Abstract
South Africa processes 5.1 million HIV CD4, viral load (VL), and tuberculosis (TB) tests annually. This pilot non-randomized trial in South Africa explored an intervention (“MatlaMobile”) to deliver laboratory results via mobile phone. Adults completing CD4, VL, and/or TB laboratory tests were enrolled—either receiving results by returning to clinic (control, n = 174) or mobile phone (intervention, n = 226). Study staff instructed control participants to return within 6 days (standard-of-care). MatlaMobile instructed intervention participants with clinically actionable results requiring intervention or treatment change (i.e., < 200 CD4 cells per milliliter, ≥ 400 viral copies per milliliter, or TB positive) to return immediately. A greater proportion of intervention participants than controls saw their results within 7 days of enrollment (73% vs. 8.6%, p < 0.001). Among participants instructed to return, more intervention participants (20%, n = 14/70) returned than controls (8.6%, n = 15/174, p = 0.02). MatlaMobile demonstrated that patients can quickly receive and respond appropriately to digital delivery of health information.
Resumen
Sudáfrica procesa 5.1 millones de pruebas de cuenta de CD4, carga viral de VIH (VL) y tuberculosis (TB) anualmente. En este estudio piloto no aleatorizado en Sudáfrica se exploró la intervención por medio de la aplicación (“MatlaMobile”) para entregar resultados de laboratorio via teléfono celular. Adultos con pruebas de CD4, VL y/o TB, fueron incluidos para recibir los resultados cuando regresaron a la clínica (control, n = 174) o en su teléfono celular (intervención, n = 226). El staff del estudio instruyó a los participantes del grupo control a regresar en los primeros 6 días (práctica estándar). MatlaMobile instruyó a los participantes que necesitaban alguna intervención o ajuste al tratamiento, de acuerdo con sus resultados (i.e., CD4 < 200, VL ≥ 400, o TB +) a regresar inmediatamente a la clínica. Mayor proporción de participantes en el grupo de intervención, vs. control, vio sus resultados en los primeros 7 días (73% vs. 8.6%, p < 0.001). De los pacientes a los que se les pidió regresar, más participantes del grupo intervención (20%, n = 17/70) lo hicieron, comparados con el grupo control (8.6%, n = 15/174, p = 0.02). MatlaMobile demostró que los pacientes pueden recibir información de salud rápidamente de forma digital y responder de manera apropiada.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The South African healthcare system is under-resourced to care for its 7.2 million patients living with human immunodeficiency virus (HIV) and 322,000 new cases of tuberculosis (TB) diagnosed annually [1, 2]. Nearly 60% of TB patients in South Africa are HIV co-infected, and though patients receive free diagnostic tests and treatment for HIV and TB at primary care clinics, the country faces a shortage of physicians to support such care [2,3,4,5].
South Africa processes the largest number of molecular diagnostic TB assays and HIV-related tests of any country—1.2 million TB sputum tests and 3.9 million combined CD4 count and viral load (VL) tests annually [6, 7]. Since 2016, national guidelines recommend that patients who are newly diagnosed with HIV should immediately receive antiretroviral therapy (ART), a CD4 count assay, and a sputum Xpert MTB/RIF test [8]. Guidelines also recommend an HIV VL test three months after ART initiation and every six months thereafter until viral suppression, at which point VL tests are reduced to an annual basis.
For each laboratory test, patients visit their clinic for sample collection and are instructed by clinic staff to return for their result after three to six working days. Patient adherence to return instructions is critical because it allows healthcare workers to promptly intervene for patients with actionable results (< 200 CD4 cells per milliliter of blood, ≥ 400 viral copies per milliliter of blood, or a TB positive). However, 60% of VL tests have undetectable levels of HIV RNA and nearly 90% of TB tests are negative. In other words, most results are non-actionable and do not merit an additional visit to the clinic, especially if that patient already visits monthly for ART refills. Yet patients with non-actionable results have no alternative to returning and hence, must join and contribute to the queue waiting to be seen by clinic staff [9].
