Abstract
We recently reported that a 12-week internet weight loss program produced greater weight losses than education control in overweight/obese people living with HIV (PLWH) (4.4 kg vs 1.0 kg; p < 0.05). This manuscript presents the changes in diet, physical activity, behavioral strategies, and cardio-metabolic parameters. Participants (N = 40; 21 males, 19 females) were randomly assigned to an internet behavioral weight loss (WT LOSS) program or internet education control (CONTROL) and assessed before and after the 12-week program. Compared to CONTROL, the WT LOSS arm reported greater use of behavioral strategies, decreases in intake (− 681 kcal/day; p = 0.002), modest, non-significant, increases in daily steps (+ 1079 steps/day) and improvements on the Healthy Eating Index. There were no significant effects on cardio-metabolic parameters. The study suggests that a behavioral weight loss program increases the use of behavioral strategies and modestly improves dietary intake and physical activity in PLWH. Further studies with larger sample sizes and longer follow-up are needed.
Clinical Trials Registration: NCT02421406.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
With the recent improvements in the treatment of HIV, individuals with this disease are now living longer and facing new challenges. Whereas concerns about wasting and lipodystrophy, or abnormal fat distribution, are less common, people living with HIV (PLWH) now struggle with obesity and obesity-related health problems, such as diabetes and heart disease [1,2,3,4]. The rates of obesity in PLWH in the US now parallel those seen in the general population [5] and the rates of diabetes and heart disease exceed those seen in adults without HIV [6,7,8,9,10]. This may be attributable to findings that many PLWH consume diets of poor quality [11] and have low levels of physical activity [12, 13], both of which contribute to the development of obesity and its comorbidities. Consequently, there is a great need to develop lifestyle interventions that improve dietary intake, increase physical activity and reduce cardio-metabolic risk in PLWH.
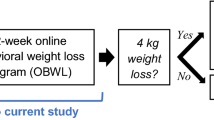
We recently reported positive weight loss outcomes in a randomized trial comparing an internet weight loss program with an internet education control for overweight/obese patients with HIV [14.] The intervention group had an average weight loss of 4.4 kg (SD = 5.4) over the 12-week program, compared to 1.0 kg (SD = 3.3) in the control condition. However, in addition to producing weight loss, an effective lifestyle intervention should also produce beneficial changes in diet, physical activity, and cardiovascular risk factors since these factors can improve overall health, independent of changes in body weight. Thus, the purpose of this paper is to examine these other outcomes within the context of this randomized trial.
Methods
Participants
Participants for this trial were recruited at the outpatient clinic of the Immunology Center at The Miriam Hospital. To be eligible, individuals needed to have HIV treated with ART, an undetectable viral load, CD4 count > 200 cells/µl, age 18–70 and BMI ≥ 27 kg/m [2]. Individuals were ineligible if they had health problems that might make the program unsafe or affect participation in the program (e.g. active substance use, currently or planning to become pregnant). Individuals receiving treatment for diabetes, hypertension, or lipid disorders were eligible and there were no eligibility criteria related to current activity level or type or duration of HIV medication.
Study Design
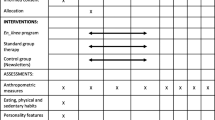
The study is registered on clinicaltrials.gov (NCT02421406) and has been described in detail [14]. In brief, those participants who met the basic eligibility criteria provided written informed consent as approved by the Lifespan Institutional Review Board. Subsequently, they were randomized in a 1:1 ratio to a 12-week internet weight loss program (WT LOSS) or internet control condition (CONTROL). All assessments were repeated after 12 weeks in the program.
