Abstract
Short-rotation coppices (SRC) and alley cropping agroforestry systems (ACS) provide ecosystem services and environmental benefits. Particularly, their role in improving soil quality has frequently been emphasised. However, the economic lifetime of fast growing tree plantations may be limited, making a transition indispensable. Since information on effects of such land-use changes on soil microbial properties is scarce, the aim of this study was to assess effects of converting a German poplar-based SRC to a silvo-arable ACS, comprising rootstock incorporation and reduced tillage, on soil quality indicators after one year. Soils were sampled under poplars and within the re-converted alleyway. Distance transects were considered to further include potential beneficial effects of remaining trees on alleyway topsoil quality. We analysed soil organic carbon (SOC), total nitrogen, soil microbial biomass C and N, ergosterol, microbial activity (enzyme activities, multi-substrate-induced respiration rates), ratios relative to SOC, fungal abundance and microbial functional diversity at two topsoil depths (0–5, 5–20 cm). Repeated measures mixed effects models were calculated for each depth, considering spatial dependence structures and potential heterogeneity of abiotic factors (pH, clay contents). SRC to ACS conversion decreased SOC, microbial biomass and activity in upper topsoils within the arable alleyway, irrespective of tree distance. Furthermore, shifts in the composition of main microbial groups towards a lower fungal abundance and functional diversity were observed. Reductions of fungal indices were also identified in lower topsoils. Unexpectedly, results demonstrated that partial conversion of a SRC back to arable cropping decreased soil quality within as little as one year.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positive effects of short-rotation coppices (SRC) and alley cropping agroforestry systems (ACS) on soil quality are being increasingly described in the temperate zone (Don et al. 2012; Jose 2009). Both land-use concepts include the implementation of trees with vigorous juvenile growth (e.g. poplars: Populus spp., willows: Salix spp.) in agricultural land for energy production (Holzmueller and Jose 2012; Kauter et al. 2003). Since tree implementation is related to changes in microclimate and above-/belowground organic matter inputs, improvements of physical, chemical and biological properties have frequently been reported (e.g. Jose 2009; Tsonkova et al. 2012). For instance, Georgiadis et al. (2017) and Beuschel et al. (2019) observed ameliorative effects of incorporated trees on SOC and microbial properties in upper topsoils under trees at various SRC and silvo-arable ACS in Denmark and Germany, respectively.
Nevertheless, the economic lifetime of tree stands may be limited to 25–30 years, as biomass yields decline probably due to increased occupied space of old non-vigorous rootstocks (Don et al. 2012; Kauter et al. 2003), making re-establishing or re-converting indispensable. However, re-conversion effects on soil properties—especially, when considering microbial parameters—have rarely been investigated before. Poeplau et al. (2011) and Toenshoff et al. (2013a) reported a decline in SOC and microbial biomass C in topsoils, respectively, after deforestation and re-conversion to arable land. Kahle et al. (2013) and Toenshoff et al. (2013b) observed no changes or even higher topsoil SOC stocks under former SRC, suggesting that ecological benefits of implemented trees may be conserved on arable soils. Conversion procedure and subsequent management can influence the magnitude of change. For example, rootstock incorporation and reduced tillage probably counteract soil deterioration (Don et al. 2012; Murty et al. 2002; Wachendorf et al. 2017). Moreover, a partial conversion of tree stands might further counterbalance deteriorating effects on soil properties, as remaining trees potentially allow the maintenance of the provision of environmental benefits (e.g. nutrient inputs via leaf litter fall, shading, water/nutrient retention, erosion protection) (Schoeneberger 2009; Tsonkova et al. 2012).
While we have recently presented effect sizes and spatial range of incorporating poplar trees in former arable land on soil quality indices within three silvo-arable ACS (Beuschel et al. 2019), the aim of this study was to estimate effects of converting a poplar-based SRC to a ACS, comprising rootstock incorporation and reduced tillage within the newly established arable alleyway, on soil quality indices after one year. Soil quality is defined as the capacity of soil to function within ecosystem boundaries, maintaining productivity, water and air quality and supporting human health and habitation (Karlen et al. 1997). Since microorganisms are proportionally linked to ecosystem functioning (Bardgett et al. 2005), the composition and activity of the soil microbial community are regarded as suitable indices for soil quality (Joergensen and Wichern 2008; Schloter et al. 2003). Fungi, in particular, which dominate the soil microbial biomass of all terrestrial ecosystems, represent a vital component of food webs and critically contribute to soil properties and processes, making fungal abundance crucial to soil quality and sustainability of agroecosystems (Joergensen and Wichern 2008; Stahl et al. 1999). Enzymes are involved in biogeochemical cycling, like organic matter degradation or nutrient mineralisation, which include multi-enzyme stepwise processes (Nannipieri et al. 2018). Enzyme activities are often thought to trace short-term dynamics of soil organic matter, due to their immediate response to land-use changes (Caldwell 2005; Stott et al. 2010). Furthermore, the assessment of land-use change effects on C dynamics requires the calculation of SOC stocks by considering bulk densities (BD) (Don et al. 2011; Lee et al. 2009). In order to elucidate conversion effects on SOC stocks, two BD datasets, which have independently been collected within the newly established silvo-arable ACS, were used for calculation of stocks. In addition, distance transects were analysed to include potential beneficial effects of trees on alleyway topsoil quality. Since abiotic factors can influence microbial properties (e.g. Wu et al. 2012) and potential site-specific heterogeneity of soil properties within/between distance transects may explain a considerable proportion of variation of soil quality indices (Beuschel et al. 2019), we further took pH and clay contents into account. We hypothesise that after SRC to ACS conversion levels of SOC, soil microbial biomass and activity, functional diversity and fungal abundance are conserved in the arable alleyway, since rootstock incorporation, reduced tillage and remaining trees counteract soil quality deterioration.