Long wait times in overburdened clinics deter patients from returning and are expensive for patients who do not need to return [10, 11]. While some patients with non-actionable results may benefit from receiving CD4, VL, and TB results in-person with a healthcare provider, others may perceive that the burdens of coming to the clinic outweigh such benefits, especially if the second visit is within a few days of the first. For example, patients often pay for travel to-and-from the clinic and may also forgo paid work on the day of the clinic visit [9, 12]. These and other barriers to care likely contribute to the 24% of patients with TB and 38% of people living with HIV in South Africa not being on TB treatment and ART respectively in 2018 [1, 13,14,15]. There is an urgent need for novel interventions to provide alternative health communication options for patients, reduce unnecessary patient visits, improve responsiveness to laboratory results requiring further clinical action, and offer accessible education to patients.
A mobile health (mHealth) intervention could be a solution for improving disease management [16], especially in South Africa [17,18,19] where 91% of individuals own a mobile phone [20]. Previous studies in southern Africa demonstrated that delivering short message services (SMS) to patients can improve linkage to care, retention in care, and adherence to ART and TB treatment [21,22,23]. Patients living with HIV in South Africa have also reported that SMS interventions were acceptable but expressed concerns about privacy and the lack of two-way communication [24, 25].
The purpose of this study was to assess MatlaMobile (“Strength by Mobile” in Setswana), an mHealth intervention to deliver CD4, VL, and TB results to patients’ mobile phones. MatlaMobile used an unstructured supplementary service data (USSD) system for participants to access PIN-protected messages that, unlike SMS, were not stored locally on the mobile phone but could still be viewed repeatedly. MatlaMobile also offered options for participants to request a call-me-back from a nurse or send their laboratory result to themselves via SMS text message. We assessed whether the intervention increased participant access to their laboratory results and encouraged more appropriate follow up visits as compared to the standard-of-care. We also assessed participant satisfaction with MatlaMobile.
Methods
Study Setting and Design
We conducted this study in Matlosana Municipality, South Africa at one urban and two peri-urban public primary care clinics with similar numbers of HIV and TB patients. For this pilot non-randomized study, we enrolled participants into the intervention arm between June and November 2017 and into the control arm between January and June 2018. Neither staff nor participants were masked to treatment assignment.
Study Participants
In both time periods, clinic nurses referred adults (≥ 18 years of age) who had ≥ 1 laboratory sample taken for a CD4 count, VL assay, or TB Xpert MTB/RIF (Cepheid) to the study staff. Clinic staff collected samples as part of routine clinical care. Study staff assessed eligibility of referred patients in a private room. Patients were eligible to participate if they were literate and owned or had access to a cell phone on which they were willing to receive their laboratory results. Study staff determined literacy by asking patients to read a sign in a language of their choice (English, Setswana, Sesotho, or Xhosa) and follow written instructions to draw a shape. Patients were excluded if they did not have a cell phone on their person at the time of screening. All participants provided written informed consent. This study was approved by the Institutional Review Board at the Johns Hopkins School of Medicine and the Human Research Ethics Committee at the University of Witwatersrand, Johannesburg.
Control and Intervention Conditions
Participants in the control arm received the standard-of-care of instructions to physically return to their clinic within 3–6 days to view their laboratory results. No mobile notifications were sent to participants in the control arm.
Participants in the intervention arm received an SMS notification on their mobile phone stating their results from MatlaMobile were available and that they could log into the MatlaMobile USSD system to view it. MatlaMobile was developed using Microsoft ASP.NET MVC technology and hosted on a shared server with a password-protected structured query language (SQL) database.
If a participant did not use MatlaMobile to see their result, study staff sent a daily SMS reminder for up to 3 days after the initial notification and then made two telephonic attempts to contact the participant. Study staff told participants in the intervention arm to return to the clinic if instructed to do so by MatlaMobile, rather than the standard-of-care instructions to always return within 3–6 days.
After receiving the SMS notification, participants dialed a code to connect with the MatlaMobile USSD system. USSD uses 140 character messages and is widely used in Africa for mobile banking and to purchase mobile phone call time, data, and messages. To access their laboratory result within the USSD system, participants entered a personal information number (PIN) they selected during enrollment, which was also linked to their mobile phone number. During enrollment, study staff also provided hands-on training and verified participants could successfully log in using their PIN. We did not directly store participant PINs in the study database. Instead, we used an algorithm to hash each PIN into a unique code prior to storage. In this way, the study staff never stored or had access to participant PINs.
