Abstract
Effective antiretroviral therapy (ART) adherence strategies for HIV+ adolescents and young adults (AYA) are needed to prevent HIV-related morbidity, mortality, and onward transmission. In the Adherence Connection for Counseling, Education, and Support (ACCESS) pilot, an exploratory sequential mixed-methods design was used to develop and test a peer-led, mobile health (mHealth) cognitive behavioral ART adherence intervention. HIV+ AYA (ages 16–29 years) with unsuppressed plasma HIV RNA (HIV viral load) were eligible for this five-session intervention directed to improving ART adherence and HIV viral load. A total of 78 peer-led remote videoconferencing sessions (via WebEx) were delivered to 16 participants. High completion rates (97.5%) and client satisfaction scores (mean = 29.13 of 32; SD = 2.45) were observed. Self-reported ART adherence improved (32% increase in doses taken; 95th CI 11.2–53.3) with an annualized average rate of 47.5% (0.28 log10) reduction in HIV viral load. We established proof of concept for the ACCESS peer-led, mHealth cognitive behavioral ART adherence intervention, with promising adherence and virologic outcome data.
Resumen
Se necesitan estrategias efectivas de adherencia a la terapia antirretroviral (TAR) para adolescentes y adultos jóvenes (AAJ) VIH+ para prevenir la morbilidad, la mortalidad y las transmisiones futuras relacionadas con el VIH. En el proyecto piloto Adherence Connection for Counseling, Education, and Support (ACCESS), se utilizó un diseño exploratorio secuencial de métodos mixtos para desarrollar y testear una intervención de adherencia cognitiva conductual de salud móvil (mHealth) dirigida por pares a la TAR. AAJ VIH+ (de 16 a 29 años de edad) con ARN del VIH (carga viral del VIH) en plasma no suprimido fueron elegibles para esta intervención de cinco sesiones dirigida a mejorar la adherencia a la TAR y la carga viral del VIH. Se dictaron un total de 78 sesiones de videoconferencias remotas dirigidas por pares (a través de WebEx) a 16 participantes. Fueron observadas tasas altas de finalización (97.5%) y puntuaciones de satisfacción del cliente (media=29.13 de 32; SD=2.45). La adherencia autoinformada a la TAR mejoró (aumento del 32% en las dosis tomadas; IC del 95=11.2 a 53.3) con una tasa promedio anualizada de reducción en la carga viral del VIH del 47.5% (0.28 log 10). Establecimos una prueba de concepto para ACCESS, la intervención de adherencia a la TAR cognitivo conductual mHealth dirigida por pares, con datos prometedores sobre la adherencia y los resultados virológicos.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Ending the HIV Epidemic: Equitable Access and Everyone’s Voice were most fitting themes for World AIDS Day on December 1, 2021, given the COVID-19 pandemic and US sociopolitical context. These themes were operationalized as a call for equitable access to HIV treatment/prevention services among underserved communities with the goal of ending the HIV epidemic [1]. However this goal remains elusive as 1.1 million individuals are living with diagnosed HIV-infection (HIV+) in the US [2]. Black and Hispanic adolescents and young adults (AYA) are disproportionately represented in the US HIV health crisis [2].
Antiretroviral therapy (ART) effectively treats HIV infection and prevents HIV-related morbidity, mortality, and sexual and perinatal transmission [3, 4]. However, improved health outcomes are related to health behavior [5], as benefits of ART are directly proportional to levels of adherence [6]. Optimal adherence is defined as 80–90% for protease-inhibitor-boosted regimens and greater than 90% for non-boosted regimens [7]. Newer ART agents such as integrase strand transfer inhibitors (INSTI) are potent medications with long half-lives; as such, 80% adherence may be sufficient for INSTI-based regimens [8]. Adherence estimates of < 80% to INSTI-based regimens, coupled with a low CD4-T-lymphocyte count, increases the risk for ART resistance [8]. Resistance to non-nucleoside reverse transcriptase inhibitors (NNRTIs) is associated with long-term failure of INSTI-based first line regimens [9]. Hence, adherence thresholds predicting ART success or failure are influenced by ART treatment experience as well as the potency, tolerability, and favorable pharmacokinetic profile of newer antiretroviral drugs, particularly INSTI-based regimens. Black and Hispanic HIV+ AYA have the lowest rates of ART adherence; nearly 40% reported suboptimal adherence (< 90%) over the past week [10]. Multiple factors are associated with these low rates of ART adherence. Decreased access to care (e.g., travel difficulty), HIV discrimination, and public stigma are important structural barriers affecting ART adherence [11,12,13,14,15]. Increased knowledge and understanding of HIV disease is associated with fewer missed ART doses [16], and self-efficacy predicts both motivational readiness for ART and ART adherence levels. Further, social support predicts self-efficacy [10]. Thus, interventions to improve ART self-efficacy and strengthen social support networks can increase motivational readiness for ART [17].
Unsuppressed plasma HIV RNA (HIV viral load) due to suboptimal ART adherence is a primary contributor to the increasing number of HIV infections [18, 19], thus representing a significant barrier to the goal of ending the HIV epidemic. Among US HIV+ AYA (ages 13–24 years), only half have achieved virologic suppression (HIV viral load < 200 copies/mL) [18]. Further, Black and Hispanic HIV+ AYA are at the highest risk for virologic failure—even if they have previously achieved a period of virologic suppression [20]—further highlighting the need for tailored adherence strategies and interventions.
To date, the development of effective ART adherence strategies for Black and Hispanic HIV+ AYA is limited; the current evidence base is dominated by underpowered pilot studies, and strategies requiring interventionists with advanced degrees [21]. The Centers for Disease Control and Prevention (CDC) published a compendium of evidence-based adherence interventions for HIV+ individuals (2005–2019) [22]. Of these 18 adherence interventions, only 2 were directed to ART adherence support of Black and Hispanic HIV+ AYA, and neither met best evidence criteria due to lack of effect on HIV viral load [23, 24].
Currently, there is an emerging body of evidence to support the role of community health workers (CHWs) in HIV behavioral interventions. These individuals typically share ethnicity, language, socioeconomic status, and life experiences with the community members they serve, and have been identified by many titles, including peer health promoters or peer health educators. For the purpose of this paper, CHWs will be identified as peer health coaches (also referred to as peers). Trained peer health coaches have competently delivered behavioral interventions to HIV+ adults [25,26,27] and adolescents [28], with improved adherence outcomes [25] and sustained viral suppression [29]. To address public health disparities among underserved populations, peer health coaches have also delivered educational or behavioral interventions using mobile health technology in a culturally sensitive manner [5, 30, 31]. Recommendations for designing and implementing peer-led interventions include a clear conceptual definition and rationale for the peer role [32], as these methodological constructs are important prerequisites to examining and explaining intervention effect [33].
Mobile health (mHealth) is defined as “health care services accessed through mobile wireless technologies” and includes healthcare interventions delivered via remote videoconferencing and smartphones [34]. For Black and Hispanic HIV+ AYA, application of mHealth strategies is developmentally appropriate, offering tremendous potential to reduce HIV health disparities [11, 13,14,15, 35]. Black and Hispanic HIV+ AYA are prolific users of technology; nearly all have a smartphone, among whom half report going online more than a few times a day [36]. Mobile platforms such as videoconferencing applications show promise in overcoming structural barriers by increasing accessibility of services [30, 37] and indirectly decreasing the effect of stigma on service access [31].