Material and methods
Study area
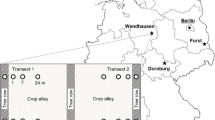
At Reiffenhausen (51°40′N, 9°59′E, Lower Saxony, Germany), a 0.4 ha SRC was established in 2011 on arable land by planting poplars (clone Max 1, Populus maximowiczi × P. Nigra) in double rows in north–west to south–east orientation. Spacings between double and single rows were 1.5 and 0.75 m, respectively. Within row spacing was 1 m, resulting in a tree density of 8890 trees ha−1. After four years, in autumn 2015, a 10-m-wide and 100-m-long tree section of the SRC (0.1 ha), containing five double rows, was harvested and re-converted to arable land for crop production, resulting in a silvo-arable ACS. Rootstocks were chopped up to 20 cm soil depth using a rotary cultivator. Reduced tillage was performed using chisel plough and disc harrow. In the alleyway, winter barley (mineral fertilizer: 238 kg N ha−1) was cultivated (harvest: July 2016), before winter oilseed rape was seeded (August 2016). Soils were classified as Eutric Cambisols (IUSS Working Group WRB 2015), revealing 59% sand, 26% silt, 15% clay and a pH (H2O) of 6.9. The site is characterised by a mean annual temperature and precipitation of 9.2 °C and 651 mm, respectively (Göttingen, 1981–2010, Deutscher Wetterdienst).
Soil sampling
In October 2016, topsoils were sampled in four replicate transects using a steel corer (diameter: 4.2 cm): under poplars and at 1 and 4.5 m tree distance from tree canopies (0.5 m distance from tree trunks). At each transect position, six soil cores were combined depth-wise (0–5, 5–20 cm) to obtain composite samples. Soils were transported to the laboratory on ice and sieved < 2 mm. No coarse woody residues were identified in soil samples. Aliquots were frozen at − 20 °C for ergosterol and enzyme activity quantification. Remaining material was stored at 4 °C. For all soil samples, soil pH, SOC, total N (TN) and soil texture were analysed. Soil pH was quantified at a soil to water ratio of 1–2.5. Methodical details of SOC, TN and soil texture determination are described in Beuschel et al. (2019). To assess conversion effects on SOC stocks at upper topsoils, two bulk density (BD) datasets (I, II) have independently been collected at all transect positions (n = 4) within the silvo-arable ACS by two working groups using 100 and 250 cm3 cylinders, respectively. SOC stocks were separately calculated using BD I and II, resulting in SOC stock datasets I and II, respectively. All results were presented on an oven-dry soil basis (105 °C, 24 h).
Microbial biomass and ergosterol
Microbial biomass C (MBC) and N (MBN) were determined according to Brookes et al. (1985) and Vance et al. (1987). MBC was calculated as EC/kEC, with EC = (organic C extracted from fumigated soil)—(organic C extracted from non-fumigated soil) and kEC = 0.45 (Joergensen 1996; Wu et al. 1990). MBN was calculated as EN/kEN, where EN = (total N extracted from fumigated soil)—(total N extracted from non-fumigated soil) and kEN = 0.54 (Brookes et al. 1985; Joergensen and Mueller 1996). Ergosterol was quantified by reverse-phase HPLC (wavelength: 282 nm) after extraction of 2 g soil with 100 ml ethanol (Djajakirana et al. 1996).
Multi-SIR
Multi-SIR (substrate-induced respiration) rates were obtained by the MicroResp™ method (Campbell et al. 2003) as described by Beuschel et al. (2019). Besides water (H2O) for basal respiration, 16 substrates were applied to upper topsoils. H2O, D-glucose, D-glucosamine, L-alanine and protocatechuic acid were also analysed in lower topsoils to assess their vertical distribution. Colour change was measured at 572 nm (4 h incubation, 25 °C) using a FLUOstar Omega microplate reader (BMG, Offenburg, Germany). Respiration rates were calculated as µg CO2-C g−1 dry weight h−1. SOC normalised multi-SIR rates are presented in Online Resource 1.