Participants could login to the MatlaMobile system multiple times. At each login, participants selected a language (English, Setswana, Sesotho, or Xhosa) and were greeted with the following options: view laboratory result (Table 1), send a call-me-back request to a nurse, or view generic educational messages about each laboratory result (Supplementary Digital Content 1). If a participant selected the option to send a call-me-back request, the MatlaMobile system automatically sent a text message to a professional nurse on the study with the participant’s phone number. The nurse responded to call-me-back requests within 24 h on workdays.
MatlaMobile messages were based on the Health Belief Model [25]. The model posits that behavior is driven by cues to action, perceived consequences, perceived benefits of acting to prevent or treat disease, barriers to acting, and one’s self-efficacy. MatlaMobile messages were cues to action. The messages reminded participants that they might need to act on their laboratory results and recommended appropriate actions (e.g., “go to the clinic now”). The messages also informed participants about possible consequences (“Your results show you have TB… You can infect your family and friends.”), and benefits related to treatment (e.g., “You may need more medicine to be healthy.”). Generic educational messages and the option to speak directly with a nurse were aimed at building patient self-efficacy toward understanding their health status and how to take action.
We categorized laboratory results as either “actionable” or “non-actionable” (Table 1) based on the national guidelines at the time of study implementation. “Actionable” results included any CD4 or VL count if the patient was not yet on ART, VL ≥ 400 copies per milliliter or CD4 ≤ 200 cells per cubic millimeter regardless of ART status, or TB results positive for Mycobacterium tuberculosis. Messages advised participants with actionable results to return to their clinic immediately. Messages advised participants with non-actionable results that their results did not require immediate clinical action and, unless they had further concerns to discuss with a provider, they did not need to return outside regularly scheduled appointments. South African healthcare providers, in collaboration with a study nurse, developed the messages. Study staff reviewed, translated, and back-translated all messages.
Study Measures
Study staff interviewed consented participants at enrollment to ascertain patient demographics (sex, age, education, household and personal income, ever having been on ART, and whether they received HIV care at one or more clinic(s)) and clinic-level characteristics (home-to-clinic travel time and wait time at the clinic). Staff also recorded the unique barcode of each laboratory specimen and observed the participant’s phone type, either smart or basic. Smartphones are able to use mobile applications (i.e. “apps”) while basic phones are typically limited to calls and texting. MatlaMobile was compatible with both smart and basic phones.
Study staff accessed the National Health Laboratory Service (NHLS) TrakCare Webview at least once daily and retrieved all available laboratory results using the unique laboratory specimen barcode and two patient identifiers (e.g. full name, date of birth, national ID). If there were near matches, study staff resolved them directly with laboratory staff who consulted original, handwritten laboratory requisition forms and the electronic Health Patient Registration System (HPRS) to check for data entry errors and alternative identifying data. Study staff also recorded the date and time each result became available in the NHLS system.
At follow up, study staff abstracted outcome data for participants using paper medical records, archived clinic files, and Tier.NET (an electronic treatment database for people living with HIV). Any time study staff entered outcome data into the study database, another staff member re-confirmed the entry against the participant’s medical records for quality assurance.
The MatlaMobile system automatically collected the following user interaction metrics: language selected, date and time that messages were received and accessed, screens viewed, whether messages were viewed in part or in full, and nurse call-me-back requests. We telephonically surveyed participants approximately one week after enrollment about their satisfaction with MatlaMobile on a 7-item scale and asked if they were aware of anyone accessing their laboratory result without their permission.
Outcomes
Our primary outcomes were whether participants, within 7 days after their laboratory test was taken, 1) had seen at least one laboratory result and 2) adhered to instructions to return to clinic. In the control arm, we defined participants as seeing their laboratory result and adhering to instructions if they returned to clinic. In the intervention arm, we counted participants as seeing their laboratory result if they used MatlaMobile to view the result. We considered intervention participants to have adhered to instruction if they returned to the clinic as instructed by their MatlaMobile message.