Interventions
Both interventions received 12 lessons via the internet. CONTROL participants received a weekly education lesson that provided basic information about diet, exercise, and weight loss. Lessons were posted on the study website and included no behavior change strategies. WT LOSS received 12 interactive multi-media lessons that targeted behavioral strategies for changing diet and activity to produce weight loss. A low-calorie, low-fat diet was prescribed (1200–1500 for those weighing < 200 lbs.; 1500–1800 for those weighing > 200 lbs.) with < 30% of calories from fat). The physical activity goal stressed 30 min of brisk walking daily. Each week, participants viewed a new lesson, submitted their daily weights, calorie and fat intake, and minutes of physical activity to the study website. A weekly automated message provided feedback to the participant on these variables (these weekly self-report measures were used as the basis for providing immediate feedback to WT LOSS participants and are considered part of the intervention; changes from baseline to end of the 12-week intervention were assessed in both groups with the measures described below). Both the WT LOSS and CONTROL intervention have been used in a prior study of weight loss in obese individuals who did not have HIV [15.]
Measures
Weight and BMI
Weight was measured to the nearest 0.1 kg at baseline and the end of the 12-week intervention using a calibrated digital scale. Standing height was measured in millimeters using a wall-mounted stadiometer and used to compute body mass index.
Use of Weight Control Strategies
Participants completed the Weight Control Strategies questionnaire, a self-report measure assessing frequency of use of 30 behavioral strategies (1 = never; 2 = occasionally; 3 = about half the time; 4 = most of the time; 5 = always). A total score and scores on the subscales for use of diet, self-monitoring, physical activity, and psychological coping strategies were calculated. This questionnaire assesses use of the key behavioral strategies taught in standard programs and higher scores on this questionnaire have been related to better weight loss outcomes [16].
Dietary Intake
To assess change in dietary intake, participants kept three-day food diaries (2 weekdays and 1 weekend day) at baseline and post-intervention. Study staff provided participants with a food diary and portion size guide and instructed them to record all foods and beverages consumed as well as the preparation, brand names and portion sizes for each at the time of consumption. With proper instruction, food records have been shown to provide an accurate record [17]. After completion, the records were reviewed by a registered dietitian, and participants were called to clarify any missing information. The three-day diet records were coded using Nutrition Data Systems for Research (NDSR, Nutrition Coordinating Center, University of Minnesota, 2016). NDSR output files were used to determine total energy intake, percent energy from each macronutrient, and diet quality, as measured by the 2015 Healthy Eating Index (HEI-2015) [18]. The HEI-2015, calculated using SAS code developed by the Nutrition Coordinating Center, measures adherence to the US Dietary Guidelines and ranges from 0 to 100, with higher scores representing healthier diets.
Physical Activity
Participants were instructed to wear the previously validated Sensewear Armband (Pittsburgh, PA) until their next visit (scheduled after approximately 1 week) at baseline and post-intervention [19, 20]. Data from this device are processed using propriety algorithms incorporated within the software (Sensewear 7.0) and metabolic equivalents (MET values) are provided for each minute the device is worn. Daily steps and time spent in moderate-to-vigorous intensity PA (MVPA, ≥ 3 METs) and bout-related MVPA (≥ 3 METs for ≥ 10 min) were computed. Only participants who wore the device for ≥ 8 h/day on ≥ 4 days were included in the analyses (n = 36).
Cardio-Metabolic Parameters
Blood pressure was measured in duplicate at baseline and after the 12-week program using a Sphygmomanometer Wall Aneroid, model 0950 (W.A. Baum Co., Inc., Copiague, NY) with an adult calibrated V-Lok cuff. Fasting blood samples were taken for assessment of glucose, insulin, HbA1c, HOMA-IR (calculated), triglycerides, total cholesterol, HDL-cholesterol, and LDL-C (calculated).
Statistical Analyses
Repeated measure analyses of variance were used to compare WT LOSS and CONTROL on changes in cardiometabolic risk factors, the use of weight control strategies, dietary intake, and physical activity after adjusting for age. We considered both the Treatment X Time interaction and the changes within each arm over time. Nominal p-values are presented. Log transformations were used for skewed variables (including measures of physical activity, insulin and triglycerides).