Soil microbial functional diversity was estimated by calculating the Shannon evenness index (Esoil): Esoil = –∑ (pi ln(pi)) / ln(k), where pi = (SIR rate of i-th substrate) / (sum of all SIR rates) and k = number of substrates (Zak et al. 1994). Metabolic quotient (qCO2) was calculated by dividing basal respiration by MBC (Anderson and Domsch 1990), expressed as µg CO2-C mg−1 MBC h−1.
Hydrolytic enzyme activities
Enzyme activities were determined according to Marx et al. (2001), using three fluorescently labelled substrates based on 4-methylumbelliferone (MUF) and one based on 7-amino-4-methylcoumarin (AMC): 4-MUF-β-D-glucopyranoside (β-glucosidase), 4-MUF-β-D-xylopyranoside (β-xylosidase), 4-MUF-N-acetyl-β-D-glucosaminide (N-acetyl-β-glucosaminidase) and L-tyrosine-7-AMC (tyrosine-aminopeptidase). Selected enzymes contribute to the degradation of organic substances (e.g. cellulose, hemicellulose, chitin, peptidoglycan, protein), resulting in the release of carbohydrates, amino sugars and amino acids (Ekenler and Tabatabai 2003; Kögel-Knabner 2002).
The soil assay was conducted as reported by Beuschel et al. (2019). Fluorescence intensity was measured at 30 min intervals for 180 min at 355 nm excitation and 460 nm emission wavelengths using a FLUOstar Omega microplate reader (BMG, Offenburg, Germany). Enzyme activities were expressed as MUF/AMC release in nmol g−1 soil h−1.
Statistics
Statistical modelling was carried out with SAS (Statistical Analysis System, version 9.4; SAS Institute Inc. 2013, Cary, NC, USA) according to the approach reported by Beuschel et al. (2019). In brief, repeated measures mixed effects models (proc MIXED, GLIMMIX) were calculated for each depth, modelling distances as repeated measures. In order to account for spatial dependence (e.g. serial correlation of observations within a transect, considering observations of different transects as being independent and assuming a decay of correlation of observations within the same transect with increasing distance), a set of candidate models with different variance–covariance structures (independent model, transect-effect model, spatial power law model) were tested (Littell et al. 2006; Piepho and Edmondson 2018). Model selection was conducted by the Akaike Information Criterion (AIC). Clay contents and pH were used as covariates to consider potential variability of abiotic factors within/between distance transects. Effects of covariates and interactions were tested by forward selection. If residuals did not match requirements (normal distribution, variance homogeneity), the response variable was transformed logarithmically, restarting modelling and verifying from residual plots that assumptions were approximately met. The LSMEANS (least squares means) statement was applied to obtain adjusted means. The option PDIFF and Tukey–Kramer tests (P < 0.05) were used for distance comparisons. Means on the logarithmic scale were back-transformed, providing estimates of medians on original scales (Piepho 2009). For comparisons of depths at each distance, the LSMEANS statement was applied using differences of response variables and abiotic factors for modelling, before P-values of t-tests were Bonferroni-Holm-corrected.
Results
Soil quality indicators after SRC to ACS conversion
SOC and TN showed a significant decline at 0–5 cm depth in the re-converted alleyway with respect to soils under trees (Table 1). MBC, MBN, ergosterol (Table 1), enzyme activities (Table 2) and multi-SIR rates (Table 3) displayed the same spatial pattern. In addition, ergosterol contents, N-acetyl-β-glucosaminidase activities and protocatechuic acid rates also reflected differences at 5–20 cm depth, with lower values under annual crops. At upper topsoils, higher BD were observed under trees with respect to arable land for dataset I, whereas dataset II showed the reverse trend (Table 4). SOC stocks, calculated from BD I, showed significantly higher values in soils under trees compared with annual crops, while no differences in SOC stocks have been identified between land-uses, when BD II was used for calculation.
At 0–5 cm depth, ratios of MBC to SOC, ergosterol to MBC (Fig. 1) and MBN to TN (Table 1) showed a significant decrease in re-converted soils compared with soils under trees. Similarly, SOC normalised enzyme activities (Fig. 2) and multi-SIR rates as well as Esoil (Table 3) displayed the same trend. This pattern was also observed at 5–20 cm depth for ergosterol/MBC ratios and SOC normalised N-acetyl-β-glucosaminidase activities and protocatechuic acid rates. qCO2 showed significantly higher values under arable land-use for both depths (Fig. 1c). All indices did not significantly differ between different alleyway distances.