Secondary outcomes included overall proportion of participants who returned to clinic regardless of whether the result was actionable or not, the median (interquartile range (IQR)) number of days from enrollment-to-return among those who returned within 30 days, the median (IQR) number of days from enrollment-to-return among those instructed to return, and the proportion of intervention participants who returned despite receiving all non-actionable results. We also calculated enrollment-to-return time distributions. Finally, we described participant interactions and satisfaction with the MatlaMobile intervention.
Statistical Analysis
We compared study arms using descriptive statistics. We used Kruskal–Wallis and chi-square tests for continuous and categorical variables respectively. For primary outcomes, we estimated adjusted risk ratios and 95% confidence intervals (CIs) using Poisson regression models with robust variance and controlling for participant demographics and clinic-level characteristics. For secondary outcomes, we compared proportions and means. Our sample size was based on detecting a 15% absolute difference in the primary outcomes between arms with a power of 80%. We conducted analyses in STATA [26], at a significance level of < 0.05.
Results
Screening and Enrollment
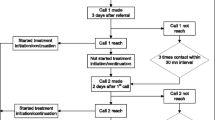
Overall, we approached 630 patients and enrolled 444 participants (Fig. 1). The primary reasons patients were ineligible for enrollment were that they did not have a mobile phone on their person (n = 88) or were illiterate (n = 54). Of the 444 enrolled, we excluded 44 from the analysis for a final sample of 226 participants in the intervention arm and 174 in the control arm. We excluded participants from the analysis due to enrollment during a laboratory strike when samples were not processed between July 21 and August 3, 2017 (n = 24), invalid or missing results (n = 6), enrollment while the clinic offered same-day TB Xpert MTB/RIF results during a non-related research study (n = 4), missing data (n = 2), and other reasons (n = 8).
Descriptive Statistics
Median age of participants was 37 years (IQR: 32–46), and the majority were women (69%, Table 2). Most were living with HIV (86.5%), with a greater proportion of people with HIV in the intervention (94.8%) than the control arm (77.6%, p < 0.001). Approximately half of participants had basic phones while the other half had touchscreen or smartphones. Overall, participants reported an hour median travel time to clinic (IQR: 0.6, 1.5) and a 3.2-h median wait time to see a healthcare provider and receive their results (IQR: 1.6, 4.5). Participants had a total of 236 CD4, 238 VL, and 114 TB Xpert MTB/RIF assays performed, with more CD4 and VL tests in the intervention group and more TB assays in the controls.
Primary Outcomes
A greater proportion of participants using MatlaMobile viewed their test result within 7 days of their enrollment (73.0%) compared to the control arm (8.6%, p < 0.001, Table 3). Controlling for demographics and clinic-level characteristics, participants in the intervention arm were 9.9 (95% CI:6.0–16.5) times more likely to view their laboratory result within 7 days of enrollment as compared to participants in the control arm. Among participants instructed to return, a greater proportion of participants using MatlaMobile did so within 7 days of enrollment (20.0%) than the control arm (8.6%, p = 0.02). Adjusting for demographics and clinic-level characteristics, participants in the intervention arm were 3.0 (95% CI: 1.3–6.8) times more likely than participants in the control arm to return within 7 days of enrollment. Though sample sizes for any one test were insufficient for a formal analysis, each trended in favor of the intervention arm.
Secondary Outcomes
A similar proportion of participants in both arms returned to clinic within 7 days of enrollment (8.6% v. 9.3%, p = 0.82). Time-to-return across study arms was similar; participants in the intervention arm returned somewhat earlier (IQR: 6.5–28 vs. 6–28, p = 0.04). Of the 156 participants in the intervention arm who received non-actionable results, seven (4.5%) returned to the clinic within 7 days of enrollment. Figures 1 and 2 of the Supplementary Digital Content 2 describe the time distribution between enrollment-to-return among participants overall and among those instructed to return.
Interactions and Satisfaction with the MatlaMobile System
Most MatlaMobile participants (88.5%) viewed their results during follow up, and of those, greater than half (64.5%) viewed results within 24-h of availability. However, few who viewed their result also read the full USSD message (27.0%). Of all MatlaMobile logins (n = 1,893), English was selected most (68.8%), followed by Setswana (15.2%), Sesotho (12.7%), and Xhosa (3.2%). A majority (95.6%) of participants signed into MatlaMobile more than once. Among the twenty-nine participants who requested a call-me-back from the study nurse, most requested help interpreting their results (55.2%) or navigating the MatlaMobile system (6.9%). Several participants (17.2%) selected the call-me-back option unintentionally or could not be reached by the study nurse (10.3%). Participants also asked when the result would be ready (3.5%), asked about symptoms of TB (3.5%), and confirmed they logged in successfully (3.5%).