Adherence Connection for Counseling, Education, and Support (ACCESS) is a proof of concept study designed to: (1) characterize feasibility and acceptability of a peer-led, mHealth cognitive behavioral intervention (CBI) delivered via remote videoconferencing using smartphones, and (2) obtain initial estimates of the biobehavioral impact of ACCESS on HIV virologic outcomes, self-reported ART adherence, beliefs and knowledge about ART treatment, adherence self-efficacy, and healthcare utilization (retention in care). Primary study outcomes included intervention feasibility, acceptability, and preliminary evidence of impact on HIV viral load. Secondary study outcomes were preliminary evidence of impact on self-reported ART adherence, beliefs and knowledge about ART treatment, adherence self-efficacy, and retention in care.
Methods
Theoretical Underpinnings
The Information-Motivation-Behavioral (IMB) Skills Model of Antiretroviral Adherence (IMB Model) [38] was used to identify the conceptual determinants of adherence behavior. We operationalized information, motivation, and behavioral skills as knowledge and beliefs about ART treatment, and adherence self-efficacy.
Study Design and Overview of Approach
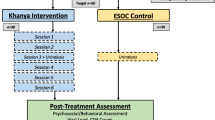
An exploratory sequential mixed-methods design was used to develop and test the ACCESS peer-led, mHealth CBI. Our research methods and study protocol, and formative qualitative findings, have been published in the Journal of Medical Internet Research Protocols [39] and Nursing Research [40], respectively. CONSORT guidelines for pilot and feasibility studies [41] were used to describe relevant methods and results. In Fig. 1, we provide an illustration of study flow and sequential qualitative and quantitative study phases, including pre- and post-intervention testing. As depicted in Fig. 1, qualitative interviews were conducted pre-intervention with 20 of the 21 enrolled participants with the primary purpose of refining the intervention. Sixteen of these 21 participants were later enrolled in the ACCESS intervention. Study procedures, including recruitment of participants, were initiated after obtaining institutional review board (IRB) approval from the three participating sites in New York City: two public hospitals and one nonprofit HIV community health plan. These sites provide care and services to individuals from diverse racial, ethnic and socioeconomic backgrounds.
Eligibility criteria for study participation were English speaking HIV+ AYA (behaviorally or perinatally infected), ages 16–29 years, with an actively prescribed ART regimen, and detectable HIV viral load of > 200 copies/mL. Neurocognitive deficits have the potential to influence participant ability to complete survey measures and or intervention components; therefore, participants were screened for neurocognitive impairments using the Folstein Mini-Mental State Exam (MMSE) [42] (those with a score of ≥ 24 were eligible to participate).
Qualitative Phase
Preliminary qualitative findings were used to inform and refine the ACCESS intervention content and implementation [40]. In brief, a qualitative inquiry was conducted to explore adherence information, motivation, and behavioral skills (IMB Model) among HIV+ AYA who met ACCESS study eligibility criteria. In this inquiry, we also explored the contextual factors of ART adherence behavior among the participating HIV+ AYA. Semi-structured, in-depth, individual interviews were completed with a convenience sample (n = 20) between December 2016 and January 2018. Directed content analysis [43] was conducted with a conceptual, and data driven (contextual) approach to coding.
As reported by our team [40], adherence information was understood in relation to HIV biomarkers (i.e., HIV viral load and CD4-T-lymphocyte counts) and conceptualized as a “war” between “good people” (i.e., CD4-T-lymphocytes) and “bad people” (i.e., HIV virus) [40]. Adherence motivation and behavioral skills were influenced by perceived stigma and social context, as it is difficult to acquire social support and self-manage a “secret” disease [40]. Relevant themes regarding ART self-management included: (1) emerging adulthood with a chronic illness, (2) stigma and disclosure concerns, (3) support systems and support deficits, (4) mental and behavioral health risks and challenges, and (5) mode of HIV transmission and perceptions of power and control. Among support systems, healthcare providers and the HIV health care context were largely described as favorably influencing ART adherence behavior [40].
ACCESS Intervention: Development and Implementation
Peer Health Coach Training For the purpose of this study, we defined a peer health coach as an individual living with HIV, taking ART, and actively engaged in HIV care (i.e., receiving primary health care services from a healthcare provider in a clinical setting) [39]. Two part-time peer health coaches were recruited from a local HIV clinic and affiliated research center, and hired with the title of Assistant Research Scientist. Requirements for this position included high school diploma, strong communication and leadership skills, knowledge and experience interfacing with HIV-related medical settings and HIV+ AYA, proficiency in use of smartphones, and basic computer skills. Peer health coaches were integral members of the ACCESS study team during its development and implementation, and were identified as key personnel on the IRB protocol upon successful completion of Collaborative Institutional Training Initiative (CITI) [44], conflict of interest, and financial disclosure documents.
Peer health coaches completed a comprehensive training program developed by the Principal Investigator (PI). The training program was a total of 40-h delivered weekly over 4 months (June 28, 2017–September 22, 2017) and led by content experts in their respective fields. Training format included didactic lectures with discussion, written practice, and active role playing using the mHealth application for synchronous videoconferencing. Didactic lectures were designed to impart knowledge of HIV-infection, treatment adherence, and stigma. Training included a discussion of the peer health coach role, professional boundaries, and ethical and human subjects considerations. Lastly, peer health coaches were trained in motivational interviewing (MI), including the stages of change model. Written practice worksheets were assigned as “homework” and reviewed in-person during didactic lectures to reinforce MI communication skills. Peer health coaches also had repeated opportunities to role play the intervention protocol, allowing for constructive feedback from members of the study team. An outline of the ACCESS peer training program is detailed in Appendix.
Intervention Refinement and Development
The ACCESS intervention was refined by integrating qualitative findings with the conceptual determinants of adherence behavior outlined in the IMB Model (i.e., adherence information, motivation, and behavioral skills) [38]. For example, in our qualitative inquiry, participants indicated that they would prefer to be introduced to the peer health coach by the PI at the start of Session 1. This introduction served to establish credibility of the peer health coach and provide the opportunity for the PI to briefly re-introduce themselves and their professional trajectory as an HIV clinician and clinical researcher. The PI also used this opportunity to re-describe the ACCESS intervention and role of the peer health coach, including boundaries for peer-participant contact outside of the five ACCESS sessions.
During qualitative interviews, the PI explored participant views of the proposed intervention delivery mode (e.g., videoconferencing), total number (e.g., five sessions) and duration (e.g., up to 60 min) of ACCESS sessions. Overall, participants agreed with the proposed intervention protocol, including number and length of sessions. One participant stated that it was important to “make someone understand that you don’t have to run the whole 60 min.” We implemented this recommendation and allowed the session length to be informed by the needs of the participant in completing the goals of the session.
We also learned through qualitative interviews that reminder devices (e.g., alarms) were not perceived as helpful for ART adherence support. As such, medication alarms and or reminder text messages were not integrated into delivery of the intervention [40]. We did, however, use phone calls to remind participants of upcoming sessions with the peer health coach, as this was the preferred method reported by participants during qualitative interviews.
Intervention Delivery
Peer health coaches delivered the ACCESS intervention to study participants between November 2017 and April 2018. For an overview of the ACCESS intervention goals and related content for each session, refer to Box 1. The peer health coaches delivered five, weekly, 60-min cognitive behavioral [45,46,47,48] and motivational [17] sessions for improved ART adherence in a private office using a WebEx equipped computer at the sponsored academic institution, while participants used their study-funded smartphone in a private location of their choosing (e.g., home). During these sessions, peer health coaches applied cognitive behavioral strategies using MI techniques to enhance problem solving, target knowledge and beliefs about ART treatment, and adherence self-efficacy, for improved adherence behavior [39].
Scheduling of ACCESS sessions was managed by the PI and a trained research assistant (RA). For example, if a participant canceled or missed a session, the RA or PI rescheduled the appointment during the same week and documented the reason for the make-up session (e.g., scheduling conflict, forgot, difficulty with technology).
