Abstract
In cross-border areas of East Africa, sexual networks include partnerships across resident, migrant, and mobile populations, and risky behaviors can coincide with fragmented health services given the challenges of cross-border coordination. Among those most at risk are female sex workers (FSWs). We map HIV prevalence among FSWs in 14 cross-border areas, estimate associations between FSW characteristics and HIV and undiagnosed HIV, and estimate progress towards the UNAIDS 90–90–90 targets. The 2016–2017 East Africa Cross-Border Integrated Health Study recruited 4040 women; 786 were classified as FSWs. Overall HIV prevalence among FSWs was 10.8% (95% CI 8.2%, 13.3%), though area-specific estimates varied considerably. Among FSWs living with HIV, 46.1% (95% CI 33.2%, 59.0%) knew their status, 80.6% (95% CI 66.3%, 94.9%) of FSWs who knew their status were on ART, and 84.8% (95% CI 66.1%, 100.0%) of FSWs on ART were virally suppressed. Results indicate a need for expanded HIV testing.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Cross-border areas have been described as “spaces of vulnerability,” where the HIV response is complicated by inadequate health services and mixing of resident, migrant, and mobile populations in an environment conducive to higher risk behaviors [1]. High HIV prevalence and risk behaviors have been documented in cross-border areas of East Africa [2, 3], while health services in these spaces remain poor, despite improvements elsewhere in East African Community Partner States [4]. In recognition of this disparity, there is a demand for information on HIV programming gaps to inform response strategies along regional transport corridors and waterways [1, 5].
The World Health Organization’s Guidelines for HIV Prevention, Diagnosis, Treatment and Care for Key Populations recommend that countries focus their HIV response on populations that are most vulnerable, have the highest prevalence of HIV, and are underserved [6]. In East Africa, female sex workers (FSWs) meet all three of these criteria. FSWs have been identified as a priority population for HIV prevention and care in the strategic plans of Uganda [7], Kenya [8], Rwanda [9], Tanzania [10], and the East African Community [11]. FSWs have an elevated risk of HIV due to behavioral, biological, and structural factors [12]; they have a considerably higher prevalence of HIV than the general population [13]; and their utilization of HIV-related services is often obstructed by the criminalization of sex work, stigma, violence, and discrimination [6].
Estimates of HIV prevalence and engagement in HIV care among FSWs can inform service improvements for this population and potentially reduce HIV transmission in the region [14]. Published estimates of HIV prevalence among FSWs [13, 15,16,17,18,19,20,21,22,23] are mostly limited, however, to highly urbanized areas. Generalizability of these estimates to FSWs in cross-border areas is uncertain. The HIV treatment cascade among FSWs has also not been well described in the region [24, 25], including in cross-border areas.
In this study, we use data from a bio-behavioral cross-sectional survey to describe HIV prevalence and other characteristics among FSWs, other women, and all women at public venues in 14 cross-border areas in Uganda, Kenya, Rwanda, and Tanzania. We also map HIV prevalence among FSWs by cross-border area. We estimate the HIV treatment cascade to identify areas of progress and unmet need among FSWs and other women living with HIV, and we evaluate progress towards the UNAIDS 90–90–90 targets, which call for 90% of people living with HIV to be diagnosed, 90% of people diagnosed to be on antiretroviral therapy (ART), and 90% of people on ART to be virally suppressed by the year 2020 [26]. Finally, we estimate associations between socio-demographic characteristics, sexual behaviors, and social vulnerability factors among FSWs and prevalent HIV and prevalent undiagnosed HIV.
Methods
Study Design and Participants
Between August 2016 and January 2017, we conducted a bio-behavioral survey for the East Africa Cross-Border Integrated Health Study (CBIHS) to assess measures of health and access to health services among populations in 14 cross-border areas in Uganda, Kenya, Rwanda, and Tanzania [27]. Cross-border areas were purposively selected by regional stakeholders, who targeted areas with significant cross-border movement and trade, high STI prevalence, known gaps in health services, and presence of key and vulnerable populations. The 14 selected areas included eight areas that surround overland border posts and six fishing villages that host cross-border trade. The selected areas were: Malaba, Kenya/Uganda; Busia, Kenya/Uganda; Sio Port/Port Victoria, Kenya; Mbita landing site and Rusinga Island, Kenya; Muhuru Bay, Kenya; Isebania, Kenya/Sirare, Tanzania; Namanga, Kenya/Tanzania; Holili, Tanzania/Taveta, Kenya; Kirongwe, Tanzania; Mutukula, Uganda/Tanzania; Kagitumba, Rwanda/Mirama Hills, Uganda; Katuna, Uganda/Gatuna, Rwanda; Kasenyi landing site, Uganda; and Majanji, Uganda.