Participants in the intervention arm were generally satisfied with MatlaMobile (Fig. 2). Of the 159 participants who responded to the follow up survey, the majority felt their information was more protected and confidential when delivered via phone than by the clinic (95%), wanted to receive other health information on their phone (96.9%), and preferred mobile delivery over a clinic visit to see laboratory results (96.9%). No participant reported MatlaMobile causing accidental disclosures or instances where others saw their laboratory results without permission.
Discussion
In this study, an mHealth program using USSD successfully and securely delivered CD4, VL, and TB laboratory results to patients’ mobile phones in South Africa. A majority of interested patients were eligible for enrollment, and among those in the intervention arm, nearly 90% viewed their PIN-protected messages and found the program highly acceptable. This is one of the first studies to evaluate, with a separate control group, sending laboratory results using USSD directly to patients’ basic and smartphones in sub-Saharan Africa.
mHealth programs using USSD systems can reach more South Africans than those relying on apps. For example, the app-based SmartLink mHealth program in South Africa piloted delivering CD4 and VL results [27]. The program excluded 90% of interested patients for not having a smartphone that could support the app with a modern Android operating system, data capabilities, and sufficient RAM [27]. In comparison, our study excluded patients only if they lacked a phone at enrollment, resulting in fewer interested patients being turned away (14%, n = 88/630). USSD systems are also more accessible because they come installed on all mobile phones by default and can be freely accessed without an Internet connection or using mobile data. In the future, mHealth programs could be made even more accessible by including voice- or image-based messages for people who cannot read or have poor eyesight [28].
This study also demonstrated that PIN-protected USSD systems are feasible and safe for delivering sensitive health information to patients with cell phones in South Africa. Although one study from Uganda suggested PINs deter patients from accessing mHealth messages [29], more than 80% of South African patients accessed their messages using a PIN in both this study and another by Maraba et al. [23]. Furthermore, no participants reported any unintentional disclosures of HIV or TB status in this study, the Maraba study, or the WelTel mHealth program in Kenya, which used HIV-neutral language without a PIN [30]. In comparison, an SMS-based mHealth program without PINs in South Africa reported 3% (n = 3) of participants having unintentional HIV disclosures [31]. Unlike SMS, USSD systems can be configured to require a PIN and do not store messages directly on patient mobile phones. Therefore, future mHealth programs should consider USSD systems, which can more reliably transmit sensitive information than SMS.
MatlaMobile and other South African mHealth programs, like MomConnect, have also been highly acceptable to patients, especially for their convenience compared to the standard-of-care [23, 32]. Nearly all MatlaMobile participants (96.9%) reported they preferred to receive results via mobile phone than at clinic. These findings are further reinforced by the Maraba study, where participants described how mHealth prevents unnecessary waiting in queues [23]. Participants in the MomConnect program also emphasized that mHealth fills a gap for rural communities that lack access to in-person services. Together, these results suggest that mHealth programs can deliver relevant health information, reduce unnecessary patient burden, and relieve clinic patient volumes. If an mHealth program like MatlaMobile is implemented in South Africa, however, researchers should evaluate the potential downsides of mHealth programs reducing face-to-face healthcare encounters among patients with non-actionable laboratory test results.
Finally, previous work demonstrated that nurses and patients in this specific region of South Africa are open to using mHealth for routine care [33]. Rollout, however, will require intensive patient education and engagement. For example, although nearly 90% of HIV patients in South Africa reported preferring two-way over one-way telephonic communication with healthcare providers, a minority of our and MomConnect participants requested call-me-backs [31, 34, 35]. MatlaMobile participants may not have realized there was a call-me-back service. Patient education or SMS reminders about the call-me-back feature, as done by MomConnect, could improve uptake [35]. It is also possible that the system to request nurse call-me-backs was not easy to use. Several participants unintentionally requested nurse call-me-backs, and the lowest scoring item on the satisfaction scale was, “It was easy to use MatlaMobile to connect to [the…] clinic.” This, in combination with the fact that fewer than a third of patients viewed the full USSD message of their result, suggest that the MatlaMobile interface could be improved.