Participants were compensated with a cash gift card of up to $150.00 for their time and travel: $15.00 for each ACCESS session attended ($75.00 total for five sessions), $25.00 for data collection pre- and post-intervention ($50.00 total), and $25 for the qualitative interview.
Data Collection
Clinical partners at each of the three recruitment sites provided the PI with a private room to conduct in-person data collection procedures (informed consent, qualitative interview, survey measures pre- and post-intervention). Demographics were collected at baseline (pre-intervention) using a data collection tool developed by the study PI.
Data on participant response rates to remote videoconferencing sessions (number of missed and rescheduled appointments, recruitment, and overall study retention and attrition rates) were collected throughout the intervention. Participant satisfaction was measured post-intervention using the Client Satisfaction Questionnaire (CSQ) [49]. To assess intervention acceptability, the peer health coach collected participant feedback at the end of ACCESS Session 5, regarding which of the ACCESS intervention sessions (Sessions 1–5), if any, were most helpful and why. This feedback was audio recorded during session 5 and transcribed verbatim by a trained RA. To assess peer health coach fidelity with the intervention protocol and related session content, audiotapes from ACCESS intervention sessions (Sessions 1–5) were reviewed by the RA.
Adherence outcomes included HIV viral load (primary adherence outcome) and 3-day self-reported adherence [50]. HIV viral load data was extracted from the participant’s medical record at the time of baseline/pre-intervention, immediately following intervention completion, and 8, 16 and 24-weeks post-intervention. Since the timing of these extracted HIV viral load measurements did not always correspond to the exact timepoint (e.g., 8-weeks post-intervention), the HIV viral load recorded closest to each timepoint was extracted. Time in days since consent was calculated for each HIV viral load measure and computed in the longitudinal analysis. Pre- and post-intervention 3-day self-reported adherence estimates were measured in-person by the study PI. We describe the feasibility, acceptability, and validity of measuring adherence with 3-day self-report and HIV viral load in our published study protocol [39].
Survey measures were collected pre- and post-intervention using reliable and valid scales [39]. Guided by the IMB Model [38], information, motivation, and behavioral skills were operationalized as knowledge about ART treatment (measured using HIV Treatment Knowledge Scale [51], beliefs about ART treatment (measured using Beliefs about Medication Scale (BAMS) [52], and adherence self-efficacy (measured using Adherence Self-Efficacy Scale [53], respectively. Retention in care was proposed as a secondary outcome [39], but not collected due to distinctions in patient check-in procedures across and within clinical sites, thereby limiting reliability and validity.
Data Verification
Under the supervision of the PI, the RA completed data cleaning and verification. Data files were randomly selected from five participants to confirm adherence outcomes (HIV viral load, 3-day self-reported adherence), CD4+ T-lymphocyte counts, and scores on the HIV Treatment Knowledge Scale, BAMS, Adherence Self-Efficacy Scale, and CSQ. Corrections were made to four single data entry points.
Data Analysis
Feasibility and acceptability of ACCESS (Study Aim 1) was examined using descriptive statistics to summarize participant response rates (recruitment, number of missed/rescheduled appointments, and retention/attrition). To evaluate participant satisfaction with the intervention, post-intervention CSQ scores [49] were computed using descriptive statistics. Intervention acceptability was assessed by evaluating and summarizing transcribed audio recordings of participant feedback regarding which of the ACCESS intervention sessions (Sessions 1–5), if any, were most helpful and why.
Descriptive statistics were generated for all demographic and study variables, with longitudinal data stratified by timepoint. Changes in 3-day self-reported adherence (missed and taken doses) were evaluated using paired t-tests. Longitudinal HIV viral load data (primary adherence outcome) was plotted over time to visualize individual-level trajectories prior to analysis. Average annualized change in log10 HIV viral load was estimated using a linear mixed model. Linear mixed models are extensions of traditional linear models that incorporate fixed and random effects to estimate the influence of group and subject-specific factors. These models are appropriate for data where observations are correlated, as is the case with repeated observations within individuals. The model included random effects for individual intercepts and a fixed effect for time, and restricted maximum likelihood was used for estimation.
Changes in other secondary outcomes (beliefs and knowledge about ART treatment, adherence self-efficacy) were compared pre- to post-intervention using paired t-tests. Confidence intervals and effect sizes are reported to convey precision and magnitude of effects. Analyses were conducted using R [54] and SPSS [55]. A 0.05 level of significance was used for all analyses.
Results
Twenty-one Black and Hispanic HIV+ AYA (mean age = 24.2 years) participated in the study (participant characteristics summarized in Table 1). Of the 21 participants, 20 completed qualitative interviews during the exploratory phase of the study [40] and sixteen participated in the ACCESS peer-led videoconferencing adherence intervention (see Fig. 1 for study flow and with depiction of participant completion and withdrawal).
Feasibility and Acceptability
Intervention Delivery and Participant Attendance
Between November 2017 and April 2018, 78 of the 80 remote videoconferencing sessions were delivered (97.5% completion rate) by two peer health coaches. Of the 16 participants, one completed 3 of the 5 sessions; thus, the remaining 15 participants completed all five sessions.
Peer-led sessions were scheduled weekly based on participant availability. As shown in Table 2, the median number of days between each session was 7 days (range = 2–28 days) with median duration for session 44:43 min (range = 34:59–60:54 min). The average number of times sessions were rescheduled was 3. Factors associated with missed or rescheduled appointments included participant no show, personal/health issues, conflict in peer health coach schedule, and technical issues using WebEx upon launch of the study.
Peer health coaches were successfully retained for the duration of the project, and all study funded smartphones were returned post-intervention. One smartphone was not functional when returned due to water damage.
Participant Satisfaction
All 16 participants completed the CSQ with high composite satisfaction scores (mean = 29.13 of 32; SD = 2.45).
Intervention Acceptability
Of the 16 participants, 15 provided feedback to the peer health coach at the conclusion of ACCESS Session 5. More than half of the participants (n = 8, 53.3%) reported ACCESS Session 3 was the most helpful because of the Heart to Heart Project educational video entitled “Understanding HIV Basics” [56]. This video reinforced their knowledge related to HIV adherence by illustrating the relationships between CD4 T-lymphocyte counts, HIV viral load, and the role of ART. Of the remaining 5 sessions, 3 participants (20%) reported Session 4 (adherence self-efficacy and effective self-management skills), and 2 participants (13.3%) reported Session 2 (beliefs about ART treatment), were the most helpful. More specifically, one participant reported that Session 2 helped them to be more honest and adherent, and 4 participants described the sessions overall as “helpful” and “enjoyable.”
Intervention Fidelity
Peer health coaches were 100% compliant with the core components of the protocol, thus all required ACCESS intervention content was presented for Sessions 1–5. One participant completed three of the five sessions, and we therefore did not review audiotapes of ACCESS Sessions 4 and 5 for this participant.
HIV Viral Load and Self-reported ART Adherence
An annualized average rate of 47.5% (0.28 log10) reduction in HIV viral load was observed (Fig. 2). Although this reduction was non-significant (t = − 1.40, p > 0.05), it is clinically meaningful, and corresponds to a small sized effect (Cohen d = 0.3). Pre versus post-intervention self-reported ART adherence improved with an average 32% increase in doses taken 95% CI [11.2, 53.3] and thus, a 32% decrease in missed doses 95% CI [− 53.2, − 11.2] [t = − 3.27, p < 0.05] (Fig. 3).
Knowledge, Beliefs, and Self-efficacy are summarized in Table 3. Participants showed significant improvements in HIV treatment knowledge and adherence self-efficacy scores post-intervention. In particular, improvement in self-reported adherence self-efficacy [53] was substantial, corresponding with a medium to large size effect [t = − 3.00, p < 0.05; Cohen d = 0.68]. Medium-sized effects were also seen on the Intent, and Positive Outcome Expectancy sub-scale of BAMS [52], though mean differences were not significant. While significant improvements were demonstrated in HIV treatment knowledge [51], the magnitude of the effect was small [t = − 3.29, p < 0.05; Cohen d = 0.27], possibly due to relatively high levels of knowledge at baseline (average score of HIV Treatment Knowledge Scale at baseline = 14.4 out of 21).