Results
The baseline characteristics of the 40 participants (21 males; 19 females) who entered the trial are shown in Table 1. On average [mean ± SD], these participants were 49.9 ± 8.8 years of age and had a BMI of 34.2 ± 6.7. On average, they had HIV for 11.9 ± 6.4 years and had a CD4 count of 743 ± 339 cells/uL at baseline. One of the 20 participants randomized to CONTROL and 2 of the 20 randomized to WT LOSS did not complete the study. The only significant baseline difference between groups was age (46.3 ± 9.8, ranging from 30.4 to 66.3 years. in WT LOSS vs 53.6 ± 6.0, ranging from 41.9 to 67.6 years. in CONTROL (p < 0.01).
Weight Changes
We previously reported the weight losses achieved [14,] but we provide them here for context in interpreting the other findings. On average participants in the WT LOSS arm lost 4.4 ± 5.4 kg over the 12-week program, compared to 1.0 ± 3.3 kg in CONTROL. Results in terms of percent of body weight lost were very similar (4.5 ± 5.8% vs 1.1 ± 3.3%).
Weight Control Strategies
Table 2 presents the use of weight control strategies at baseline and 12 weeks in WT LOSS vs CONTROL. Compared to CONTROL participants, those in the WT LOSS arm reported significantly greater increases in the use of strategies related to physical activity, psychological coping, and self-monitoring. They also reported greater overall (total) use of the strategies taught in the program. Both groups reported greater use of diet strategies at the end of the program, relative to when they entered the program; strategies that increased in both arms included having several servings of fruit and vegetables each day, avoiding fried foods, and eating lower fat meats.
Dietary Intake
The WT LOSS arm decreased their intake from 2198 ± 704 kcal/day at baseline to 1517 ± 491 kcal/day at 12 weeks (p = 0.002), whereas the decrease in CONTROL was not significant, 1847 ± 868 to 1732 ± 539 kcal/day, (p = 0.35). The difference in caloric reduction between the two arms was not statistically significant (− 681 ± 407 kcal/day in WT LOSS vs 216 ± 591 kcal/day in CONTROL, p = 0.13). The macronutrient distribution was similar in the two conditions at baseline (35% fat, 17% protein, and 48% carbohydrates) and did not change significantly over time. Diet quality (Table 3), assessed using the HEI, improved in both groups over time, but the changes were not statistically significant. Among the component scores of the HEI 2015, only the change in Greens and Beans score significantly differed across groups (0.9 increase in WT LOSS vs a − 0.5 decrease in CONTROL, p = 0.05).
Physical Activity
On average, participants wore the armband for 21.3 ± 0.9 h/day and 8.4 ± 1.6 days at baseline and 20.0 ± 2.1 h/day and 8.2 ± 1.6 days at follow-up. Wear time did not differ between CONTROL and WT LOSS (p’s > 0.26). The increase in total minutes per day of moderate-to-vigorous physical activity (WT LOSS: 9.4 ± 42.4 vs CONTROL: 3.2 ± 5.5; p = 0.87) or MVPA in 10-minute bouts (WT LOSS: 10.6 ± 31.4 vs CONTROL: 3.5 ± 12.5; p = 0.90) did not differ between groups. Participants in the WT LOSS group increased their daily steps from 5311 ± 2583 to 6390 ± 3548, an increase of 1079 ± 2321 steps/day whereas the CONTROL group decreased their daily steps from 4906 ± 2159 to 4668 ± 2482, a decrease of 238 ± 1509 steps/day. Analyses of log transformed data showed that the difference between groups for change in daily steps was significant at p = 0.03, but it was no longer significant (p = 0.08) after adjusting for age and wear time.
Cardio-Metabolic Parameters
As shown in Table 4, there were no significant differences between WT LOSS and CONTROL for changes in blood pressure or any of the cardio-metabolic outcomes over the 12-week program.
Discussion
Findings from this study suggest that a 12-week internet behavioral weight loss program, relative to a 12-week control program, was effective in producing a 4 kg weight loss and also helped overweight/obese PLWH increase their use of healthy weight control strategies, reduce their caloric intake, and increase their physical activity. However, the lifestyle intervention did not lead to significant improvements in laboratory measures of cardiovascular risk factors.