For example, future iterations should include messages that sufficiently motivate patients to return to clinic. mHealth interventions from this and the Maraba study yielded low patient return rates, despite systematic reviews demonstrating that mHealth interventions can improve adherence to health appointments and medications [27, 36, 37]. Messages may have been overly complex, since half of our nurse call-me-back requests were for help interpreting laboratory results, or inaccessible, since the target population did not contribute to writing the messages before implementing the intervention. Instead, MatlaMobile messages were designed by clinicians and revised by multiple staff members familiar with the target population. Despite this weakness, MatlaMobile offered colloquial messages in four languages each time participants logged in, whereas MomConnect only allowed users to use one language, picked at enrollment [32]. To optimize MatlaMobile, future work should examine the details of how participants engaged with the system (e.g., signing in multiple times) and work closely with patients to craft persuasive, easy-to-understand messages.
Our study has limitations. Clinics in this region primarily used paper medical records to document clinic visits. Clinic staff, who sometimes misplaced records, also did not always note when patients returned to clinic to retrieve laboratory results. This was especially true of patients whose GeneXpert tests were negative for TB. Therefore, it may be possible that our outcome data are incomplete. To mitigate these risks, we hired trained HIV counselors familiar with the systems for filing medical records and made at least two attempts across multiple sources to retrieve each participant’s record. Our study may also contain secular biases because we recruited study arms sequentially. We believe biases are minimal because we recruited participants during similar seasons and there were no major health policy changes during the study. Additionally, our telephonic acceptability survey results could include social desirability bias. The quantitative results presented here, however, align with a forthcoming analysis of qualitative interviews exploring perceptions about MatlaMobile. Self-reported data are often limited, especially for identifying breaches of confidentiality, as incidents may have occurred without the participants’ knowledge.
Conclusion
Results from MatlaMobile, though promising, will require further implementation research, buy-in from stakeholders, strong long-term governmental commitments, and financial investments to scale the program nationwide [38,39,40]. Key considerations for scaling include: patterns of SIM card and phone ownership in South Africa; potential supply chain impacts due to increased demand for ART or TB treatment; and importantly, interoperability with laboratory databases and future electronic medical records [36, 41, 42]. Our data suggest a USSD system is an inclusive solution to deliver results directly to patients with most types of cell phone. Therefore, a more comprehensive trial is needed and should include an integrated version of MatlaMobile where laboratory results with improved motivational messages are automatically sent to patients [23]. Providing patients direct access to their health information can promote valuable knowledge and engagement in care.
References
World Health Organization. Global tuberculosis report. Geneva, Switzerland; 2019. https://www.who.int/tb/publications/global_report/tb19_Report_country_profiles_15October2019.pdf
UNAIDS Data 2018. Geneva, Switzerland; 2018. https://www.unaids.org/sites/default/files/media_asset/unaids-data-2018_en.pdf
Republic of South Africa. Act No. 61: National Health Act. South Africa; 2003.
The 2017 update, Global Health Workforce Statistics. World Health Organization. Geneva, Switzerland; [cited 2019 Apr 13]. Available from: https://www.who.int/hrh/statistics/hwfstats/en/
Breier, M. The Shortage of Medical Doctors in South Africa. (2008).
Takuva S, Brown A, Macleod W, et al. Disparities in Engagement Within HIV Care in South Africa. In: Conference on Retroviruses and Opportunistic Infections. Seattle, WA; 2015. Available from: https://www.croiconference.org/sessions/disparities-engagement-within-hiv-care-south-africa
Mametja D. Plenary Talk. In: Fifth Annual South African Tuberculosis Conference. Durban, South Africa; 2018.
Meintjes G, Moorhouse MA, Carmona S, et al. Adult antiretroviral therapy guidelines 2017. South Afr J HIV Med. 2017;18(1)
Stime KJ, Garrett N, Sookrajh Y, et al. Clinic flow for STI, HIV, and TB patients in an urban infectious disease clinic offering point-of-care testing services in Durban, South Africa. BMC Health Serv Res. 2018;18(1):363.