Discussion
We established proof-of-concept for the first known peer-led, mHealth cognitive behavioral ART adherence intervention with HIV+ AYA. ACCESS demonstrated feasibility, acceptability, and improvements in HIV viral load and 3-day self-reported ART adherence. Study findings show that mobilizing peer health coaches using WebEx videoconferencing technology is a viable method to deliver an HIV behavioral intervention targeting ART adherence [57, 58] for a population facing stigma and barriers to care [59]. In comparing pre- versus post-intervention outcomes, our virologic and adherence data are very promising. An annualized average rate of 47.5% (0.28 log10) reduction in HIV viral load was observed, and pre- versus post-intervention self-reported ART adherence improved with an average 32% increase in doses taken (95th CI 11.2–53.3). We also observed significant improvements in HIV treatment knowledge, and adherence self-efficacy.
We attribute these findings to a number of factors, including the mixed-methods study design with formative qualitative work [40]. In-depth interviews allowed for HIV+ AYA expression and integration of their voices in the design, refinement, and implementation of the ACCESS intervention. The inclusion of two adherence outcomes (HIV viral load and 3-day self-reported adherence) strengthened the study, as many larger adherence trials do not include virologic outcome data [60]. Other strategies key to our success included a robust theory based protocol with clear conceptualization of study variables, establishment of functional partnerships across three domains (academic, clinical, and community), and a comprehensive peer training program [39]. Peer health coaches were retained for the entirety of intervention and demonstrated high fidelity to the intervention protocol, most likely related to a comprehensive training program with didactic and practicum components. Peer health coaches were also integrated as core members of the study team, with a clearly defined scope of work and role, thereby supporting their professional identity and confidence. Evidence to support the importance of training and work-place assimilation for CHWs is well described [61, 62].
Of note, graduate and undergraduate nursing students completing research residencies were members of our study team. These students served in the role of actors for peer training practicums and role-playing sessions, allowing for both peers and students alike to gain experience and knowledge in intervention delivery. We highly recommend these immersion experiences as they allow students to connect and operationalize philosophical and theoretical underpinnings from the classroom to a real-world research lab, thereby preparing the next generation of scientists [63] and building capacity in the HIV behavioral sciences.
To date, HIV behavioral interventions have low completion rates, resulting in less impactful clinical and research outcomes; participant completion rates of 63% are described as high for a 3-session HIV prevention video meta-intervention with young adults [64]. Our observed high rates of intervention completion (97.5%) among HIV+ AYA study participants are thus remarkable, considering ACCESS included five sessions. Technology-enabled adherence interventions incorporating multiple strategies show the greatest promise for improving HIV care [65]. The ACCESS intervention incorporated social support and videoconferencing technology to promote its success. As such, participants were offered mobile access to individual-level social capital through peer support, facilitated by a neutral context for safe HIV self-disclosure and discussion of ART self-management [66, 67].
The ACCESS intervention was developed and implemented before the current COVID-19 pandemic, and during a time when videoconferencing platforms were not routinely used for social connection and communication. The COVID-19 pandemic underscores the importance of leveraging mobile approaches for optimal health care delivery [68]. In sharing our experience and lessons learned, we offer a blueprint for future peer-led interventions using technology directed to HIV+ AYA. Smartphone ownership is nearly universal among US AYA across race, gender, ethnic and socioeconomic backgrounds [36]. However, researchers need to acknowledge the digital health divide (gap between those with and without access to digital technology), especially among older individuals from lower socioeconomic strata and less educational attainment [69, 70].
Study participants of mHealth interventions need functional smartphones with uninterrupted internet access, and financial resources to pay for continued services. We addressed these structural barriers in the ACCESS pilot. Our study participants were provided with study funded smartphones to “bridge” this digital divide and ensure uninterrupted access to the study intervention. Upon study completion, all smartphones were returned by participants. Our success in participants returning study funded smartphones was dissimilar to the experiences of Jones et al. [71]; return of study funded phones in this trial was limited due to damage, theft, and attrition.
The strengths of using a videoconferencing platform for peer-participant connection helped minimize structural barriers by increasing accessibility of services [30, 37] and indirectly decreasing the effect of stigma on service access [31]. By reducing the structural barriers of travel and stigma, technology-enabled interventions hold promise in reducing health disparities associated with access to services [14]. Yet, despite this promise, evidence from a recent integrative review showed that only nine technology-enabled ART adherence interventions were conducted in the US during the past decade with HIV+ AYA; most of which were small pilots, and only three randomized controlled trials (RCTs) [72]. Four of these nine interventions used SMS text messaging, with initial evidence of efficacy shown in two studies [72]. Similarly, texting is commonly implemented for ART adherence support in Low and Middle-Income Countries (LMIC) (i.e., Sub-Saharan Africa) [73, 74]. Multi-level interventions combining individual- and community-level targets are most likely to improve adherence in LMIC [74]. One such intervention for HIV+ AYA in Western Kenya, demonstrated feasibility and acceptability; participants were provided with study funded smartphones preprogramed with the WhatsApp mobile platform to connect with peer support and counseling [75]. The scalability and sustainability of technology-enabled interventions for people living with HIV in LMIC will require training of health workers, infrastructure development, and evaluation of cost [76].
Approaches incorporating health behavior theories are strongly recommended for HIV mobile interventions, and yet not universal in practice [77]. Evidence from a systematic review of mHealth behavioral interventions with underserved populations shows similar findings with less than half of included studies integrating theoretical constructs [78]. A key strength of the ACCESS intervention is the application of the Information-Motivation-Behavioral Skills Model [38] to elucidate the mechanisms of adherence behavior. Information (knowledge about ART treatment) and behavioral skills (adherence self-efficacy) were identified as needs of participating HIV+ AYA, and were responsive to the intervention. Yet when applied to our qualitative inquiry [40], key constructs of this model did not fully explain the essence of adherence behavior, thus providing support for the need for more comprehensive models that incorporate multi-level contextual factors.
Retention in HIV care was proposed as a secondary outcome for analysis [39] by calculating the proportion of kept to scheduled HIV care visits (range 0–100%); the denominator excluded canceled visits [79, 80]. Retention data were to be extracted from the medical record of study participants; however, this was not feasible due to heterogeneity in documentation of clinical encounters. Our challenges are not unique given evidence from a systematic review of interventions designed to improve HIV linkage, retention/re-engagement in care, and adherence to ART [81]. Of the studies that examined retention in HIV care, outcome measures were highly diverse and characterized by four categories: changes in clinic visits, laboratory tests, ART use, and HIV viral load suppression. Therefore, there is a need for consistent health care utilization metrics and definitions across interventions as part of the larger goal to end the HIV epidemic [81, 82].
Limitations
In addition to a non-randomized and underpowered study design, the sample population in this pilot included participants recruited from a discrete, urban geographic location, and may not be generalizable to other regions of the U.S. Our sample population is primarily represented by HIV+ AYA with perinatal HIV transmission. However, there is evidence to show similarity in adherence outcomes between perinatally and behaviorally infected AYA [10]. Since the ACCESS intervention was a proof-of-concept study, pilot testing was conducted in one language and exclusive to English speaking participants. Self-reported ART adherence estimates were subject to the potential for social desirability bias, but inclusion of HIV biomarkers (i.e., HIV viral load) strengthened rigor, reliability, and validity. Five HIV+ AYA completed the exploratory phase (qualitative interview), but did not later participate in the ACCESS intervention. This finding is most likely explained by the extended time interval between the first qualitative interview and implementation of the ACCESS intervention. Therefore, researchers using mixed-methods exploratory designs need to carefully consider the overall timeline for completion of the exploratory qualitative phase, which is influenced by recruitment rates and data saturation.
