacteriology. American Society for Microbiology, Washington, DC, pp 647–654
Tamura K, Stecher G, Peterson D, Filipski A, Kumar S (2013) MEGA6: molecular evolutionary genetics analysis version 6.0. Mol Biol Evol 30:2725–2729
Thompson JD, Higgins DG, Gibson TJ (1994) CLUSTAL W: improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res 22:4673–4680
Traiwan J, Park M-K, Kim W (2011) Paenibacillus puldeungensis sp. nov., isolated from a grassy sandbank. Int J Syst Evol Microbiol 61:670–673
Weisburg WG, Barns SM, Pelletier DA, Lane DJ (1991) 16S ribosomal DNA amplification for phylogenetic study. J Bacteriol 173:697–703
Xu X-W, Huo Y-Y, Wang C-S, Oren A, Cui H-L, Vedler E, Wu M (2011) Pelagibacterium halotolerans gen. nov., sp. nov. and Pelagibacterium luteolum sp. nov., novel members of the family Hyphomicrobiaceae. Int J Syst Evol Microbiol 61:1817–1822
Zhou Y, Gao S, Wei DQ, Yang LL, Huang X, He J, Zhang YJ, Tang SK, Li WJ (2012) Paenibacillus thermophilus sp. nov., a novel bacterium isolated from a sediment of hot spring in Fujian province, China. Antonie Van Leeuwenhoek 102:601–609
Acknowledgments
We would like to thank Professor Aharon Oren from The Hebrew University of Jerusalem for assistance with Latin in deriving the specific epithet for strain LAM0504T. We also thank Professor Min Wu from Zhejiang University for his technique assistant on the chemotaxonomic analyses. This work was supported by National Nonprofit Institute Research Grant of CAAS (No. 2014-30), Fund for Innovation team project of environmental pollution preventing and controlling technology (No. 670100656), National Key Technology R&D Program of China (Nos. 2013BAD05B04F02 and 2011BAD11B05), Foundation of the Key Laboratory of Development and Application of Rural Renewable Energy (MOA, China) (No. 2013002), and the Science Foundation of Modern Farming Group (No. MF20100518).
Author information
Authors and Affiliations
Corresponding authors
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Chen, XR., Shao, CB., Wang, YW. et al. Paenibacillus vini sp. nov., isolated from alcohol fermentation pit mud in Sichuan Province, China. Antonie van Leeuwenhoek 107, 1429–1436 (2015). https://doi.org/10.1007/s10482-015-0438-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10482-015-0438-y