Wouters E, Heunis C, van Rensburg D, et al. Patient satisfaction with antiretroviral services at primary health-care facilities in the Free State, South Africa—a two-year study using four waves of cross-sectional data. BMC Health Serv Res. 2008;8(1):210.
Maughan-Brown B, Kuo C, Galárraga O, et al. Stumbling Blocks at the Clinic: Experiences of Seeking HIV Treatment and Care in South Africa. AIDS Behav. 2018;22(3):765–73.
Mudzengi D, Sweeney S, Hippner P, et al. The patient costs of care for those with TB and HIV: a cross-sectional study from South Africa. Health Policy Plan. 2017;32(suppl_4):iv48–56.
UNAIDS. Country Factsheets South Africa. 2017 [cited 2019 Apr 13]. Available from: https://www.unaids.org/en/regionscountries/countries/southafrica
Ngangue P, Bedard E, Zomahoun HTV, et al. Returning for HIV test results: a systematic review of barriers and facilitators. Int Sch Res Notes. 2016;2016:1–24.
Meehan S-A, Sloot R, Draper HR, et al. Factors associated with linkage to HIV care and TB treatment at community-based HIV testing services in Cape Town, South Africa. PLoS ONE. 2018;13(4):e0195208.
Cole-Lewis H, Kershaw T. Text Messaging as a tool for behavior change in disease prevention and management. Epidemiol Rev. 2010;32(1):56–69.
van Heerden AC, Norris SA, Richter LM. Using mobile phones for adolescent research in low and middle income countries: preliminary findings from the Birth to Twenty Cohort. South Africa J Adolesc Heal. 2010;46(3):302–4.
Clouse K, Schwartz SR, Van Rie A, et al. High mobile phone ownership, but low Internet and email usage among pregnant, HIV-infected women attending antenatal care in Johannesburg. J Telemed Telecare. 2015;21(2):104–7.
Crankshaw T, Corless IB, Giddy J, et al. Exploring the patterns of use and the feasibility of using cellular phones for clinic appointment reminders and adherence messages in an antiretroviral treatment clinic, Durban, South Africa. AIDS Patient Care STDS. 2010;24(11):729–34.
Silver L & Johnson C. Internet Connectivity Seen as Having Positive Impact on Life in Sub-Saharan Africa: But Digital Divides Still Persist. 2018. Available from: https://www.pewresearch.org/global/2018/10/09/internet-connectivity-seen-as-having-positive-impact-on-life-in-sub-saharan-africa/
Mills EJ, Lester R, Thorlund K, et al. Interventions to promote adherence to antiretroviral therapy in Africa: a network meta-analysis. Lancet HIV. 2014;1(3):e104–e111111.
Kemp CG, Velloza J. Implementation of eHealth interventions across the HIV care cascade: a review of recent research. Curr HIV/AIDS Rep. 2018;15(6):403–13.
Maraba N, Hoffmann CJ, Chihota VN, et al. Using mHealth to improve tuberculosis case identification and treatment initiation in South Africa: Results from a pilot study. PLoS ONE. 2018;13(7):e0199687.
Georgette N, Siedner MJ, Petty CR, et al. Impact of a clinical program using weekly Short Message Service (SMS) on antiretroviral therapy adherence support in South Africa: a retrospective cohort study. BMC Med Inform Decis Mak. 2017;17(1):18.
Nachega J, Skinner D, Jennings L, et al. Acceptability and feasibility of mHealth and community-based directly observed antiretroviral therapy to prevent mother-to-child HIV transmission in South African pregnant women under Option B+: an exploratory study. Patient Prefer Adherence. 2016 Apr;683. Available from: https://www.dovepress.com/acceptability-and-feasibility-of-mhealth-and-community-based-directly--peer-reviewed-article-PPA
StataCorp. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP; 2015.
Venter W, Coleman J, Chan VL, et al. Improving linkage to HIV care through mobile phone apps: randomized controlled trial. JMIR mHealth uHealth. 2018;6(7):e155.
Anstey Watkins JOT, Goudge J, Gómez-Olivé FX, et al. Mobile phone use among patients and health workers to enhance primary healthcare: a qualitative study in rural South Africa. Soc Sci Med. 2018;198:139–47.