The study protocol was approved by the institutional review boards of the University of North Carolina at Chapel Hill, Makerere University in Uganda, the Kenya Medical Research Institute, the Rwanda Military Hospital, and the National Institute for Medical Research in Tanzania. All study participants provided verbal informed consent.
Procedures
Following a mapping readiness assessment to assess acceptability of the study and mapping in cross border sites, the three-step Priorities for Local AIDS Control Efforts (PLACE) method [28] was used to sample and recruit participants at each cross-border area. In the first step, interviewers generated a list of public venues where people meet new sexual partners and where female sex workers, men who have sex with men, people who inject drugs, mobile populations, and other vulnerable groups socialize. Interviewers approached community informants according to targets for each of approximately 20 types of informants (e.g., taxi drivers, security guards, fisher folks, vendors). Targets were set at each area based on expectations of how knowledgeable potential types of community informants were about social interactions at local venues where people meet new sexual partners or where vulnerable groups socialize. Following recruitment, community informants were invited to list up to 10 such venues known to them. Up to 200 informants were interviewed at each area to generate an exhaustive list of venues. A list of unique venues was compiled from the community informant reports, and venues were sorted into four priority strata based on reported presence of key populations, on-site sexual activity, and the number of times a venue was reported. In the second step, interviewers visited listed venues to verify their existence and characterize the venues. If 100 or fewer venues were listed in a cross-border area, all venues were visited. Otherwise, interviewers visited a stratified random sample of 100 venues, oversampling venues from higher priority strata.
In the third step, 40 of the verified and operational venues on the list were sampled for bio-behavioral interviews, again oversampling higher priority venues. At each venue, interviewers recruited a stratified random sample of respondents according to targets for male and female patrons and workers. Venue-level respondent targets were set proportional to venue size. Approximately 960 respondents were recruited in each cross-border area, with the exceptions of Muhuru Bay, Kenya; Kirongwe, Tanzania; Sio Port/Port Victoria, Kenya; and Majanji, Uganda where venue and respondent targets were halved due to initial plans to pool data from these areas.
Consenting respondents reported on their socio-demographic characteristics, health status, access to health services, and sexual and health-seeking behaviors. Most questions were completed face-to-face with an interviewer, though some sensitive questions were repeated in a self-completed section wherein the interviewer dictated each question and the respondent selected an image corresponding to their intended response. All participants were offered an on-site rapid HIV test and counseling. In each cross-border area, local health workers administered rapid HIV tests consistent with those in use at local public sector health facilities at the time of data collection. Respondents with a reactive test were linked to care and asked to provide dried blood spots (DBS) for HIV-1 RNA viral load testing. Viral load testing was performed in accordance with national guidelines in each country, and results were communicated back to the local health facilities. Respondents who gave DBS were given a card with a unique identifier and instructions for obtaining their viral load results.
Statistical Analysis
Sampling weights were estimated and used in all analyses to account for known differences in sampling probabilities among respondents. For results related to HIV prevalence and viral suppression, we also used inverse probability weights to account for missing data due to refusals of the HIV and viral load tests [29]. Details regarding estimation of the inverse probability weights are provided in the Supplemental Digital Content. The reweighted data represent the distribution of characteristics among women socializing at public venues across the 14 cross-border areas. All standard errors were estimated using Taylor series linearization to account for the complex survey design [30].
FSWs were defined at the analysis stage as adult women, ages 18 years or older, who reported that they had received money in exchange for sex in the preceding 12 months. We estimated the unweighted and weighted distributions of socio-demographic characteristics and HIV prevalence among FSWs and, for comparison, among all adult women and among adult women who reported never exchanging sex for money in the preceding 12 months. We estimated prevalence ratios to examine the association between sex work and prevalent HIV.
We estimated and mapped HIV prevalence among FSWs in each cross-border area where 20 or more FSWs participated in the on-site HIV testing. Because age was associated with prevalent HIV, we also estimated age-standardized prevalence for areas where 20 or more FSWs ages 20 to 49 were tested. Estimates were standardized to the age distribution of women ages 20 to 49 years in eastern Africa [31] using, typically, 5-year age groups; sparse data were collapsed for women ages 40 to 49 years and, on an area-by-area basis, across age groups where no FSWs were tested. We also calculated prevalence ratios to examine the association between FSWs characteristics and prevalent HIV.