Participants in the WT LOSS arm reported significantly greater increases than the CONTROL group in the use of behavioral strategies related to self-monitoring, physical activity, and psychological coping. These domains reflect specific strategies that were taught only in the weight loss program, suggesting that participants in this group were adhering to the program recommendations. Since both groups received basic education related to healthy eating, it is not surprising that both groups reported increased use of low calorie, low-fat diet strategies. The changes reported in the use of these behavioral strategies, if maintained over time, should help improve long-term weight loss outcomes. Moreover, many of these changes, such as eating more high fiber foods, exercising for 30 min/day, and rewarding oneself for meeting goals, might also be expected to impact cardiovascular disease (CVD) risk factors.
Data from the 3-day food records suggest that the WT LOSS group reduced their daily energy intake by almost 700 calories a day whereas the CONTROL group reduced their intake by only 200 calories per day. The decrease in caloric intake in the WT LOSS group suggests that these participants adhered to the calorie restriction prescription. However, despite recommendations to decrease fat intake to < 30% of total energy to support a low calorie, low fat diet, participants in the WT LOSS arm did not change the macronutrient distribution of their diet. The total HEI showed nonsignificant improvements over time, with the intervention group changing from a total HEI of 47.9 to 53.2 and the CONTROL group from 46.7 to 51. Recent data from the National Health and Nutrition Examination Survey suggest that the mean HEI-2015 score for American adults ages 18–64 is 58.3 [21], suggesting that this population’s overall diet quality is still below the national average. However, the fact that HEI improved through a behavioral weight control intervention is consistent with several other studies [22,23,24]. We are only aware of one randomized trial of a lifestyle behavioral intervention that targeted the HEI in PLWH along with increased physical activity [25]. This study found no significant improvements in HEI or accelerometry measured physical activity, although the intervention group did lose significantly more weight than the control group (− 0.732 kg vs + 0.153, p = 0.03). Further effort is needed to better understand the barriers to improving healthy eating in this population.
There have been a fair number of studies showing positive effects of supervised exercise sessions on fitness, body composition and quality of life in PLWH [26, 27]. There are far fewer studies of lifestyle physical activity in this population [28], and thus we have less information available on the extent to which this population will increase their physical activity on their own. Fitch et al. [28] tested a lifestyle intervention that focused on healthy eating and physical activity. Like the current study, these investigators recommended increased walking with a goal of 10,000 steps/day and 3 h of activity per week and participants completed the activity on their own. Greater increases in self-reported physical activity were seen in the intervention group than in the control group over the 6-month study, but no differences were observed for VO2 max or lipid levels. These findings are in contrast to the current study which found that the changes in MVPA minutes did not differ significantly between WT LOSS and CONTROL [29]. Shah et al. [29] tested a physical activity intervention based on self-determination in older PLWH and found significant increases in self-reported activity and improved physical function, but that study did not focus on weight loss.
The present study is one of the first to examine changes in lifestyle physical activity using an objective measure of physical activity in PLWH. While the change in bout-related MVPA minutes did not significantly differ between conditions, the WT LOSS group increased their bout-related MVPA by 10.6 min/day whereas the CONTROL group increased by 3.5 min/day. This is equivalent to a 49 min/week difference in the change in bout-related MVPA between groups, suggesting that the use of the PA-related behavioral strategies may have contributed to the adoption of more PA over time among WT LOSS participants. Likewise, we found that on average, participants in the WT LOSS group increased the number of steps/day by 1000 steps, equivalent to a half mile, whereas the CONTROL group had a slight decrease in daily steps. In order to place these changes in context, we note that the participants in our study were achieving about 5000 steps per day at baseline (< 5000 steps/day is considered a sedentary lifestyle; 5000–7499 “low active”, 7500–9999 “somewhat active” and 10,000 steps is “active”) [30.] Although the increase we found, from 5000 to 6000 steps per day, is important, on average participants in the WT LOSS arm continued to be in the Low Active category. These results contrast with data reported using the same type of armbands in a weight loss study of over 200 obese patients who did not have HIV [31]. In that trial, the average number of steps increased from 7500 at baseline to almost 10,000 steps per day. These data suggest that PLWH may have particularly low levels of physical activity, a suggestion confirmed by other studies [12, 13], and that the internet program may produce only modest increases in physical activity in this population.