Other considerations for this exploratory sequential mixed-methods design include HIV+ AYA participation in both the formative qualitative work and intervention. This decision was pragmatic, given the inherent challenges of recruiting and engaging the HIV+ AYA population in research. However, limitations of this approach are related to the potential effects of qualitative participation on variables of interest prior to initiating the ACCESS intervention. In terms of sampling and generalizability, another consideration is that participants who are willing to engage in both qualitative and intervention research components may not be representative of the broader HIV+ AYA population for whom the intervention is designed.
Our peer training paradigm was successful as evidenced by retention of the two peer health coaches and 100% fidelity to the intervention protocol. We do recognize the limitations of this peer training paradigm, including the time and personnel resources needed. For example, the ACCESS study PI and other team members were physically on-site and available for discussion of participant sessions, pre and post intervention delivery. This approach may not be feasible in some resource limited contexts, and feasibility in the clinical setting remains to be determined.
Conclusion and Next Steps
We developed ACCESS for Black and Hispanic HIV+ AYA in response to critical methodological challenges with conventional approaches [72, 83] and persistent health disparities leading to poor HIV virologic control [84, 85]. Improving ART adherence and HIV viral load suppression for Black and Hispanic HIV+ AYA is an urgent public health matter, particularly in the face of the COVID-19 pandemic. Given evidence from our qualitative inquiry [40] and other recent studies showing influence of contextual factors (HIV stigma, psychological distress and related substance use) on ART adherence, there is a pressing need for multi-level frameworks, expanded intervention content, and novel strategies to address these challenges.
In resource limited countries, there is greater availability of CHWs for HIV care, and research is being directed to developing, testing, and establishing community based models for support of adherence and retention in care [86]. However, recommendations to optimize the CHW role for HIV support in LMICs calls for improved training and supervision, better care coordination, integration of patient centered mHealth approaches, and increased funding [87, 88]. In the US, the CHW role in HIV care and research continues to evolve, and best practices for training, retaining and integrating CHWs into clinical and research teams remain to be determined. In fact, the evidence base is sparse with regard to efficacy data and clinical/virologic outcomes of peer-led HIV interventions [27, 33]. Hence, findings from the ACCESS intervention provide important information on the preliminary efficacy of a peer-led intervention on HIV biomarkers (HIV viral load), self-reported adherence, knowledge and beliefs about ART treatment, and adherence self-efficacy. Moreover, findings from the ACCESS intervention provide support for a comprehensive training protocol and the integration of peer health coaches as core members of the study team.
Research aimed to better understand moderating effects of peer-led interventions and mediators of the processes by which peer-led interventions facilitate HIV-related outcomes is also needed [33]. We recognize the necessity for transparency in the level and type of training offered to CHWs; this description is essential to understand their impact on HIV study outcomes. Moving forward, standardized training protocols delineating core requisites of peer-led behavioral interventions are essential. Additionally, based on our experience with undergraduate and graduate students in the ACCESS research lab, we recommend these immersion experiences as part of the larger goal of building student competencies and workforce capacity in evidence-based practice and research [89].
In conclusion, we established proof of concept for the first known peer-led, mHealth, cognitive behavioral ART adherence intervention, and showed promising virologic and adherence outcome data. Our multicomponent approach (i.e., technology and peer health coaches) is highly promising for HIV behavioral science, with potential broad effect on clinical treatment and prevention. Additionally, our success with implementation of synchronous videoconferencing for intervention delivery to HIV+ AYA lends itself to application by trained research staff for related study procedures (i.e., electronic consent, qualitative and quantitative data collection). Yet the sustainability and scalability of technology-enabled ART adherence interventions for HIV+ AYA will require economic evaluation of cost-effectiveness [90], clinical trials of efficacy, increased collaboration, and information sharing among HIV behavioral scientists [14].
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Code Availability
Not applicable.
References
Centers for Disease Control and Prevention. HIV prevention in the United States: mobilizing to end the epidemic [Internet]. 2021. Available from: https://www.cdc.gov/hiv/policies/strategic-priorities/mobilizing/funding-communities.html.
Centers for Disease Control and Prevention. HIV surveillance report, 2019 [Internet]. 2021 May. Report No.: vol 32. Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
Eisinger RW, Dieffenbach CW, Fauci AS. HIV viral load and transmissibility of HIV infection: undetectable equals untransmittable. J Am Med Assoc. 2019;321:451–2.
Gross IM, Hosek S, Richards MH, Fernandez MI. Predictors and profiles of antiretroviral therapy adherence among African American adolescents and young adult males living with HIV. AIDS Patient Care STDs. 2016;30(7):324–38.
Coates TJ. An expanded behavioral paradigm for prevention and treatment of HIV-1 infection. J Acquir Immune Defic Syndr. 2013;63 Suppl 2(0 2):S179-82.
Boussari O, Subtil F, Genolini C, Bastard M, Iwaz J, Fonton N, et al. Impact of variability in adherence to HIV antiretroviral therapy on the immunovirological response and mortality. BMC Med Res Methodol. 2015;15:10.
Gordon LL, Gharibian D, Chong K, Chun H. Comparison of HIV virologic failure rates between patients with variable adherence to three antiretroviral regimen types. AIDS Patient Care STDs. 2015;29:384–8.
Lepik KJ, Harrigan PR, Yip B, Wang L, Robbins MA, Zhang WW, et al. Emergent drug resistance with integrase strand transfer inhibitor-based regimens. AIDS Lond Engl. 2017;31(10):1425–34.
Siedner MJ, Moorhouse MA, Simmons B, de Oliveira T, Lessells R, Giandhari J, et al. Reduced efficacy of HIV-1 integrase inhibitors in patients with drug resistance mutations in reverse transcriptase. Nat Commun. 2020;11(1):5922.
MacDonell KK, Jacques-Tiura AJ, Naar S, Fernandez MI, ATN 086/106 Protocol Team. Predictors of self-reported adherence to antiretroviral medication in a multisite study of ethnic and racial minority HIV-positive youth. J Pediatr Psychol. 2016;41:419–28.
Kaufman MR, Cornish F, Zimmerman RS, Johnson BT. Health behavior change models for HIV prevention and AIDS care: practical recommendations for a multi-level approach. J Acquir Immune Defic Syndr. 2014;66 Suppl 3(Suppl 3):S250-8.
Philbin MM, Tanner AE, Chambers BD, Ma A, Ware S, Lee S, et al. Transitioning HIV-infected adolescents to adult care at 14 clinics across the United States: using adolescent and adult providers’ insights to create multi-level solutions to address transition barriers. AIDS Care. 2017;29:1227–34.
Philbin MM, Tanner AE, DuVal A, Ellen J, Kapogiannis B, Fortenberry JD. Linking HIV-positive adolescents to care in 15 different clinics across the United States: creating solutions to address structural barriers for linkage to care. AIDS Care. 2014;26(1):12–9.
Mulawa MI, LeGrand S, Hightow-Weidman LB. eHealth to enhance treatment adherence among youth living with HIV. Curr HIV/AIDS Rep. 2018;15(4):336–49.
Sweeney SM, Vanable PA. The association of HIV-related stigma to HIV medication adherence: a systematic review and synthesis of the literature. AIDS Behav. 2016;20(1):29–50.
Chenneville T, Clutter MO, Hintz S, Walsh A, Emmanuel P, Lujan-Zilberman J, et al. Decisional capacity and medication adherence among youth with HIV. AIDS Care. 2015;27(3):338–41.
