Abstract
We examined three waves of National HIV Behavioral Surveillance surveys of persons who inject drugs (PWID) in San Francisco to assess meeting UNAIDS 90–90–90 targets. Diagnosis of PWID living with HIV increased from 64.4% in 2009 to 80.5% in 2015. Antiretroviral treatment among those diagnosed did not improve (63.8% in 2009, 62.9% in 2015). Programs in San Francisco have not achieved the first two UNAIDS targets for PWID by 2015. In a context of a rising opioid epidemic, there is urgent need for increased case finding of PWID living with HIV who are undiagnosed with rapid linkage to treatment.
Resumen
Examinamos encuestas de comportamiento en personas que se inyectan drogas en San Francisco para evaluar el cumplimiento de los objetivos de ONUSIDA 90-90-90. El diagnóstico en personas que viven con VIH aumentó de 64.4% en 2009 a 80.5% en 2015. El tratamiento antirretroviral no mejoró (63.8% en 2009, 62.9% en 2015). Los programas no han alcanzado los objetivos de ONUSIDA para personas que se inyectan drogas. En un contexto de aumento de la epidemia de opiáceos, existe una necesidad de un mayor esfueurzo de encontrar casos de VIH que no han sido diagnosticados con un vínculo rápido con el tratamiento.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2014, UNAIDS proposed goals for 2020 for treating infections and preventing onward transmission now widely known as the “90–90–90” targets [1]. These are that 90% of persons living with HIV will be diagnosed, of whom 90% will receive antiretroviral treatment (ART), and of whom 90% will be virally suppressed. For some cities, including San Francisco, “fast track” 95–95–95 targets have been set to help end the epidemic by 2030 [2]. A review of data from 69 countries found that none had met the 90–90–90 targets as of August 2015 [3]. Some select locations recently report meeting 90–90–90 targets, such as in New South Wales, Australia [4]. Even as UNAIDS 90–90–90 targets are achieved overall, some groups may be left behind. For example, the first target (> 90% diagnosed) had been met for white MSM in San Francisco by 2008 and the second (> 90% on ART) by 2014 [5]. However, neither target had been achieved for African American MSM in San Francisco by 2014 [5].
Persons who inject drugs (PWID) are another population experiencing disparities in HIV prevention and care in San Francisco. There were an estimated 3323 PWID living with HIV in 2016 who were diagnosed in San Francisco, and 18% of the 223 persons newly diagnosed with HIV that year had a history of injecting drugs [6]. Data reported from clinics find that only 59% of PWID initiating ART had achieved viral suppression within 1 year of diagnosis compared to 82% of MSM [6]. Population- or community- based surveys are needed to measure the first of the 90–90–90 targets, interpret the next two targets, and identify disparities for different populations at different steps along the continuum of engagement in care [7]. We report on progress towards the UNAIDS 90–90–90 targets for the population of PWID in San Francisco using three waves of cross-sectional surveys from 2009 to 2015.
Methods
We examined steps in the cascade of engagement in HIV care using data from the National HIV Behavioral Surveillance (NHBS) surveys for PWID in San Francisco conducted in 2009, 2012, and 2015. The standardized methods for NHBS have been published elsewhere [8]. In brief, NHBS comprises serial cross-sectional surveys in several US cities coordinated by the Centers of Disease Control and Prevention (CDC). The surveys track HIV prevalence, service utilization, and risk and preventive behaviors in key populations at risk for HIV. The surveys use respondent driven sampling (RDS) to recruit a targeted 500 PWID per wave per city [8, 9].