Mean ratios of a soil microbial biomass C (MBC) to soil organic carbon (SOC) b ergosterol to MBCa and c metabolic quotient (qCO2) at different distances from the trees at two soil depths after SRC to ACS conversion. Means with no letter in common indicate significant differences between distances, where capital and small letters represent 0–5 cm and 5–20 cm depth, respectively (n = 4); error bars show standard errors based on mixed effects models; mean standard errors of differences between distances of depth 0–5 cm for (a) 0.19 (b) 0.10b (c) 0.28 and 5–20 cm for (a) 0.20 (b) 0.10b (c) 0.20; asterisks show significant differences between depths at each distance (***P < 0.001, **P < 0.01, *P < 0.05); P-values for clay for (b) 0.02 (0–5, 5–20 cm) and (b) 0.01 (5–20 cm); if covariates (clay, pH) were significant, covariate-adjusted means are presented. aBack-transformed data from the logarithmic scale are presented as medians. Error bars show variance approximations by delta-method using standard errors on the logarithmic scale. bMean standard error of differences between distances on the logarithmic scale
Back-transformed values from the logarithmic scale presented as medians of SOC normalised enzyme activities a β-glucosidase (β-GLU) b β-xylosidase (β-XYL) cN-acetyl-β-glucosaminidase (NAG) and d tyrosine-aminopeptidase (TYR) at different distances from the trees at two soil depths after SRC to ACS conversion. For statistical details and abbreviations, see Fig. 1. Error bars show variance approximations by delta-method using standard errors on the logarithmic scale; logarithmic mean standard errors of differences between distances of depth 0–5 cm for (a) 0.03 (b) 0.04 (c) 0.03 (d) 0.04 and 5–20 cm for (a) 0.04 (b) 0.03 (c) 0.04 (d) 0.04; P-values for clay for (b) 0.02 (5–20 cm) and (d) 0.02 (5–20 cm); if covariates were significant, covariate-adjusted values are presented
Differences between depths
In arable soils, no differences of SOC and TN were detected between depths, whereas contents significantly decreased with depth under trees. A similar vertical distribution was also mirrored by MBC, MBN, ergosterol (Table. 1), enzyme activities (Table 2) and multi-SIR rates (Table 3). Patterns remained unchanged when data were normalised to soil organic matter (Figs. 1a and 2, Table 1). Ergosterol/MBC ratios also showed the same trend (Fig. 1b). In contrast, qCO2 displayed lower values at 0–5 compared with 5–20 cm depth under poplar trees. In re-converted soils, no differences between topsoil layers were identified (Fig. 1c).
Discussion
Soil quality indicators after SRC to ACS conversion
Conversion from SRC to ACS distinctly reduced SOC, TN, MBC and MBN at 0–5 cm depth in the newly established arable alleyway within one year. Changes from perennial to annual plants are often related to depletion of soil organic matter and microbial biomass (Poeplau et al. 2011; Stahl et al. 1999; Toenshoff 2013a). The loss of permanent vegetation cover and re-introduction of tillage may have reduced physical stabilisation and protection by disturbance of soil macroaggregates, microbial habitats as well as tree root and fungal hyphae networks (Guggenberger et al. 1999; Kahle et al. 2013). This might have masked beneficial effects resulting from rootstock incorporation. Rowena Gerjets (personal communication) determined 448 g living and 48 g dead poplar roots under trees in comparison with 23 g winter oilseed rape roots and 8 g dead poplar roots m−2 in arable land (0–20 cm) in May 2017 at Reiffenhausen, indicating a reduced root biomass after conversion. Schmidt et al. (2019) quantified 85.1 g of poplar leaf litter under trees, declining to 9.6 and 0.7 g m−2 a−1 at 1 and 4 m distance, respectively, whereas estimates of harvest residues (winter barley), resulting from grain and straw yields, were about 570 g m−2 in the alleyway. In combination with tree root biomass, data show that amounts of organic matter inputs remained almost unchanged. Thus, the often mentioned assumption that re-conversion is related to declining organic matter inputs could not be confirmed at this site. However, to fully estimate the link between quantity of organic matter inputs and soil quality changes, a more detailed assessment of above- and belowground inputs is indispensable, as e.g. herbaceous vegetation within tree understoreys may considerably contribute to C inputs (Cardinael et al. 2018).