Finally, we estimated the HIV treatment cascade among FSWs, among women who did not exchange sex for money in the last 12 months, and among all women living with HIV. The HIV treatment cascade depicts the proportion of those with a reactive HIV test who knew their status, the proportion of those who knew their status who had ever received HIV care (were “linked to care”), the proportion of those linked to care who were on ART, and the proportion of those on ART who achieved viral suppression (defined as a viral load below 1,000 copies/mL). These results were also used to describe progress towards the 90–90–90 targets.
Analyses were conducted in SAS version 9.4 (SAS Institute; Cary, NC) and R version 4.0.5.
Results
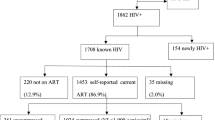
Across the 14 cross-border areas, 11,567 patrons and workers were approached for the bio-behavioral survey, and 11,427 were eligible and consented to participate. Among the participants, 4182 (36.6%) were women, of whom 4040 (96.6%) were adults ages 18 years or older. Among the adult women who participated in the survey, 786 met the study definition of a FSW, while 3224 reported that they did not exchange sex for money in the preceding 12 months, and 30 (0.7%) did not provide sufficient information to be classified one way or the other. Of the adult women, 3725 (92.2%) participated in the on-site HIV test. Of the 786 women classified as FSWs, 715 (91.0%) participated in the HIV test, 85 had a reactive HIV test result, and 73 provided a DBS sample with valid results. Of the 85 FSWs with reactive HIV test results, 37 reported their HIV-positive status in the bio-behavioral survey.
The unweighted and weighted distributions of socio-demographic characteristics among the 786 FSWs and 3224 other adult women in the study sample are presented in Table 1. The median age of FSWs was 25.0 (IQR 21.3, 29.5) years. Less than 20% (18.8%; 95% CI 14.5%, 23.1%) of FSWs completed secondary school, 32.3% (95% CI 28.0%, 36.6%) were married, and 39.3% (95% CI 34.6%, 44.0%) had been previously married but were not married at the time of the survey. Three-quarters (76.8%; 95% CI 71.9%, 81.7%) of FSWs visited venues where people drink, socialize, or meet new sexual partners at least once per week. The median ages at first sex and at first sex work among FSWs were 15.7 (IQR 14.2, 17.5) and 19.2 (IQR 17.3, 22.4) years, respectively. Over half (51.9%; 95% CI 46.5%, 57.2%) of FSWs used a condom at last sex with their main partner, while approximately two-thirds (65.5%; 95% CI 60.3%, 70.6%) used a condom with their last sex work client. In the three months before the survey, 13.6% (95% CI 10.3%, 17.0%) of FSWs experienced physical intimate partner violence, and 10.7% (95% CI 8.1%, 13.3%) were forced to have sex against their will. As compared to women who reported that they did not participate in sex work, FSWs were less likely to be currently married and more likely to have been previously married. FSWs visited venues more often than other women, had less difficulty obtaining condoms, and were more likely to have used a condom at last sex with their main partner. FSWs were also more likely to have experienced intimate partner violence and forced sex. With an estimated HIV prevalence of 10.8% among FSWs, FSWs were 1.6 (95% CI 1.2, 2.1) times as likely as other adult women to be living with HIV.