Related to the increase in objectively measured physical activity, we also found significant increases in the use of behavioral strategies to increase activity in the WT LOSS group relative to the CONTROL. These strategies included setting goals for activity, planning when to be active, and using stimulus control strategies (such as putting out exercise clothes) to prompt physical activity.
In the present study, we found no evidence of significant changes in cardio-metabolic measures in WT LOSS relative to the CONTROL group. There are several possible explanations for this finding. First, the magnitude of weight loss may have been inadequate to observe changes in these parameters. Studies in individuals who are not HIV-infected suggest that a 3–5% weight loss is sufficient to improve blood pressure, but a 10–15% weight loss may be needed for lipids [32]. In the present study, those patients who achieved a 5% weight loss did experience significantly greater improvement in systolic blood pressure relative to those who had poorer weight loss outcomes, with no difference in lipids or other physiological parameters (data not presented); thus these findings support the hypothesis that those randomized to the weight loss intervention would have had to achieve a greater average weight loss in order to see beneficial health effects. The 12-week intervention may also have been too short and the sample size too small to observe changes in these parameters.
Alternatively, the HIV infection itself and the inflammation associated with it, may blunt the effect of weight loss on these parameters. This suggestion is supported by an earlier study of the effects of weight loss on metabolic and cardiovascular outcomes in PLWH. Engelson et al. [33] evaluated changes in 18 obese women with HIV who completed a 12-week weight loss program (out of 31 who started the program). The women exercised in three supervised exercise sessions per week (each session lasting 90 min) and were placed on a 1200 cal, 30% fat diet. On average, the 18 completers lost 6.7 kg (7.3%) and showed improvements in body composition. However, there was no evidence of improved insulin sensitivity or changes in lipids with this weight loss. Thus, these authors concluded that the beneficial effects of weight loss on CVD may be less in those with HIV. However, the findings may also be due to the older ART treatment regimens used in this study. To specifically address the question of whether the benefits of weight loss are blunted in PLWH, Reeds and colleagues [34] studied 19 women (16 PLWH and 6 not PLWH) before and after they achieved a 6–8% weight loss (mean of 7.7% vs 7.3%, respectively.) It took significantly longer for PLWH participants to achieve this goal (18 vs 12 weeks; p = 0.006). Weight loss caused a greater decline in fat free mass in PLWH but led to similar significant improvements in insulin sensitivity and blood pressure in both groups of women. Glucose levels, LDL-C and triglycerides did not change significantly in either group, and HDL-C decreased significantly in both. Further research with larger sample sizes and a more intensive behavioral weight loss program is needed to determine whether PLWH are able to achieve similar amounts of weight loss and whether both groups benefit comparably from these weight losses.
Strengths of this study include the randomized trial design, the high retention of participants, and the use of state-of-the-art measures of diet and physical activity. Moreover, in contrast to the past studies of the effects of supervised exercise and studies that followed only participants who achieved a specific amount of weight loss, we sought to determine the efficacy of randomization to a lifestyle intervention. As part of this intervention, patients were taught behavioral strategies for changing diet and exercise to produce weight loss but were required to make these changes on their own and data are presented for the full group of participants, regardless of the magnitude of weight loss achieved. Use of the internet program, which can be easily disseminated to large numbers of patients, is also an important strength.
There are several important limitations to this study. The sample size was small and included individuals with a variety of health conditions (e.g. diabetes) and medication regimens; changes in medication during the program were not assessed. Although all analyses adjusted for age, there were significant differences between the two groups for age, which may have affected outcomes. A larger study is needed to more precisely determine the effects of weight loss and patient characteristics on changes in CVD risk factors. In addition, the study was short and thus we could not examine the maintenance of the effects. The WT LOSS and CONTROL interventions were not tailored to those with HIV and did not address specific barriers that PLWH might face in adhering to a weight loss program (e.g. food insecurity).