Lower soil microbial activity and functional diversity in arable soils indicate a reduced capability of the microbial community to degrade organic matter and to use different substrates for metabolism, negatively affecting nutrient cycling (Degens et al. 2000; Zak et al. 2003). Land-uses that are associated with annual cultivation and soil organic matter depletion are often linked to lower microbial activity and functional diversity (Degens et al. 2000; Kremer and Li 2003; Six et al. 2006). Decreasing plant species richness in the alleyway compared with trees, containing understorey vegetation, may also have promoted a functionally less distinct microbial community (Rowe et al. 2009; Zak et al. 2003). Furthermore, higher qCO2 under arable crops reflect a lower proportional incorporation of substrate C of heterotrophic microorganisms into their biomass, while mineralising a higher percentage of substrate C to CO2 to meet energy demands (Anderson and Domsch 1990; Lavahun et al. 1996). As microbial properties are positively associated with organic matter concentrations (Lavahun et al. 1996), microbial parameters were normalised to SOC. Unchanged patterns of microbial activity after SOC normalisation indicate that changes occur regardless of SOC concentrations, presuming that quality and accessibility of organic matter might also have played important roles (e.g. Lagomarsino et al. 2011; Zak et al. 2003). The loss of previously stabilised extracellular enzymes within the soil matrix probably has further contributed to the decline in microbial activity (Nannipieri et al. 2018; Paudel et al. 2011). Observed shifts may have resulted from changes in biochemical characteristics of organic matter inputs and soil physical disturbance (Ekenler and Tabatabai 2003; Myers et al. 2001). Moreover, reduced MBC/SOC and MBN/TN ratios illustrate decreasing substrate availabilities, which might have been promoted by exhaustion of easily available C and N sources in arable soils and woody residues within one year after land-use transition (Toenshoff et al. 2013a).
Reduced ergosterol values and ergosterol/MBC ratios reflect a lower absolute and relative abundance of saprotrophic and ectomycorrhizal fungi (Joergensen and Wichern 2008) after land-use transition. A decline in fungal biomass can be attributed to tillage induced disturbance of hyphae networks (Frey et al. 1999; Stahl et al. 1999). Particularly, poplars can comprise ectomycorrhizal and arbuscular mycorrhizal fungi (Smith and Read 2008). Results support previous studies that reported lower hyphal length, ergosterol contents (Stahl et al. 1999) and fungal abundance (Stauffer et al. 2014) in cultivated cropland compared with uncultivated soils. As particularly members of basidiomycetes prevail the decomposition of recalcitrant organic material (Baldrian and Valášková 2008), declining protocatechuic acid rates reflect a reduced potential to decompose recalcitrant material (e.g. lignin). Fungi related indices also showed differences in lower topsoils, indicating that effect sizes of re-conversion on fungi are more pronounced in contrast to other components of the soil microbial biomass pool (Frey et al. 1999; Stahl et al. 1999). Additionally, bacteria might have been promoted by increasing land-use intensity and fertilizer inputs in the alleyway (Six et al. 2006).
No differences of soil quality indices between distances within the re-converted arable alleyway were identified, which reflect no evidence of extending beneficial effects of remaining trees, lacking the potential to counteract soil deterioration after SRC to ACS conversion to date. This may be attributed to the early sampling date after land-use transition and negligible C inputs from litter fall. Results are in line with previous studies, reporting no differences within alleyways in three silvo-arable ACS after 5–8 years (Beuschel et al. 2019) and in a re-cultivated post-mining ACS after 9 years (Nii-Annang et al. 2009). However, examination of topsoils avoided a detection of potential tree effects linked to root growth into the alleyway below the tillage layer in this study.
Differences between depths
Improved soil quality indices in upper compared with lower topsoils under trees can be attributed to the cessation of tillage, an extensive root and fungal hyphae network under permanent vegetation cover and changes in availability and quality of organic substrates at 0–5 cm depth. Even a low tree biomass at Reiffenhausen (1.1 t ha−1, 2011–2015, Schmidt et al. 2019) resulted in increased soil quality parameters five years after tree planting, supporting previous studies for SRC (e.g. Stauffer et al. 2014; Tariq et al. 2018) and ACS (e.g. Beuschel et al. 2019; Paudel et al. 2012).
Missing differences of soil quality indices between depths in the alleyway may have resulted from the initial conversion procedure related to mechanical mixing of soil. Reduced tillage positively influences the soil microbial community, e.g. by remaining larger amounts of plant residues concentrated in upper soil layers for microbial metabolism (Kandeler et al. 1999). However, the time after land-use change until soil sampling might have been too short to detect typical vertical soil quality gradients.
SOC stocks
After SRC to ACS conversion, BD showed lower and higher values in the arable alleyway at 0–5 cm depth compared with soils under remaining trees for datasets I and II, respectively. Similarly, Georgiadis et al. (2017) described lower BD in topsoils under trees in several SRC in comparison with arable soils, while Rowe et al. (2016) have reported the opposite pattern. Cessation of tillage and increasing C contents as well as annual tillage may decline BD in soils under trees and arable land-use, respectively. In contrast, increased thickness-growth of coarse tree roots may result in soil compaction. Moreover, coarse root distribution and tree trunk distance during soil sampling can affect results.