Area-specific estimates of HIV prevalence among FSWs ranged from 1.7% (95% CI 0.0%, 3.4%) in Busia, Kenya/Uganda to 22.0% (95% CI 14.3%, 29.7%) in Mutukula, Uganda/Tanzania. Area-specific estimates of undiagnosed HIV prevalence—i.e., the percent of all FSWs who are living with HIV but do not know of their HIV-positive status—ranged from 1.3% (95% CI 0.0%, 2.9%) in Busia, Kenya/Uganda to 10.2% (95% CI 4.4%, 16.0%) in Isebania, Kenya/Sirare, Tanzania. The estimated percentage of FSWs living with HIV in each cross-border area is shown in Fig. 1a, while the estimated percentages living with undiagnosed HIV are shown in Fig. 1b. Due to sparse data in some cross-border areas and age groups, prevalence estimates were not computed for 3 cross-border areas, age-standardization was not done for 4 areas when estimating HIV prevalence and 5 areas when estimating undiagnosed HIV prevalence, and data were collapsed across women ages 35 to 49 years prior to standardizing estimates for Kasenyi. Both before and after age-standardizing, prevalence estimates varied substantially by geographic area, even among areas in close proximity, such as those near the northeast corner of Lake Victoria. HIV prevalence was highest among FSWs at venues in Mutukula, Uganda/Tanzania; Muhuru Bay, Kenya; and Malaba, Kenya/Uganda. Though confidence intervals were wide, point estimates tended to be higher in cross-border areas near the lake. The prevalence of undiagnosed HIV among FSWs was highest in Malaba, Kenya/Uganda, Isebania, Kenya/Sirare, Tanzania; and Kasenyi landing site, Uganda. In some cross-border areas, there were large differences in the percentage of FSWs living with HIV and the percentage of FSWs living with undiagnosed HIV. For example, Muhuru Bay had one of the highest HIV prevalence estimates of all cross-border areas in the study, with an estimated 20.9% (95% CI 4.2%, 37.6%) of FSWs living with HIV. Due to the level of awareness of HIV-positive status among FSWs in Muhuru Bay, however, we estimated that only 5.3% (95% CI 0.0%, 12.3%) of FSWs in Muhuru Bay were living with undiagnosed HIV.
Among female sex workers (FSWs) at public venues in cross-border areas in the 2016–2017 East Africa Cross-Border Integrated Health Study, estimated prevalence of (a) HIV and (b) undiagnosed HIV. Prevalence estimates and corresponding 95% confidence intervals (CIs) were calculated for cross-border areas where 20 or more FSWs were tested. For cross-border areas where 20 or more FSWs ages 20–49 years were tested, HIV prevalence estimates were also age-standardized to the distribution of women ages 20–49 years in East Africa; standardized results are shown in square brackets. Data were weighted and standard errors adjusted to account for survey design and informative refusals of the on-site HIV test
Table 2 shows HIV prevalence, undiagnosed HIV prevalence, and prevalence ratios across levels of sociodemographic characteristics among FSWs. Of the characteristics examined, age, type of employment, and difficulty obtaining condoms were most strongly associated with a reactive HIV test. As compared to FSWs 18 to 24 years of age, FSWs 30 years of age and older were 1.9 (95% CI 1.0, 3.5) times as likely to have a reactive HIV test (Fig. 2). FSWs who were informally employed were 2.6 (95% CI 1.5, 4.6) times as likely to have a reactive result as those employed in the formal sector. FSWs reporting that condoms are not easy to obtain were 2.4 (95% CI 1.3, 4.3) times as likely to have a reactive result and 2.3 (95% CI 1.0, 5.0) times as likely to have undiagnosed HIV, compared to those who reported that it was easy to obtain a condom. Aside from these characteristics, the 95% confidence intervals around most prevalence ratios for HIV and undiagnosed HIV contained the null value.
Estimated HIV prevalence by age group among adult women (unweighted n = 3725) at venues in 14 cross-border areas in East Africa, also disaggregated for female sex workers (FSWs, unweighted n = 715) and other adult women (unweighted n = 2983). Data were weighted to account for survey design and informative refusals of the on-site HIV test, and standard errors were adjusted to account for survey design. Point estimates of prevalence (%) are presented along with corresponding 95% confidence intervals, shown parenthetically (East Africa Cross-Border Integrated Health Study, 2016–2017)
Of the 85 FSWs who had a reactive HIV test, 48 had not been previously diagnosed with HIV. Among FSWs with undiagnosed HIV, 13.3% (95% CI 1.0%, 25.6%) had never been tested for HIV, and 64.1% (95% CI 47.5%, 80.7%) had a negative test result in the past 12 months. Weighting for survey design and informative refusals, 46.1% (95% CI 33.2%, 59.0%) of FSWs living with HIV knew their status, 80.6% (95% CI 66.3%, 94.9%) of FSWs who knew of their HIV-positive status were on ART, and 84.8% (95% CI 66.1%, 100.0%) of FSWs on ART were virally suppressed. The HIV care cascade in Fig. 3 shows the proportion of women at each stage of HIV care and treatment and the gaps between these values and the 90–90–90 targets. Results are presented for all women and stratified by FSW classification.