Recruitment was initiated through the selection of “seeds” who were diverse with respect to demographic and behavioral characteristics. Seeds were instructed to recruit three to five eligible PWID from their social networks to the study with referral coupons. To be eligible, participants needed to be 18 years of age or older, report injecting illicit drugs in the past 12 months, and have a referral coupon from another participant. Recruits in turn referred other eligible PWID to the study, and the process continued until the desired sample size and stability (i.e., the make-up of the sample did not change with further recruitment) were achieved. Eligible PWID were given $50 for their participation and $10 for each eligible PWID they successfully recruited. After providing written informed consent, trained staff conducted structured face-to-face interviews recording responses on pre-programmed electronic devices. Upon completion of the interview, HIV counseling and testing was conducted. The current analysis focuses on the proportion of persons who previously were diagnosed with HIV and the proportion currently taking ART among those who tested positive in the current survey. In 2015, persons reporting ART use were also asked what was the result of their most recent viral load test. Data were analyzed using RDS-Analyst (Gile’s estimator) to compare survey-weighted point estimates for population characteristics including HIV prevalence [10]. The Cochrane Q test was used to assess differences in the samples using RDS-Analyst adjusted confidence limits. For the analysis among HIV-positive participants (i.e., prior diagnosis, currently on ART), data are presented as the crude sample means due to small numbers and few cross-recruitments within the sub-groups needed for adjustments [5]. The study protocol was reviewed and approved by the Internal Review Board (IRB) of the University of California San Francisco.
Results
The samples of PWID recruited across the three survey waves were comparable in size (n = 535 in 2009, 570 in 2012, and 538 in 2015) and characteristics with three exceptions. There were more PWID age 18–30 years in 2015 compared to 2009 (RDS-Analyst adjusted 17.0% vs. 6.7%, respectively, p = 0.032); income tended to be higher in 2015 compared to 2009 (58.8% had annual income above $10,000 in 2015 vs 42.2% in 2009, p = 0.003); and more had health insurance in 2015 compared to 2009 (88.5% vs 68.1%, respectively, p < 0.001). RDS-Analyst adjusted HIV prevalence was 11.4% (95% confidence interval [CI] 7.0–16.8) in 2009, 12.0% (95% CI 7.5–16.8) in 2012, and 16.8% (95% CI 11.7–21.8) in 2015. The apparent increase in HIV prevalence in 2015 was not statistically significant (p = 0.244).
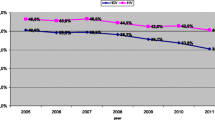
Among PWID testing HIV positive, the crude percentage who reported already being diagnosed was 64.4% (n = 47/73) in 2009 and 59.1% (n = 39/66) in 2012 before increasing to 80.5% (n = 62/77) in 2015 (p = 0.023, 2009 vs. 2015, Fig. 1). Also among all PWID living with HIV, the percentage who reported currently taking ART was 41.1% (n = 30/73) in 2009, 40.9% (n = 27/66) in 2012, and 50.6% (n = 39/77) in 2015. These differences in ART use in the population living with HIV were not statistically different (p = 0.244, 2009 vs. 2015). Changing the denominator to be among those previously diagnosed (as per UNAIDS 90–90–90 convention), the corresponding figures for ART use were 63.8% (n = 30/47) in 2009, 69.2% (n = 27/39) in 2012, and 62.9% (n = 39/62) in 2015. When asked about their most recent viral load test result, 87.2% (n = 34/39) of PWID on ART in 2015 reported that it was “undetectable.”
Discussion
Our community-based surveys point to failures in achieving UNAIDS 90–90–90 targets for the PWID population in San Francisco. While there was apparent improvement in the diagnosis of HIV between 2012 and 2015, the estimate of 80.5% among PWID was notably lower than the 97% of MSM with HIV who knew their status in our 2014 NHBS survey for this population [11]. Moreover, there was a persistent gap in the use of ART among PWID diagnosed with HIV, falling short of the second UNAIDS 90–90–90 target (i.e., 62.9% in 2014). Again, for comparison, 95% of MSM diagnosed with HIV reported being currently on ART in the 2014 NHBS survey [11]. Finally, the third UNAIDS target, the percent virally suppressed, was estimated as 87.2% among PWID on ART. However, this last measure is limited by being self-reported and asked only in 2015.