Dinaj-Koci V, Wang B, Naar-King S, MacDonell KK. Adolescent Medicine Trials Network for HIVAI. A multi-site study of social cognitive factors related to adherence among youth living with HIV in the new era of antiretroviral medication. J Pediatr Psychol. 2019;44(1):98–109.
Centers for Disease Control and Prevention. Monitoring selected national HIV prevention and care objectives by using HIV surveillance data—United States and 6 dependent areas, 2017. [Internet]. Report No.: HIV Surveillance Supplemental Report 2019; 24(No. 3). Available from: http://www.cdc.gov/hiv/library/reports/hiv-surveillance.html.
Ellen JM, Kapogiannis B, Fortenberry JD, Xu J, Willard N, Duval A, et al. HIV viral load levels and CD4+ cell counts of youth in 14 cities. AIDS Lond Engl. 2014;28(8):1213–9.
Wood SM, Lowenthal E, Lee S, Ratcliffe SJ, Dowshen N. Longitudinal viral suppression among a cohort of adolescents and young adults with behaviorally acquired human immunodeficiency virus. AIDS Patient Care STDs. 2017;31:377–83.
Shaw S, Amico KR. Antiretroviral therapy adherence enhancing interventions for adolescents and young adults 13–24 years of age: a review of the evidence base. J Acquir Immune Defic Syndr. 2016;72(4):387–99.
HIV Prevention Research Synthesis Project. Compendium of evidence-based interventions and best practices for HIV prevention. Centers for Disease Control and Prevention; 2022. Available from: https://www.cdc.gov/hiv/research/interventionresearch/compendium/index.html.
Bouris A, Jaffe K, Eavou R, Liao C, Kuhns L, Voisin D, et al. Project nGage: results of a randomized controlled trial of a dyadic network support intervention to retain young Black men who have sex with men in HIV care. AIDS Behav. 2017;21(12):3618–29.
Garofalo R, Kuhns LM, Hotton A, Johnson A, Muldoon A, Rice D. A randomized controlled trial of personalized text message reminders to promote medication adherence among HIV-positive adolescents and young adults. AIDS Behav. 2016;20(5):1049–59.
Enriquez M, Cheng AL, Banderas J, Farnan R, Chertoff K, Hayes D, et al. A peer-led HIV medication adherence intervention targeting adults linked to medical care but without a suppressed viral load. J Int Assoc Provid AIDS Care. 2015;14(5):441–8.
Enriquez M, Cheng AL, McKinsey D, Farnan R, Ortego G, Hayes D, et al. Peers keep it real: re-engaging adults in HIV care. J Int Assoc Provid AIDS Care. 2019;18:2325958219838858.
Cabral HJ, Davis-Plourde K, Sarango M, Fox J, Palmisano J, Rajabiun S. Peer support and the HIV continuum of care: results from a multi-site randomized clinical trial in three urban clinics in the United States. AIDS Behav. 2018. https://doi.org/10.1007/s10461-017-1999-8.
Naar-King S, Outlaw A, Green-Jones M, Wright K, Parsons JT. Motivational interviewing by peer outreach workers: a pilot randomized clinical trial to retain adolescents and young adults in HIV care. AIDS Care. 2009;21(7):868–73.
Cunningham WE, Weiss RE, Nakazono T, Malek MA, Shoptaw SJ, Ettner SL, et al. Effectiveness of a peer navigation intervention to sustain viral suppression among HIV-positive men and transgender women released from jail: the LINK LA randomized clinical trial. JAMA Intern Med. 2018;178(4):542–53.
Davidson TM, Ruggiero KJ, Egede LE. Promoting reach, dissemination, and engagement of technologies for addressing mental health care disparities among underserved populations. Clin Psychol Sci Pract. 2019;26(1): e12273.
Ralston AL, Andrews AR III, Hope DA. Fulfilling the promise of mental health technology to reduce public health disparities: review and research agenda. Clin Psychol Sci Pract. 2019;26(1): e12277.
Simoni JM, Franks JC, Lehavot K, Yard SS. Peer interventions to promote health: conceptual considerations. Am J Orthopsychiatry. 2011;81(3):351–9.
Simoni JM, Nelson KM, Franks JC, Yard SS, Lehavot K. Are peer interventions for HIV efficacious? A systematic review. AIDS Behav. 2011;15(8):1589–95.
Kvedar J, Coye MJ, Everett W. Connected health: a review of technologies and strategies to improve patient care with telemedicine and telehealth. Health Aff (Millwood). 2014;33(2):194–9.
Philbin MM, Tanner AE, Chambers BD, Ma A, Ware S, Lee S, et al. Transitioning HIV-infected adolescents to adult care at 14 clinics across the United States: using adolescent and adult providers’ insights to create multi-level solutions to address transition barriers. AIDS Care. 2017;29(10):1227–34.
Lenhart A. Teens, social media and technology overview. Washington, D.C.: Pew Internet Research Center; 2015.
Saberi P, Dawson Rose C, Wootton AR, Ming K, Legnitto D, Jeske M, et al. Use of technology for delivery of mental health and substance use services to youth living with HIV: a mixed-methods perspective. AIDS Care. 2019. https://doi.org/10.1080/09540121.2019.1622637.
Fisher JD, Fisher WA, Amico KR, Harman JJ. An information-motivation-behavioral skills model of adherence to antiretroviral therapy. Health Psychol. 2006;25:462–73.
Dunn Navarra AM, Viorst Gwadz M, Bakken S, Whittemore R, Cleland CM, D’Eramo MG. Adherence connection for counseling, education, and support: research protocol for a proof-of-concept study. JMIR Res Protoc. 2019;8: e12543.
Dunn Navarra AM, Whittemore R, Bakken S, Rosenberg MJ, Gormley M, Bethea J, et al. Adherence self-management and the influence of contextual factors among emerging adults with human immunodeficiency virus. Nurs Res. 2020;69(3):197–209.
Eldridge SM, Chan CL, Campbell MJ, Bond CM, Hopewell S, Thabane L, et al. CONSORT 2010 statement: extension to randomised pilot and feasibility trials. BMJ. 2016;355: i5239.
Folstein MF, Folstein SE, McHugh PR. “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–98.
Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15:1277–88.
The CITI Program. Courses [Internet]. Available from: https://about.citiprogram.org/courses/.
Hansen DJ, Zamboanga BL, Sedlar G. Cognitive-behavior therapy for ethnic minority adolescents: broadening our perspectives. Cogn Behav Pract. 2000;7(1):54–60.
Kennard B, Brown L, Hawkins L, Risi A, Radcliffe J, Emslie G, et al. Development and implementation of health and wellness CBT for individuals with depression and HIV. Cogn Behav Pract. 2014;21(2):237–46.
Olem D, Sharp KM, Taylor JM, Johnson MO. Overcoming barriers to HIV treatment adherence: a brief cognitive behavioral intervention for HIV-positive adults on antiretroviral treatment. Cogn Behav Pract. 2014;21(2):206–23.
Pantalone DW. Introduction: using evidence-based cognitive and behavioral principles to improve HIV-related psychosocial interventions. Cogn Behav Pract. 2014;21(2):145–8.
Larsen DL, Attkisson CC, Hargreaves WA, Nguyen TD. Assessment of client/patient satisfaction: development of a general scale. Eval Program Plan. 1979;2(3):197–207.
Garvie PA, Wilkins ML, Young JC. Medication adherence in adolescents with behaviorally-acquired HIV: evidence for using a multimethod assessment protocol. J Adolesc Health. 2010;47(5):504–11.
Balfour L, Kowal J, Tasca GA, Cooper CL, Angel JB, Macpherson PA, et al. Development and psychometric validation of the HIV treatment knowledge scale. AIDS Care. 2007;19(9):1141–8.