The HIV treatment cascade among adult women (unweighted n = 274) at venues in 14 cross-border areas in East Africa, also disaggregated for female sex workers (FSWs, unweighted n = 85) and other adult women (unweighted n = 187). The cascade depicts, among those with a reactive HIV test, the estimated percent who knew their HIV status, were linked to care, were on antiretroviral therapy (ART), and were virally suppressed. The 95% confidence intervals around the point estimates are shown parenthetically. Data were weighted to account for survey design, informative refusals of the on-site HIV test, and missing viral load data. Standard errors were adjusted to account for survey design. For reference, dashed bars indicate the proportion of women with HIV who would need to know their HIV status, be on ART, and be virally suppressed for the 90–90-90 targets to be met (East Africa Cross-Border Integrated Health Study, 2016–2017)
Discussion
In this study, we found that FSWs at venues in cross-border areas in East Africa are more likely than other women to be living with HIV. We also found variation in prevalence among cross-border areas, which substantiates concerns about the generalizability of other HIV prevalence estimates to cross-borders areas. At 10.8% (95% CI 8.2%, 13.3%), HIV prevalence among FSWs in this study was generally lower than other published prevalence estimates among FSWs elsewhere in Kenya, Tanzania, Rwanda, and Uganda [17, 18, 20,21,22, 32]. The relatively low prevalence found in several cross-border areas in this study could reflect local successes in the HIV response, reduced risk behaviors among FSWs despite the service delivery challenges and risk behaviors documented in cross-border areas, or lower exposure to HIV. In this study, women who reported exchanging sex for money in the preceding 12 months were classified as FSWs, regardless of whether the women would self-identify as sex workers. The lower prevalence of HIV observed among FSWs in this study may also be partly attributable to the inclusion of FSWs who may not identify as sex workers, if, compared to self-identified sex workers, these women experience fewer behavioral and structural risk factors for HIV infection overall.
Results of this study suggest that less than half of FSWs living with HIV (46.1%; 95% CI 33.2%, 59.0%) were aware of their status. Among other women, the proportion who knew their status was even smaller (38.3%; 95% CI 29.5%, 47.1%). These gaps are greater than those reported elsewhere in the region, such as in the SEARCH Study in rural Kenya and Tanzania, where 69.7% of women living with HIV had been previously diagnosed [33]. We also found that most FSWs living with undiagnosed HIV had been tested in the 12 months prior to the survey. While further research is needed to identify optimal testing strategies for cross-border areas, the number of new HIV diagnoses among FSWs in the CBIHS (48 of 786 FSWs) illustrates the value of mobile and venue-based testing for FSWs in the cross-border context. Furthermore, the maps of HIV prevalence and undiagnosed HIV prevalence among FSWs provided in this study highlight that areas with the highest unmet need for HIV testing are not necessarily the same areas as those with highest HIV prevalence.
Though not knowing one’s HIV status was found to be a major impediment to the 90–90–90 targets, results for the latter targets were encouraging. Approximately 80% of FSWs who knew their status were on ART, and among FSWs on ART, approximately 85% were virally suppressed. Though confidence intervals were wide, FSWs were somewhat more likely than other women to know their status and to be linked to care, on ART, and virally suppressed. These comparisons remind of the continuing need for HIV-related services even among women who do not participate in sex work, while possibly indicating some success in focusing HIV services for FSWs.
The CBIHS has several strengths. These include its multisite design, which allowed for comparison of results across cross-border areas, and implementation of the study in multiple countries, which avoided arbitrary exclusion of people in cross-border areas who would be found on only one side of the border. Venue-based recruitment also allowed for inclusion of populations that may be missed in routine monitoring or alternative study designs such as household surveys. As compared to studies that rely on observed behaviors or self-identification to recruit FSWs, we expect that the CBIHS reduced selection bias by recruiting women irrespective of perceived sex worker status. This also allowed for the consideration of alternative definitions of sex work. We found consistency in the direction and strength of HIV prevalence ratios using broader definitions of sex work. For example, women who had ever exchanged sex for money were 1.7 (95% CI 1.3, 2.3) times as likely to have a reactive HIV test, and women who ever exchanged sex for money, goods, gifts, or favors were 1.5 (95% CI 1.2, 2.0) times as likely as other women to have a reactive test.