We recognize other limitations. Sample sizes were small for the sub-groups of PWID living with HIV, affecting statistical power to measure differences as well as precluding generation of robust weights for the adjustment of the RDS data. The wording of the question for prior diagnosis was asked in 2015 as “Have you ever tested positive for HIV?”, whereas the previous surveys asked “What was the result of your most recent HIV test?” The change may account for some of the difference in prior diagnosis from 2012 to 2015. Another limitation is that our RDS surveys may not be representative of all PWID or biased in ways that are difficult to determine or correct. For example, the cash incentive for participation may result in differential recruitment for lower income PWID. The peer-referral recruitment may differentially recruit older and longer-term PWID who have more social network connections to other PWID than younger and newer injectors.
Despite these limitations, we interpret our data as a signal that the population of PWID will require additional and novel approaches to achieve UNAIDS 90–90–90 targets. PWID are already marginalized with stark disparities in HIV-related mortality and co-morbidity [6]. San Francisco and other jurisdictions need to increase efforts to engage PWID for HIV testing, linking them to ART, and retaining them in care to achieve sustained viral suppression for their own health benefits as well as for preventing onward transmission. While San Francisco has made significant progress in achieving UNAIDS targets for some populations, others are being left behind. In the context of a rising opioid epidemic in the US, we risk falling even further behind for PWID [12].
References
90-90-90: an ambitious treatment target to help end the AIDS epidemic. Joint United Nations Programme on HIV/AIDS (UNAIDS). http://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (2014). Accessed 3 May 2018.
Fast-track: ending the AIDS epidemic by 2030. The Joint United Nations Programme on HIV/AIDS (UNAIDS). http://www.unaids.org/sites/default/files/media_asset/JC2686_WAD2014report_en.pdf (2014). Accessed 3 May 2018.
Levi J, Raymond A, Pozniak A, Vernazza P, Kohler P, Hill A. Can the UNAIDS 90-90-90 target be achieved? A systematic analysis of national HIV treatment cascades. BMJ Global Health. 2016;1(2):e000010.
Keen P, Gray R, Telfer B, Guy R, Schmidt H, Whittaker B, et al. The 2016 HIV diagnosis and care cascade in New South Wales, Australia: meeting the UNAIDS 90-90-90 targets. J Int AIDS Soc. 2018;21(4):e25109.
Okeke N, McFarland W, Raymond H. Closing the gap? The HIV continuum in care for African-American men who have sex with men, San Francisco, 2004–2014. AIDS Behav. 2016;21(6):1741–4.
HIV Epidemiology Annual Report. San Francisco: San Francisco Department of Public Health. https://www.sfdph.org/dph/files/reports/RptsHIVAIDS/AnnualReport2015-20160831.pdf (2016). Accessed 3 May 2018.
Hladik W, Benech I, Bateganya M, Hakim A. The utility of population-based surveys to describe the continuum of HIV services for key and general populations. Int J STD AIDS. 2015;27(1):5–12.
Lansky A, Abdul-Quader A, Cribbin M, Hall T, Finlayson T, Garfein R, et al. Developing an HIV behavioral surveillance system for injecting drug users: The National HIV Behavioral Surveillance System. Public Health Rep. 2007;122(1):48–55.
Heckathorn D. Respondent-driven sampling: a new approach to the study of hidden populations. Soc Probl. 1997;44(2):174–99.
Hard-to-Reach Population Methods Research Group. Wiki.stat.ucla.edu. http://wiki.stat.ucla.edu/hpmrg/index.php/Main_Page (2018). Accessed 3 May 2018.
Raymond H, Scheer S, Santos G, McFarland W. Examining progress toward the UNAIDS 90-90-90 framework among men who have sex with men, San Francisco, 2014. AIDS Care. 2016;28(9):1177–80.
Rudd R, Seth P, David F, Scholl L. Increases in drug and opioid-involved overdose deaths—United States, 2010–2015. Morb Mortal Wkly Rep. 2016;65(5051):1445–52.
Funding
This study was funded by the Centers for Disease Control and Prevention (grant number 5U1BPS003247).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
All authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Kim, N., Welty, S., Reza, T. et al. Undiagnosed and Untreated HIV Infection Among Persons Who Inject Drugs: Results of Three National HIV Behavioral Surveillance Surveys, San Francisco, 2009–2015. AIDS Behav 23, 1586–1589 (2019). https://doi.org/10.1007/s10461-018-2284-1
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10461-018-2284-1