Consequently, calculated SOC stocks demonstrate that differences between soils under perennial and annual vegetation are strengthened (SOC I) or levelled (SOC II), depending on the underlying BD dataset. Kahle et al. (2013) also detected a decline in SOC contents at 0–10 cm depth one year after SRC re-conversion, but no changes in stocks at 0–30 cm depth, assuming that tillage solely caused vertical redistribution. According to changes in BD, however, the use of fixed sampling depths resulted in a comparison of different soil masses, which may entail inaccurate estimations of stocks (Don et al. 2011; Murty et al. 2002). Our results demonstrate that optimised methods, like use of representative elementary volumes for BD determination, consideration of coarse root distribution and correction of stocks for equivalent soil masses, are indispensable in future studies. This will help to prevent erroneous conclusions being drawn from land-use changes (Don et al. 2011; Lee et al. 2009).
Conclusions
Conversion of SCR to ACS caused a distinct decline in soil microbial indicators in arable alleyway topsoils within as little as one year. Results do not support previous assumptions that rootstock incorporation and reduced tillage counteract soil deterioration under arable cropping. Microbial indices suggest that land-use change is linked to decreasing soil quality, negatively affecting soil ecosystem functioning. As a decline was observed, irrespective of tree distance, no evidence of short-term beneficial effects of remaining trees on alleyway topsoil quality were identified. To elucidate the main processes explaining C dynamics in ACS, studies are required that include subsoils and equivalent soil masses as well as detailed quantifications of above-/belowground organic matter inputs. Moreover, the assessment of mid- and long-term effects of converting SRC to silvo-arable ACS on soil quality indices are vital to refine our short-term results.
References
Anderson TH, Domsch KH (1990) Application of eco-physiological quotients qCO2 and qD on microbial biomasses from soils of different cropping histories. Soil Biol Biochem 22:251–255
Baldrian P, Valášková V (2008) Degradation of cellulose by basidiomycetous fungi. FEMS Microbiol Rev 32:501–521
Bardgett RD, Bowman WD, Kaufmann R, Schmidt SK (2005) A temporal approach to linking aboveground and belowground ecology. Trends Ecol Evol 20:634–641
Beuschel R, Piepho HP, Joergensen RG, Wachendorf C (2019) Similar spatial patterns of soil quality indicators in three poplar-based silvo-arable alley cropping systems in Germany. Biol Fertil Soils 55:1–14
Brookes PC, Landman A, Pruden G, Jenkinson DS (1985) Chloroform fumigation and the release of soil nitrogen: a rapid direct extraction method for measuring microbial biomass nitrogen in soil. Soil Biol Biochem 17:837–842
Caldwell BA (2005) Enzyme activities as a component of soil biodiversity: a review. Pedobiologia 49:637–644
Campbell CD, Chapman SJ, Cameron CM, Davidson MS, Potts JM (2003) A rapid microtiter plate method to measure carbon dioxide evolved from carbon substrate amendments so as to determine the physiological profiles of soil microbial communities by using whole soil. Appl Environ Microbiol 69:3593–3599
Cardinael R, Guinet B, Chevallier T, Dupraz C, Cozzi T, Chenu C (2018) High organic inputs explain shallow and deep SOC storage in a long-term agroforestry system—combining experimental and modelling approaches. Biogeosciences 15:297–317
Degens BP, Schipper LA, Sparling GP, Vojvodic-Vukovic M (2000) Decreases in organic C reserves in soils can reduce the catabolic diversity of soil microbial communities. Soil Biol Biochem 32:189–196
Djajakirana G, Joergensen RG, Meyer B (1996) Ergosterol and microbial biomass relationship in soil. Biol Fertil Soils 22:299–304
Don A, Schumacher J, Freibauer A (2011) Impact of tropical land-use change on soil organic carbon stocks—a meta-analysis. Glob Change Biol 17:1658–1670
Don A, Osborne B, Hastings A, Skiba U, Carter MS, Drewer J, Flessa H, Freibauer A, Hyvönen N, Jones MB, Lanigan GJ, Mander Ü, Monti A, Djomo SN, Valentine J, Walter K, Zegada-Lizarazu W, Zenone T (2012) Land-use change to bioenergy production in Europe: implications for the greenhouse gas balance and soil carbon. Glob Change Biol Bioenergy 4:372–391
Ekenler M, Tabatabai MA (2003) Tillage and residue management effects on β-glucosaminidase activity in soils. Soil Biol Biochem 35:871–874
Frey SD, Elliott ET, Paustian K (1999) Bacterial and fungal abundance and biomass in conventional and no-tillage agroecosystems along two climate gradients. Soil Biol Biochem 31:573–585