Limitations of this study include that some factors, like reporting sex work or a previous HIV diagnosis, were subject to recall and social desirability biases common to most behavioral surveys. Among unweighted survey respondents, 19.6% reported that they received money for sex. A higher proportion of women may have engaged in other forms of transactional sex, such as sex for goods, gifts, favors, services; however, these forms of transactional sex were outside the scope of this analysis. If, among all women who exchange money for sex, those who self-identify as sex workers are more likely to report this behavior, our results describing FSW characteristics may be biased towards the characteristics of self-identified sex workers. For example, if self-identified FSWs are more likely than other FSWs to access HIV testing through targeted outreach, our estimate that 46.1% of FSWs with HIV know of their HIV-positive status would overestimate the true parameter among all FSWs. This study also does not capture all dimensions of sex work relevant to HIV transmission. Programs seeking to reduce HIV incidence among FSWs and their clients may benefit from future studies on frequency of sex work, number of unique partners, and the use of prevention strategies. Furthermore, for safety reasons, interviewers did not recruit respondents late into the evenings, so our findings may not generalize to FSWs and other women who attend venues exclusively late at night. There is also potential for bias related to differential likelihood of participation in the CBIHS by HIV status or engagement in care. We accounted for informative refusals of the HIV and viral load tests among survey participants by offering the survey to those refusing the tests and then weighting the data; these weights cannot, however, account for any bias related to complete avoidance of the survey.
Conclusions
This study contributes to a fuller understanding of the characteristics of FSWs in cross-border spaces, indicating continuing demand for HIV prevention and treatment services and describing gaps in current HIV control strategies. FSWs at venues in cross-border areas in East Africa are more likely than other women to be living with HIV; however, there is considerable geographic heterogeneity in HIV prevalence estimates among FSWs throughout the region, which emphasizes the relevance of geography to the efficient provision of HIV prevention and treatment interventions. Despite lower than expected prevalence among FSWs, HIV transmission potential is sustained in cross-borders areas by deficits in HIV testing services. Finally, among FSWs, there is a dual need for programs that reach those who have never been tested for HIV and for programs that increase the frequency of testing.
As East African nations pursue greater movement of people, goods, and services across the region, it is important to examine how the continuing HIV epidemic and national and regional HIV control efforts affect cross-border areas. These cross-border areas, and members of key populations who are present there, may be particularly impacted by changes in population mobility that affect transmission dynamics for HIV and other infectious diseases. Results of this and future studies can be used to improve the effectiveness and efficiency of infectious disease control efforts for people living in and transiting through cross-border areas in East Africa.
References
International Organization for Migration. A Response Analysis of HIV/AIDS Programming along Transport Corridors in Uganda. 2013. http://uganda.iom.int/wp-content/uploads/2016/02/ARESPONSEANALYSISOFHIVPROGRAMMING.pdf. Accessed 24 Sep 2017.
East African Community Secretariat. Health and HIV and AIDS Along the East African Community (EAC) Transport Corridors. 2015. https://www.afidep.org/download/03.03.2016.PB.SiTan_CBHIPPfinal.pdf. Accessed 27 Nov 2017.
Ferguson A, Kriitmaa K. HIV Hot-spot mapping and situational analysis along the Kampala-Juba transport route. 2008. https://www.iom.int/jahia/webdav/shared/shared/mainsite/activities/health/hiv-population/HIV-Hotspot-Mapping-Situational-Analysis-Kampala-Juba-Transport-Route-2008.pdf. Accessed 24 Sept 2017.
International Organization for Migration. A Rapid Assessment of Access to Health Care at Selected One Stop Border Posts in East Africa. 2013. https://www.iom.int/files/live/sites/iom/files/Country/docs/IOM-OSPB-Report-2013.pdf. Accessed 24 Sept 2017.
East African Community. Health and HIV/AIDS along the East African Community Transport Corridors: A Situational Analysis. 2015. https://www.afidep.org/download/03.03.2016.PB.SiTan_CBHIPPfinal.pdf. Accessed 24 Sept 2017.
World Health Organization. Consolidated Guidelines on HIV Prevention, Treatment and Care for Key Populations. 2014.
Uganda AIDS Commission. National HIV and AIDS Strategic Plan 2015/2016–2019/2020. 2015. http://library.health.go.ug/publications/service-delivery-diseases-control-prevention-communicable-diseases/hivaids/national-h-1. Accessed 24 Sept 2017.
National AIDS Control Council. Kenya AIDS Strategic Framework 2014/2015–2018/2019. 2014]. pp. 1–84. http://nacc.or.ke/wp-content/uploads/2015/09/KASF_Final.pdf. Accessed 24 Sept 2017.