Conclusions
Our data suggest that PLWH who are assigned to an internet WT LOSS program, relative to those in CONTROL, made significant improvements in their use of behavioral strategies and in calorie intake. Improvements in physical activity were more limited and there were no significant effects on laboratory cardiovascular risk factors. Further research with a greater number of participants and a more intensive and longer weight loss program is needed to understand the extent to which PLWH benefit from behavioral weight loss interventions and to examine the factors that may limit the improvements in behavior and CVD risk factors seen in this population.
References
Mankal PK, Kotler DP. From wasting to obesity, changes in nutritional concerns in HIV/AIDS. Endocrinol Metab Clin North Am. 2014;43(3):647–63.
Amorosa V, Synnestvedt M, Gross R, et al. A tale of 2 epidemics: the intersection between obesity and HIV infection in Philadelphia. J Acquir Immune Defic Syndr. 2005;39(5):557–61.
Crum-Cianflone N, Tejidor R, Medina S, Barahona I, Ganesan A. Obesity among patients with HIV: the latest epidemic. AIDS Patient Care STDS. 2008;22(12):925–30.
Tate T, Willig AL, Willig JH, et al. HIV infection and obesity: where did all the wasting go? Antivir Ther. 2012;17(7):1281–9.
Thompson-Paul AM, Wei SC, Mattson CL, et al. Obesity among HIV-infected adults receiving medical care in the United States: data from Cross-Sectional Medical Monitoring Project and National Health and Nutrition Examination Survey. Medicine. 2015;94(27):e1081.
Koethe JR, Grome H, Jenkins CA, Kalams SA, Sterling TR. The metabolic and cardiovascular consequences of obesity in persons with HIV on long-term antiretroviral therapy. AIDS. 2016;30(1):83–91.
Koethe JR, Jenkins CA, Lau B, et al. rising obesity prevalence and weight gain among adults starting antiretroviral therapy in the United States and Canada. AIDS Res Hum Retrovir. 2016;32(1):50–8.
De Wit S, Sabin CA, Weber R, et al. Incidence and risk factors for new-onset diabetes in HIV-infected patients: the Data Collection on Adverse Events of Anti-HIV Drugs (D:A:D) study. Diabetes Care. 2008;31(6):1224–9.
Butt AA, McGinnis K, Rodriguez-Barradas MC, et al. HIV infection and the risk of diabetes mellitus. AIDS. 2009;23(10):1227–34.
Capeau J, Bouteloup V, Katlama C, et al. Ten-year diabetes incidence in 1046 HIV-infected patients started on a combination antiretroviral treatment. AIDS. 2012;26(3):303–14.
Duran AC, Almeida LB, Segurado AA, Jaime PC. Diet quality of persons living with HIV/AIDS on highly active antiretroviral therapy. J Hum Nutr Diet. 2008;21(4):346–50.
Vancampfort D, Mugisha J, De Hert M, et al. Global physical activity levels among people living with HIV: a systematic review and meta-analysis. Disabil Rehabil. 2016;40:1–10.
Vancampfort D, Mugisha J, De Hert M, Probst M, Stubbs B. Sedentary behavior in people living with HIV: a systematic review and meta-analysis. J Phys Act Health. 2017;14(7):571–7.
Becofsky K, Wing EJ, McCaffery J, Bodreau M, Wing RR. A Randomized, controlled trial of a behavioral weight loss program for HIV-infected patients. Clin Infect Dis. 2017;65(1):154–7.
Thomas JG, Leahey TM, Wing RR. An automated internet behavioral weight-loss program by physician referral: a randomized controlled trial. Diabetes Care. 2015;38(1):9–15.
Pinto AM, Fava JL, Raynor HA, LaRose JG, Wing RR. Development and validation of the weight control strategies scale. Obesity (Silver Spring). 2013;21(12):2429–36.
Thompson F, Subar A. Dietary assessment methodology. In: Coulston A, Boushey C, Ferruzz M, editors. Nutrition in the prevention and treatment of disease. 3rd ed. London: Elsevier; 2013.