Siedner MJ, Santorino D, Haberer JE, et al. Know your audience: predictors of success for a patient-centered texting app to augment linkage to HIV care in rural Uganda. J Med Internet Res. 2015;17(3):e78.
Lester RT, Ritvo P, Mills EJ, et al. Effects of a mobile phone short message service on antiretroviral treatment adherence in Kenya (WelTel Kenya1): a randomised trial. Lancet. 2010;376(9755):1838–45.
Georgette N, Siedner MJ, Zanoni B, et al. The acceptability and perceived usefulness of a weekly clinical SMS program to promote HIV antiretroviral medication adherence in KwaZulu-Natal, South Africa. AIDS Behav. 2016;20(11):2629–38.
Skinner D, Delobelle P, Pappin M, et al. User assessments and the use of information from MomConnect, a mobile phone text-based information service, by pregnant women and new mothers in South Africa. BMJ Glob Health. 2018;3(Suppl 2):e000561.
DiAndreth L, Krishnan N, Elf JL, et al. Formative research for an mHealth program to improve the HIV care continuum in South Africa. AIDS Care. 2019. https://doi.org/10.1080/09540121.2019.1640850.
Barron P, Peter J, LeFevre AE, Sebidi J, et al. Mobile health messaging service and helpdesk for South African mothers (MomConnect): history, successes and challenges. BMJ Glob Health. 2018;3(Suppl 2):e000559.
Fischer AE, Sebidi J, Barron P, et al. The MomConnect nurses and midwives support platform (NurseConnect): a qualitative process evaluation. JMIR mHealth uHealth. 2019;7(2):e11644.
Cooper V, Clatworthy J, Whetham J, et al. mHealth interventions to support self-management in HIV: a systematic review. Open AIDS J. 2017;11(1):119–32.
Horvath T, Azman H, Kennedy GE, et al. Mobile phone text messaging for promoting adherence to antiretroviral therapy in patients with HIV infection. Cochrane Database Syst Rev. 2012. https://doi.org/10.1002/14651858.CD009756.
Seebregts C, Dane P, Parsons AN, et al. Designing for scale: optimising the health information system architecture for mobile maternal health messaging in South Africa (MomConnect). BMJ Glob Heal. 2018;3(Suppl 2):e000563.
Labrique AB, Wadhwani C, Williams KA, et al. Best practices in scaling digital health in low and middle income countries. Glob Health. 2018;14(1):103.
Chib A, van Velthoven MH, Car J. mHealth adoption in low-resource environments: a review of the use of mobile healthcare in developing countries. J Health Commun. 2015;20(1):4–34.
Barron P, Pillay Y, Fernandes A, et al. The MomConnect mHealth initiative in South Africa: early impact on the supply side of MCH services. J Public Health Policy. 2016;37(S2):201–12.
Jarrett B, Kabasia J, Sekwele M, et al. Acceptability of a comprehensive clinic-based intervention to increase health care provider prescription of isoniazid preventive therapy in South Africa. In: The 48th Union World Conference on Lung Health. Guadalajara, Mexico; 2017
Acknowledgments
The authors thank the study participants who volunteered their time, the North West Provincial Department of Health for hosting the study, and the study staff. This study was partially funded by 13 student fellowships through the Global Established Multidisciplinary Sites (GEMS) program at the Johns Hopkins Center for Global Health.
Author information
Authors and Affiliations
Contributions
All authors contributed to the design, planning, and implementation of the study. LD, BAJ, JLE, JEG, and NAM analyzed the data. All authors contributed to the writing and review of the manuscript and approve this version.
Corresponding author
Ethics declarations
Conflict of interest
No conflicts of interest declared.
Ethical Approval
All authors are accountable for all aspects of the work, including investigating and resolving any questions related to its accuracy or integrity.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
DiAndreth, L., Jarrett, B.A., Elf, J.L. et al. Secure Delivery of HIV-Related and Tuberculosis Laboratory Results to Patient Cell Phones: A Pilot Comparative Study. AIDS Behav 24, 3511–3521 (2020). https://doi.org/10.1007/s10461-020-02912-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-020-02912-3