Georgiadis P, Vesterdal L, Stupak I, Raulund-Rasmussen K (2017) Accumulation of soil organic carbon after cropland conversion to short-rotation willow and poplar. Glob Change Biol Bioenergy 9:1390–1401
Guggenberger G, Frey SD, Six J, Paustian K, Elliott ET (1999) Bacterial and fungal cell-wall residues in conventional and no-tillage agroecosystems. Soil Sci Soc Am J 63:1188–1198
Holzmueller EJ, Jose S (2012) Biomass production for biofuels using agroforestry: potential for the North Central Region of the United States. Agrofor Syst
IUSS Working Group WRB (2015) World Reference Base for soil resources 2014, update 2015. International soil classification system for naming soils and creating legends for soil maps. World soil resources reports 106. FAO, Rome
Joergensen RG (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEC value. Soil Biol Biochem 28:25–31
Joergensen RG, Mueller T (1996) The fumigation-extraction method to estimate soil microbial biomass: calibration of the kEN value. Soil Biol Biochem 28:33–37
Joergensen RG, Wichern F (2008) Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol Biochem 40:2977–2991
Jose S (2009) Agroforestry for ecosystem services and environmental benefits: an overview. Agrofor Syst 76:1–10
Kahle P, Möller J, Baum C, Gurgel A (2013) Tillage-induced changes in the distribution of soil organic matter and the soil aggregate stability under a former short rotation coppice. Soil Till Res 133:49–53
Kandeler E, Tscherko D, Spiegel H (1999) Long-term monitoring of microbial biomass, N mineralization and enzyme activities of a Chernozem under different tillage management. Biol Fertil Soils 28:343–351
Karlen DL, Mausbach MJ, Doran JW, Cline RG, Harris RF, Schuman GE (1997) Soil quality: a concept, definition, and framework for evaluation (a guest editorial). Soil Sci Soc Am J 61:4–10
Kauter D, Lewandowski I, Claupein W (2003) Quantity and quality of harvestable biomass from Populus short rotation coppice for solid fuel use—a review of the physiological basis and management influences. Biomass Bioenergy 24:411–427
Kögel-Knabner I (2002) The macromolecular organic composition of plant and microbial residues as inputs to soil organic matter. Soil Biol Biochem 34:139–162
Kremer RJ, Li J (2003) Developing weed-suppressive soils through improved soil quality management. Soil Till Res 72:193–202
Lagomarsino A, Benedetti A, Marinari S, Pompili L, Moscatelli MC, Roggero PP, Lai R, Ledda L (2011) Soil organic C variability and microbial functions in a Mediterranean agro-forest ecosystem. Biol Fertil Soils 47:283–291
Lavahun MFE, Joergensen RG, Meyer B (1996) Activity and biomass of soil microorganisms at different depths. Biol Fertil Soils 23:38–42
Lee J, Hopmans JW, Rolston DE, Baer SG, Six J (2009) Determining soil carbon stock changes: simple bulk density corrections fail. Agric Ecosyst Environ 134:251–256
Littell RC, Milliken GA, Stroup WW, Wolfinger RD, Schabenberger O (2006) SAS for mixed models, 2nd edn. SAS Institute Inc., Cary, NC, USA
Marx MC, Wood M, Jarvis SC (2001) A microplate fluorimetric assay for the study of enzyme diversity in soils. Soil Biol Biochem 33:1633–1640
Murty D, Kirschbaum MUF, McMurtie RE, McGilvray H (2002) Does conversion of forest to agricultural land change soil carbon and nitrogen? a review of the literature. Glob Change Biol 8:105–123
Myers RT, Zak DR, White DC, Peacock A (2001) Landscape-level patterns of microbial community composition and substrate use in upland forest ecosystems. Soil Sci Sic Am J 65:359–367
Nannipieri P, Trasar-Cepeda C, Dick RP (2018) Soil enzyme activity: a brief history and biochemistry as a basis for appropriate interpretations and meta-analysis. Biol Fertil Soils 54:11–19
Nii-Annang S, Grünewald H, Freese D, Hüttl RF, Dilly O (2009) Microbial activity, organic C accumulation and 13C abundance in soils under alley cropping systems after 9 years of recultivation of quaternary deposits. Biol Fertil Soils 45:531–538
Paudel BR, Udawatta RP, Anderson SH (2011) Agroforestry and grass buffer effects on soil quality parameters for grazed pasture and row-crop systems. Appl Soil Ecol 48:125–132
Paudel BR, Udawatta RP, Kremer RJ, Anderson SH (2012) Soil quality indicator responses to row crop, grazed pasture, and agroforestry buffer management. Agrofor Syst 84:311–323
Piepho HP (2009) Data transformation in statistical analysis of field trials with changing treatment variance. Agron J 101:865–869
Piepho HP, Edmondson R (2018) A tutorial on the statistical analysis of factorial experiments with qualitative and quantitative treatment factor levels. J Agron Crop Sci 204:429–455