Krebs-Smith SM. Health Eating Index 2015. J Acad Nutr Diet. 2018;118:1622.
Papazoglou D, Augello G, Tagliaferri M, et al. Evaluation of a multisensor armband in estimating energy expenditure in obese individuals. Obesity. 2006;14(12):2217–23.
Jakicic JM, Marcus M, Gallagher KI, et al. Evaluation of the SenseWear Pro Armband to assess energy expenditure during exercise. Med Sci Sports Exerc. 2004;36(5):897–904.
Guenther PM, Casavale KO, Reedy J, et al. Update of the Healthy Eating Index: HEI-2010. J Acad Nutr Diet. 2013;113(4):569–80.
Ambrosini GL, Solis-Trapala I, Ahern AL, et al. Greater improvements in diet quality among overweight participants following a group-based commercial weight loss programme than those receiving support to lose weight in primary care. Nutr J. 2018;17(1):64.
O’Brien KM, Hutchesson MJ, Jensen M, Morgan P, Callister R, Collins CE. Participants in an online weight loss program can improve diet quality during weight loss: a randomized controlled trial. Nutr J. 2014;13(1):82.
Anderson C, Harrigan M, George SM, et al. Changes in diet quality in a randomized weight loss trial in breast cancer survivors: the lifestyle, exercise, and nutrition (LEAN) study. NPJ Breast Cancer. 2016;2:16026.
Webel AR, Moore SM. Randomized controlled trial of the systemCHANGE intervention on behaviors related to cardiovascular risk in HIV + adults. J Acquir Immune Defic Syndr. 2018;78(1):23–33.
Gomes NCC, et al. Effects of combined aerobic and resistance exercise on exercise capacity, muscle strength and quality of life in HIV-infected patients: a systematic review and meta-analysis. PLoS ONE. 2015;10(9):e0138066.
O’Brien KK, Tynan AM, Nixon SA, Glazier RH. Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol. BMC Infect Dis. 2016;16:182.
Fitch KV, Anderson EJ, Hubbard JL, et al. Effects of a lifestyle modification program in HIV-infected patients with the metabolic syndrome. AIDS. 2006;20(14):1843–50.
Shah KN, Majeed Z, Yoruk YB, et al. Enhancing physical function in HIV-infected older adults: a randomized controlled clinical trial. Health Psychol. 2016;35(6):563.
Tudor-Locke C, Craig CL, Aoyagi Y, et al. How many steps/day are enough? For older adults and special populations. Int J Behav Nutr Phys Act. 2011;8(1):80.
Creasy SA, Lang W, Tate DF, Davis KK, Jakicic JM. Pattern of daily steps is associated with weight loss: secondary analysis from the step-up randomized trial. Obesity. 2018;26(6):977–84.
Jensen MD, Ryan DH, Apovian CM, et al. 2013 AHA/ACC/TOS guideline for the management of overweight and obesity in adults: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and The Obesity Society. Circulation. 2014;129(25 Suppl 2):S102–38.
Engelson ES, Agin D, Kenya S, et al. Body composition and metabolic effects of a diet and exercise weight loss regimen on obese, HIV-infected women. Metabolism. 2006;55(10):1327–36.
Reeds DN, Pietka TA, Yarasheski KE, et al. HIV infection does not prevent the metabolic benefits of diet-induced weight loss in women with obesity. Obesity (Silver Spring). 2017;25(4):682–8.
Acknowledgements
We wish to thank Whitney Howie for her contribution to the dietary analyses. This project was supported by the Providence/Boston Center for AIDS Research (Grant Number P30 A1042853); the ACTG Clinical Research Site 2951 (The Miriam Hospital; Grant Number UM1AI069412); and the Cardiovascular Behavioral and Preventive Medicine Training Grant (Grant Number KB T32 HL076134).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Wing, R.R., Becofsky, K., Wing, E.J. et al. Behavioral and Cardiovascular Effects of a Behavioral Weight Loss Program for People Living with HIV. AIDS Behav 24, 1032–1041 (2020). https://doi.org/10.1007/s10461-019-02503-x
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-019-02503-x