Ministry of Health. Rwanda HIV and AIDS National Strategic Plan July 2013–June 2018. 2013. pp. 19–118. http://www.nationalplanningcycles.org/sites/default/files/country_docs/Rwanda/final_nsp_2013-2018.pdf. Accessed 24 Sept 2017.
National AIDS Control Programme. Third Health Sector HIV and AIDS Strategic Plan (HSHSP III) 2013–2017. 2013. http://ihi.eprints.org/2291/1/HSHSP_III_-_FINAL_Draft_15SEP2013__final_output_to_NACP_withNBTS_update04112013.pdf. Accessed 24 Sept 2017.
East African Community. HIV and AIDS/STI and TB Multisectoral Strategic Plan and Implementation Framework 2015–2020. 2015. https://static1.squarespace.com/static/5519047ce4b0d9aaa8c82e69/t/555312bee4b0368962bef3b3/1431507646132/EAC+HIV+and+AIDS++Strategic+Plan.pdf. Accessed 24 Sept 2017.
Shannon K, Goldenberg SM, Deering KN, Strathdee SA. HIV infection among female sex workers in concentrated and high prevalence epidemics. Curr Opin HIV AIDS. 2014;9(2):174–82.
Baral S, Beyrer C, Muessig K, Poteat T, Wirtz AL, Decker MR, et al. Burden of HIV among female sex workers in low-income and middle-income countries: A systematic review and meta-analysis. Lancet Infect Dis. 2012;12(7):538–49.
Mountain E, Pickles M, Mishra S, Vickerman P, Alary M, Boily M-C. The HIV care cascade and antiretroviral therapy in female sex workers: implications for HIV prevention. Expert Rev Anti Infect Ther. 2014;12(10):1203–19.
Muldoon KA. A systematic review of the clinical and social epidemiological research among sex workers in Uganda. BMC Public Health. 2015;15(1):1226.
Beyrer C, Crago A-L, Bekker L-G, Butler J, Shannon K, Kerrigan D, et al. An action agenda for HIV and sex workers. Lancet. 2015;385(9964):287–301.
Musyoki H, Kellogg TA, Geibel S, Muraguri N, Okal J, Tun W, et al. Prevalence of HIV, sexually transmitted infections, and risk behaviours among female sex workers in Nairobi, Kenya: results of a respondent driven sampling study. AIDS Behav. 2015;19(1):46–58.
Menon S, van den Broeck D, Rossi R, Ogbe E, Mabeya H. Multiple HPV infections in female sex workers in Western Kenya: implications for prophylactic vaccines within this sub population. Infect Agent Cancer. 2017;12(1):2.
McClelland RS, Richardson BA, Cherutich P, Mandaliya K, John-Stewart G, Miregwa B, et al. A 15-year study of the impact of community antiretroviral therapy coverage on HIV incidence in Kenyan female sex workers. AIDS. 2015;29(17):2279–86.
Mutagoma M, Nyirazinyoye L, Sebuhoro D, Riedel DJ, Ntaganira J. Syphilis and HIV prevalence and associated factors to their co-infection, hepatitis B and hepatitis C viruses prevalence among female sex workers in Rwanda. BMC Infect Dis. 2017;17(1):525.
Hladik W, Baughman AL, Serwadda D, Tappero JW, Kwezi R, Nakato ND, et al. Burden and characteristics of HIV infection among female sex workers in Kampala, Uganda—a respondent-driven sampling survey. BMC Public Health. 2017;17(1):565.
Kapiga SH, Ewings FM, Ao T, Chilongani J, Mongi A, Baisley K, et al. The epidemiology of HIV and HSV-2 infections among women participating in microbicide and vaccine feasibility studies in Northern Tanzania. PLoS ONE. 2013;8(7):e68825.
Chersich MF, Bosire W, Kingola N, Temmerman M, Luchters S. Effects of hazardous and harmful alcohol use on HIV incidence and sexual behaviour: a cohort study of Kenyan female sex workers. Global Health. 2014;10(1):22.
Mountain E, Mishra S, Vickerman P, Pickles M, Gilks C, Boily M-C. Antiretroviral therapy uptake, attrition, adherence and outcomes among HIV-infected female sex workers: a systematic review and meta-analysis. PLoS ONE. 2014;9(9):e105645.
Risher K, Mayer KH, Beyrer C. HIV treatment cascade in MSM, people who inject drugs, and sex workers. Curr Opin HIV AIDS. 2015;10(6):420–9.