Poeplau C, Don A, Vesterdal L, Leifeld J, van Wesemaels B, Schumacher J, Gensior A (2011) Temporal dynamics of soil organic carbon after land-use change in the temperate zone—carbon response functions as a model approach. Glob Change Biol 17:2415–2427
Rowe RL, Street NR, Taylor G (2009) Identifying potential environmental impacts of large-scale deployment of dedicated bioenergy crops in the UK. Renew Sust Energ Rev 13:271–290
Rowe RL, Keith AM, Elias D, Dondini M, Smith P, Oxley J, McNamara NP (2016) Initial soil C and land-use history determine soil C sequestration under perennial bioenergy crops. Glob Change Biol Bioenergy 8:1046–1060
SAS Institute Inc. (2013) SAS/STAT user’s guide, version 9.4, SAS Institute Inc., Cary, NC, USA
Schloter M, Dilly O, Munch JC (2003) Indicators for evaluating soil quality. Agric Ecosyst Environ 98:255–262
Schmidt M, Göbel L, Corre MD, Veldkamp E (2019) BonaRes SIGNAL, Site: Reiffenhausen, biomass production 2016/2017. BonaRes Data Portal, https://doi.org/10.20387/bonares-1b9y-806w
Schoeneberger MM (2009) Agroforestry: working trees for sequestering carbon on agricultural lands. Agrofor Syst 75:27–37
Six J, Frey SD, Thiet RK, Batten KM (2006) Bacterial and fungal contributions to carbon sequestration in agroecosystems. Soil Sci Soc Am J 70:555–569
Smith ES, Read D (2008) Mycorrhizal symbiosis, 3rd edn. Academic Press, New York
Stahl PD, Parkin TB, Christensen M (1999) Fungal presence in paired cultivated and uncultivated soils in central Iowa, USA. Biol Fertil Soils 29:92–97
Stauffer M, Leyval C, Brun JJ, Leportier P, Berthelin J (2014) Effect of willow short rotation coppice on soil properties after three years of growth as compared to forest, grassland and arable land uses. Plant Soil 377:423–438
Stott DE, Andrews SS, Liebig MA, Wienhold BJ, Karlen DL (2010) Evaluation of β-glucosidase activity as a soil quality indicator for the soil management assessment framework. Soil Sci Soc Am J 74:107–119
Tariq A, Gunina A, Lamersdorf N (2018) Initial changes in soil properties and carbon sequestration potential under monocultures and short-rotation alley coppices with poplar and willow after three years of plantation. Sci Total Environ 634:963–973
Toenshoff C, Joergensen RG, Stuelpnagel R, Wachendorf C (2013) Dynamics of soil organic carbon fractions one year after the re-conversion of poplar and willow plantations to arable use and perennial grassland. Agric Ecosyst Environ 174:21–27
Toenshoff C, Stuelpnagel R, Joergensen RG, Wachendorf C (2013) Carbon in plant biomass and soils of poplar and willow plantations - implications for SOC distribution in different soil fractions after re-conversion to arable land. Plant Soil 367:407–417
Tsonkova P, Böhm C, Quinkenstein A, Freese D (2012) Ecological benefits provided by alley cropping systems for production of woody biomass in the temperate region: a review. Agrofor Syst 85:133–152
Vance ED, Brookes PC, Jenkinson DS (1987) An extraction method for measuring soil microbial biomass C. Soil Biol Biochem 19:703–707
Wachendorf C, Stuelpnagel R, Wachendorf M (2017) Influence of land use and tillage depth on dynamics of soil microbial properties, soil carbon fractions and crop yield after conversion of short-rotation coppices. Soil Use Manage 33:379–388
Wu J, Joergensen RG, Pommerening B, Chaussod R, Brookes PC (1990) Measurement of microbial biomass C by fumigation extraction—an automated procedure. Soil Biol Biochem 22:1167–1169
Wu YT, Gutknecht J, Nadrowski K, Geißler C, Kühn P, Scholten T, Both S, Erfmeier A, Böhnke M, Bruelheide H, Wubet T, Buscot F (2012) Relationships between soil microorganisms plant communities, and soil characteristics in Chinese subtropical forests. Ecosystems 15:624–636
Zak JC, Willig MR, Moorhead DL, Wildman HG (1994) Functional diversity of microbial communities: a quantitative approach. Soil Biol Biochem 26:1101–1108
Zak DR, Holmes WE, White DC, Peacock AD, Tilman D (2003) Plant diversity, soil microbial communities, and ecosystem function: are there any links? Ecology 84:2042–2050
Acknowledgements
Research was funded by the Federal Ministry of Education and Research within the project “SIGNAL—sustainable intensification of agriculture through agroforestry”, related to BonaRes. We thank Mick Locke for the careful correction of our English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Beuschel, R., Piepho, HP., Joergensen, R.G. et al. Effects of converting a temperate short-rotation coppice to a silvo-arable alley cropping agroforestry system on soil quality indicators. Agroforest Syst 94, 389–400 (2020). https://doi.org/10.1007/s10457-019-00407-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-019-00407-2