Riekert KA, Drotar D. The beliefs about medication scale: development, reliability, and validity. J Clin Psychol Med Settings. 2002;9(2):177–84.
Johnson MO, Neilands TB, Dilworth SE, Morin SF, Remien RH, Chesney MA. The role of self-efficacy in HIV treatment adherence: validation of the HIV treatment adherence self-efficacy scale (HIV-ASES). J Behav Med. 2007;30(5):359–70.
R Core Team. R: a language and environment for statistical computing. Vienna: R Foundation for Statistical Computing; 2019. Available from: https://www.R-project.org/.
IBM Corp. SPSS statistics for windows, version 24.0. Armonk: IBM Corp.; 2016.
Understanding HIV: basics [Internet]. [cited 2021 Jan 22]. Available from: https://vimeo.com/208140568.
Pellowski JA, Kalichman SC. Recent advances (2011–2012) in technology-delivered interventions for people living with HIV. Curr HIV/AIDS Rep. 2012;9(4):326–34.
Belzer ME, Naar-King S, Olson J, Sarr M, Thornton S, Kahana SY, et al. The use of cell phone support for non-adherent HIV-infected youth and young adults: an initial randomized and controlled intervention trial. AIDS Behav. 2014;18(4):686–96.
Kenya S, Jones J, Arheart K, Kobetz E, Chida N, Baer S, et al. Using community health workers to improve clinical outcomes among people living with HIV: a randomized controlled trial. AIDS Behav. 2013;17(9):2927–34.
Shah R, Watson J, Free C. A systematic review and meta-analysis in the effectiveness of mobile phone interventions used to improve adherence to antiretroviral therapy in HIV infection. BMC Public Health. 2019;19(1):915.
Rajabiun S, Baughman A, Sullivan M, Poteet B, Downes A, Davich JAW, et al. A participatory curricula for community health workers and supervisors to increase HIV health outcomes. Front Public Health. 2021;9: 689798.
Lee LK, Ruano E, Fernández P, Ortega S, Lucas C, Joachim-Célestin M. Workforce readiness training: a comprehensive training model that equips community health workers to work at the top of their practice and profession. Front Public Health. 2021;9: 673208.
Morris NS, Wassef ME, Sullivan-Bolyai S, Bova C, Kane AT. Making explicit the development of PhD-prepared nurses to steward the discipline. Nurs Outlook. 2021;69(1):50–6.
Albarracín D, Wilson K, Durantini MR, Sunderrajan A, Livingood W. A meta-intervention to increase completion of an HIV-prevention intervention: results from a randomized controlled trial in the state of Florida. J Consult Clin Psychol. 2016;84(12):1052–65.
Quintana Y, Gonzalez Martorell EA, Fahy D, Safran C. A systematic review on promoting adherence to antiretroviral therapy in HIV-infected patients using mobile phone technology. Appl Clin Inform. 2018;9(2):450–66.
Kalichman SC, Banas E, Katner H, Hill M, Kalichman MO. Individual social capital and the HIV continuum of care in a rural setting of the southeast United States. J Rural Ment Health. 2020;44(2):75–86.
Chapman Lambert C, Tarver WL, Musoke PL, Stringer KL, Whitfield S, Turan B, et al. Complexities of HIV disclosure in patients newly entering HIV Care: a qualitative analysis. J Assoc Nurses AIDS Care. 2020;31(2):208–18.
Badawy SM, Radovic A. Digital approaches to remote pediatric health care delivery during the COVID-19 pandemic: existing evidence and a call for further research. JMIR Pediatr Parent. 2020;3(1): e20049.
Pew Research Center: Internet, Science & Tech. 53% of Americans say the internet has been essential during the COVID-19 outbreak [Internet]. 2020 [cited 2021 Feb 16]. Available from: https://www.pewresearch.org/internet/2020/04/30/53-of-americans-say-the-internet-has-been-essential-during-the-covid-19-outbreak/.
Campbell BR, Ingersoll KS, Flickinger TE, Dillingham R. Bridging the digital health divide: toward equitable global access to mobile health interventions for people living with HIV. Expert Rev Anti Infect Ther. 2019;17(3):141–4.
Jones R, Lacroix LJ. Streaming weekly soap opera video episodes to smartphones in a randomized controlled trial to reduce HIV risk in young urban African American/black women. AIDS Behav. 2012;16(5):1341–58.
Navarra AM, Gwadz MV, Whittemore R, Bakken SR, Cleland CM, Burleson W, et al. Health technology-enabled interventions for adherence support and retention in care among US HIV-infected adolescents and young adults: an integrative review. AIDS Behav. 2017;21:3154–71.
Ødegård ES, Langbråten LS, Lundh A, Linde DS. Two-way text message interventions and healthcare outcomes in Africa: systematic review of randomized trials with meta-analyses on appointment attendance and medicine adherence. PLoS ONE. 2022;17(4): e0266717.
Pugh LE, Roberts JS, Viswasam N, Hahn E, Ryan S, Turpin G, et al. Systematic review of interventions aimed at improving HIV adherence to care in low- and middle-income countries in Sub-Saharan Africa. J Infect Public Health. 2022;15(10):1053–60.
Chory A, Callen G, Nyandiko W, Njoroge T, Ashimosi C, Aluoch J, et al. A pilot study of a mobile intervention to support mental health and adherence among adolescents living with HIV in western Kenya. AIDS Behav. 2022;26(1):232–42.
Phan JM, Kim S, Linh ĐTT, Cosimi LA, Pollack TM. Telehealth interventions for HIV in low- and middle-income countries. Curr HIV/AIDS Rep. 2022. https://doi.org/10.1007/s11904-022-00630-0.
Simoni JM, Ronen K, Aunon FM. Health behavior theory to enhance eHealth intervention research in HIV: rationale and review. Curr HIV/AIDS Rep. 2018;15(6):423–30.
Anderson-Lewis C, Darville G, Mercado RE, Howell S, Di Maggio S. mHealth technology use and implications in historically underserved and minority populations in the United States: systematic literature review. JMIR mHealth uHealth. 2018;6(6): e128.
Mugavero MJ. Improving engagement in HIV care: what can we do? Top HIV Med Publ Int AIDS Soc USA. 2008;16(5):156–61.
Mugavero M, Westfall A, Zinski A, Davila J, Drainoni ML, Gardner L, et al. Measuring retention in HIV care: the elusive gold standard. J Acquir Immune Defic Syndr. 2012;61(5):574–80.
Risher KA, Kapoor S, Daramola AM, Paz-Bailey G, Skarbinski J, Doyle K, et al. Challenges in the evaluation of interventions to improve engagement along the HIV care continuum in the United States: a systematic review. AIDS Behav. 2017;21(7):2101–23.
Blanco N, Lavoie MCC, Koech E, Riedel DJ, Ngeno C, Adebajo S, et al. Re-engagement into HIV Care: a systematic review. AIDS Behav. 2021. https://doi.org/10.1007/s10461-021-03365-y.
Wu YP, Hommel KA. Using technology to assess and promote adherence to medical regimens in pediatric chronic illness. J Pediatr. 2014;164(4):922–7.
Lally MA, van den Berg JJ, Westfall AO, Rudy BJ, Hosek SG, Fortenberry JD, et al. HIV continuum of care for youth in the United States. J Acquir Immune Defic Syndr. 2018;77(1):110–7.
Wong K, Zucker J, Fernandes H, Cennimo D. Adolescent HIV viral load in an urban hospital in Newark, New Jersey. Int J Pediatr Adolesc Med. 2016;3(3):103–8.