UNAIDS. Ending AIDS: Progress Towards the 90–90–90 Targets . 2017. http://www.unaids.org/en/resources/documents/2017/20170720_Global_AIDS_update_2017. Accessed 24 Sept 2017.
Edwards JK, Arimi P, Ssengooba F, Mulholland GE, Markiewicz M, Bukusi EA, et al. The HIV care continuum among resident and non-resident populations found in venues in East Africa cross-border areas. J Int AIDS Soc. 2019;22(1):e25226.
Weir SS, Tate JE, Zhusupov B, Boerma JT. Where the action is: monitoring local trends in sexual behaviour. Sex Transm Infect. 2004;80:ii63–8.
Seaman SR, White IR. Review of inverse probability weighting for dealing with missing data. Stat Methods Med Res. 2013;22(3):278–95. https://doi.org/10.1177/0962280210395740.
Binder DA. On the variances of asymptotically normal estimators from complex surveys. Int Stat Rev / Rev Int Stat. 1983;51(3):279.
Department of Economic and Social Affairs Population Division. The 2017 Revision of World Population Prospects. 2017. https://esa.un.org/unpd/wpp/
Vu L, Misra K. High burden of HIV, syphilis and HSV-2 and factors associated with HIV infection among female sex workers in Tanzania: implications for early treatment of HIV and Pre-exposure prophylaxis (PrEP). AIDS Behav. 2018;22(4):1113–21.
Petersen M, Balzer L, Kwarsiima D, Sang N, Chamie G, Ayieko J, et al. Association of implementation of a universal testing and treatment intervention with HIV diagnosis, receipt of antiretroviral therapy, and viral suppression in East Africa. JAMA. 2017;317(21):2196. https://doi.org/10.1001/jama.2017.5705.
Acknowledgements
This work was supported by the United States Agency for International Development (USAID) under the terms of MEASURE Evaluation Cooperative Agreement AID-OAA-L-14-00004. MEASURE Evaluation is implemented by the Carolina Population Center, University of North Carolina at Chapel Hill in partnership with ICF International; John Snow, Inc.; Management Sciences for Health; Palladium; and Tulane University. Views expressed are not necessarily those of USAID or the United States government.
Funding
This work was supported by the United States Agency for International Development (USAID) under the terms of MEASURE Evaluation cooperative agreement AID-OAA-L-14-00004. MEASURE Evaluation is implemented by the Carolina Population Center, University of North Carolina at Chapel Hill in partnership with ICF International; John Snow, Inc.; Management Sciences for Health; Palladium; and Tulane University.
Author information
Authors and Affiliations
Contributions
All authors collaborated in the design of the study. JE, GM, and MM developed the survey instruments, and FS oversaw data collection. GM conducted the literature search, conducted the tabular and graphic analyses, and wrote initial drafts. JE provided input on analyses. All authors contributed to interpretation of the results, reviewed the manuscript and provided comments.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
The questionnaire and methodology for this study was approved by the institutional review boards of the University of North Carolina at Chapel Hill, Makerere University in Uganda, the Kenya Medical Research Institute, the Rwanda Military Hospital, and the National Institute for Medical Research in Tanzania.
Consent to participate
Verbal informed consent was obtained from all participants prior to the interview.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Appendix
Appendix
For measures related to HIV testing, data were weighted by the product of the survey sampling weights and HIV test refusal weights. The HIV test refusal weight was estimated as the marginal probability of refusing the HIV test divided by the probability of refusing the test conditional on informative covariates. Probabilities were estimated using multivariable logistic regression, with the conditional probability of refusing the test modeled as a function of a respondent’s sex, age group, cross-border area where interviewed, employment at the place of interview, and timing and reported result of last HIV test. Categorical covariates were modeled using indicator variables. For viral suppression results, missing viral load weights were similarly estimated among the women, dividing the marginal probability of a missing viral load by the conditional probability, conditional on the woman’s age group, cross-border area where interviewed, and employment at the place of interview. In estimating viral suppression results, data were weighted by the product of three component weights: the survey sampling weight, the HIV test refusal weight, and the missing viral load weight.
Rights and permissions
About this article
Cite this article
Mulholland, G.E., Markiewicz, M., Arimi, P. et al. HIV Prevalence and the HIV Treatment Cascade Among Female Sex Workers in Cross-Border Areas in East Africa. AIDS Behav 26, 556–568 (2022). https://doi.org/10.1007/s10461-021-03411-9
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-021-03411-9