Limbada M, Bwalya C, Macleod D, Floyd S, Schaap A, Situmbeko V, et al. A comparison of different community models of antiretroviral therapy delivery with the standard of care among stable HIV+ patients: rationale and design of a non-inferiority cluster randomized trial, nested in the HPTN 071 (PopART) study. Trials. 2021;22(1):52.
Ngcobo S, Scheepers S, Mbatha N, Grobler E, Rossouw T. Roles, barriers, and recommendations for community health workers providing community-based HIV care in Sub-Saharan Africa: a review. AIDS Patient Care STDs. 2022;36(4):130–44.
Khumalo GE, Lutge EE, Naidoo P, Mashamba-Thompson TP. Barriers and facilitators of rendering HIV services by community health workers in sub-Saharan Africa: a meta-synthesis. Fam Med Community Health. 2021;9(4): e000958.
Meeks S, Getz BR, Hess LS, Kostiwa IM, Ludwin BM, Rodgers JR, et al. The BE-ACTIV Project: how research, professional training, education, and practice were integrated in a single clinical trial. Gerontol Geriatr Educ. 2015;36(3):318–29.
Badawy SM, Kuhns LM. Economic evaluation of text-messaging and smartphone-based interventions to improve medication adherence in adolescents with chronic health conditions: a systematic review. JMIR mHealth uHealth. 2016;4(4): e121.
Acknowledgements
We acknowledge the time and contribution of the participants, peer health coaches, Jacobi Research Office Staff, namely Walter Vasquez and Aline Baday, David Resto and the NYU Meyers information technology team, Eva Liang, John Bethea, Laura Ridge, Sima Toussi, Jerry Ernst, Gail Shust, Adita Kaul, and Susan Abramowitz for their support of this project.
Funding
The Adherence Connection Counseling, Education, and Support (ACCESS) study was funded by the National Institute of Nursing Research (K23NR015970; R01NR019535 PI: Dunn Navarra).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by A-MDN, MGR, MG, JF, and CC. The first draft of the manuscript was written by A-MDN and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflict of interest to report.
Ethical Approval
All study procedures were approved by the New York University School of Medicine Institutional Review Board (IRB) in accordance with the US Federal Policy for the Protection of Human Subjects, including the 1964 Helsinki declaration and its later amendments.
Consent to Participate
All participants provided written informed consent.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix: Training Protocol for ACCESS Peer Health Coaches
Appendix: Training Protocol for ACCESS Peer Health Coaches
Dates | PHASE I: Didactics—Instructive Lectures & Interactive Discussions |
|---|---|
6/28/2017 (2 h) | Week 1: ACCESS Research Study Overview ∙ Study overview—presented by principal investigator ∙ Ethical considerations (Health Insurance Portability and Accountability Act (HIPAA), NYS AIDS Law) ∙ WebEx lecture—presented by information technology specialist |
7/5/2017 (2 h) | Week 2: HIV Bootcamp ∙ Basic HIV epidemiology ∙ Adherence to HIV medications |
7/12/2017 (2 h) | Week 3: Peer Mentoring Role in the ACCESS Study ∙ Research ethics and compliance ∙ Introduction to Collaborative Institutional Training Initiative (CITI) ∙ Peer mentoring and the ACCESS Study ∙ Working on a research team ∙ Communication skills & techniques ∙ Assessment of harm and related reporting procedures ∙ Cultural competency; Stigma and HIV ∙ Stages of change ∙ Introduction to open questions, affirmation, reflective listening, and summarizing (OARS) ∙ Homework—complete Institutional Review Board (IRB) CITI requirementsa |
Dates | PHASE II: Motivational Interviewing (MI) Didactics, Written Practice, & Role Playing |
|---|---|
7/19/2017 (2.5 h) | Week 4: Motivational Learning Guest speaker: Kathleen Sciacca, M.A ∙ Motivational Interviewing (MI)—history, past and present techniques, definitions ∙ Handouts—glossary of terms, MI Resource List, OARS, Change Talk ∙ Homework—responding to open questions with affirmation and reflection |
7/26/2017 (3 h) | Week 5: Motivational Learning Guest Speaker: Kathleen Sciacca, M.A ∙ Motivational Interviewing (Therapists’ Pitfalls in Traditional counseling) ∙ Four elements & strategies of Motivational Interviewing ∙ OARS—core skills ∙ Reflective listening ∙ Body & Soul MI videoc (20-min presentation) ∙ EARS (elaboration, affirming, reflecting, summarizing) ∙ Change-talk exercises based on Body & Soul MI video |
8/2/2017 & 8/9/2017 (2 h) | Self-Directed Learning Activities—No In-Person Meeting/Training ∙ Peer counselor guide (read—adapted from National Cancer Institute)b ∙ Homework—responding to change talk—practice exercises |
8/16/2017 (2 h) | Week 6: Peer Health Coach Practice Exercises ∙ Review and discuss homework ∙ OARS model—essential communication skills |
8/23/2017 (2 h) | Week 7: Peer Health Coach OARS/EARS Exercises ∙ Review Team Members’ Fall 2017 Schedule ∙ Role play—peer health coaches—practice with each other ∙ Video clip exercises—values & motivationc (30 min) ∙ Role of the peer |
Weeks 8 & 9 Transition to Integration and Application of MI Skills with ACCESS Protocol | |
8/30/2017 (2 h) | Week 8: Role Play ∙ Review of last week (refresher) ∙ Review informed consent ∙ Review homework—practice exercise using OARS & EARS ∙ How to manage different questions coming from peers? ∙ Role play—(Peer Health Coaches—practice with each other for 60 min) |
9/8/2017 (2 h) | Week 9: Role Play; Review Protocol ∙ Review project timeline ∙ Warm-up review—OARS exercises ∙ Role play—intervention session 1 (60 min) |
Dates | PHASE III: ACCESS Protocol Practice: Live Role Play using Student Actors & WebEx |
|---|---|
9/13/2017 (2 h) | Week 10: Practice/Role Play Session 1 ∙ WebEx role play—intervention session 1 (60 min) ∙ Question & answer about intervention protocol |
9/22/2017 (2 h) | Week 11: Practice/Role Play Session 1 & 2 ∙ WebEx role play—ACCESS intervention session 1 ∙ WebEx role play—ACCESS intervention session 2 |
9/29/2017 (2 h) | Week 12: Practice/Role Play Session 2 & 3 ∙ WebEx role play—ACCESS intervention session 2 ∙ WebEx role play—ACCESS intervention session 3 |
10/4/2017 (1 h) | Self-Directed Learning Activities—No In-Person Meeting/Training ∙ Independent practice of ACCESS sessions 2 & 3 |
10/11/2017 (2 h) | Week 13: Practice/Role Play Session 3 ∙ WebEx role play—ACCESS intervention session 3 |
10/18/2017 (2 h) | Week 14: Practice/Role Play Session 4 ∙ WebEx role play—ACCESS intervention session 4 |
10/25/2017 (1 h) | Self-Directed Learning Activities—No In-Person Meeting/Training ∙ Independent practice of ACCESS sessions 4 & 5 |
11/01/2017 (2 h) | Week 15: Practice/Role Play Session 5 ∙ WebEx role play—ACCESS intervention session 5 |
11/08/2017 (2 h) | Week 16: Practice/Role Play—Revisit Session 1 ∙ WebEx role play—ACCESS intervention session 1 review |
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Navarra, AM.D., Rosenberg, M.G., Gormley, M. et al. Feasibility and Acceptability of the Adherence Connection Counseling, Education, and Support (ACCESS) Proof of Concept: A Peer-Led, Mobile Health (mHealth) Cognitive Behavioral Antiretroviral Therapy (ART) Adherence Intervention for HIV-Infected (HIV+) Adolescents and Young Adults (AYA). AIDS Behav 27, 1807–1823 (2023). https://doi.org/10.1007/s10461-022-03913-0
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-022-03913-0