Abstract
Agroforestry has become an important land use type in Central and South America. It is important to study agroforestry systems because of their ability to sequester carbon. This study investigates plantations that are located in the foothills of the Peruvian Andes, and it evaluates the aboveground and soil carbon storage of agroforestry coffee plantations with different dominant shading trees, including Inga spp., Pinus spp. (both 15 years old) and Eucalyptus spp. (7 years old). These agroforestry systems were also compared to a coffee plantation without shading trees. Biomass and carbon were estimated for trees and coffee shrubs using allometric equations. Soil (within depth of 30 cm) and litter carbon were estimated using field sampling and laboratory analyses. The total carbon stock for the site dominated by Inga spp. was 119.9 ± 19.5 Mg ha−1, while for the sites dominated by Pinus spp. it was 177.5 ± 14.1 Mg ha−1 and for the site dominated by Eucalyptus spp. it was 162.3 ± 18.2 Mg ha−1. In the Sun coffee site the ecosystem carbon stock was 99.7 ± 17.2 Mg ha−1. Most carbon was fixed in the soil compartment (57–99 %), followed by aboveground tree biomass (23–32 %), tree belowground biomass (8–9 %), coffee shrubs (0.2–2 %) and litter (1 %).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the last few years, the importance of research on mitigation, i.e. reducing the sources or enhancing the sinks of greenhouse gases, especially mitigation of CO2, has been increasing due to climate change effects. Unsuitable land use activities (mainly deforestation) are the second major source of anthropogenic CO2 emissions (IPCC 2013), and agroforestry systems seem to be an acceptable management of crop production, as well as for CO2 mitigation through an increase in carbon stocks (Schroth et al. 2002).
In agroforestry systems, trees or shrubs are grown around or among crops or pastureland (Nair 1993), and the recognition of this system as a greenhouse gas–mitigation strategy under the Kyoto Protocol has earned it the right to be part of the strategy for biological carbon sequestration (Nair et al. 2009a). Research on carbon sequestration in different types of land use provide information for the Reduced Emissions from Deforestation and Forest Degradation (REDD) programs that would likely allow some countries to sell carbon credits to interested buyers or received financial support from funds (The World Bank 2011). Agroforestry systems help REDD by reducing pressure for further forest conversion to agriculture (Noponen et al. 2013) and by serving as a source of fuel-wood and construction material (Rice and Ward 2008). This system has special importance because of its applicability in agricultural lands as well as in reforestation programs (Ruark et al. 2003). Moreover, according to Smith and Scherr (2003), community-based agroforestry carbon projects have the highest potential for local livelihood benefits and pose less risk to communities than large-scale industrial plantations and strict forest protection.
Many studies have investigated the effect of agroforestry systems on carbon storage. Some of these studies have compared different types of pastures and crops and have assessed the effect of tree shading (Soto-Pinto et al. 2010; Avila et al. 2001). Schmitt-Harsh et al. (2012) examined the carbon pools of smallholders of coffee plantations in agroforestry systems and compared them with mixed dry forest systems in Guatemala. The results showed that secondary forests store more carbon (199 Mg ha−1) than coffee agroforestry systems (128 Mg ha−1). Similar results were found by Noordwijk et al. (2002) for secondary forests and agroforestry coffee systems in Sumatra. However, in this study, the difference in carbon storage was even larger (262 Mg ha−1 for secondary forests and 82 Mg ha−1 for agroforestry coffee systems). Hergoulac´h et al. (2012) compared the greenhouse gas balance in two coffee plantations: a monoculture and a culture shaded by Inga densiflora, and found that the aboveground carbon stocks in the coffee monoculture and the agroforestry system amounted to 9.8 ± 0.4 and 25.2 ± 0.6 Mg ha−1, respectively. In a study by Häger (2012) from Costa Rica, carbon stock in coffee agroforestry farms was 93 ± 29 Mg ha−1.
Soil plays a crucial role in carbon sequestration. Among many different types of land use focused on agronomical production, agroforestry systems can be considered as systems with high effectiveness of soil carbon storage, as well as with low vulnerability, compared with other types of intensive land management (Nair et al. 2009b). Despite continuous crop exploitation, agroforestry production conserves the soil environment due to more closed nutrients and water turnover via tree cover producing litter and shading the soil surface, as well as sustaining the less-eroded soil body. Moreover, in some studies, soil organic matter was found to have increased over 10 years by 16–42 Mg ha−1 in the 0–45 cm layer (Beer et al. 1998), which is about 8–21 Mg C ha−1, depending on the planted shading species and commodities. On the other hand, soil organic matter dynamics are driven by many factors, in general by climate, soil type and land use management, which determine the physical, chemical and biological controls of soil carbon sequestration and turnover (Feller and Beare 1997).
Coffee (Coffea spp.) is very important cash crop cultivated in an area of slightly more than 100,000 km2 (Lewin et al. 2004; Leff et al. 2004). It is traditionally grown under the shade of trees, forming typical agroforestry systems (Wintgens 2004). Shade trees provide numerous benefits; there is an added value of wood production, which can be expressed either in terms of woody biomass or carbon fixation (Batjes and Sombroek 1997; Hergoulac´h et al. 2012; Häger 2012), and these plantations may also act as a critical refuge for forest biota, including birds, insects, mammals and reptiles (Perfecto et al. 1996; Moguel and Toledo 1999).
In Peru, the conversion of forests and grasslands to agricultural purposes is the main source of GHG emissions (MINAM 2010). According to Stephen (2005), 1879 km2 of forests were lost annually between 1985 and 1990 in Peru. Oliveira et al. (2007) reported that between 1999 and 2005, disturbance and deforestation rates throughout the Peruvian Amazon were between 632 and 645 km2 per year. According to Meza et al. (2006), about 80 % of the deforested area was left unmanaged, while the rest was used for agricultural production in Peru in 2002. This indicates a potential for agroforestry system expansion in this country.
The local leguminous species of the genus Inga are often used as the principal shade trees in Peru (Rice and Ward 2008). Inga species fix N and also produce a great deal of litter, which enriches the soil with organic material (Brack 1999; Rhoades et al. 1998). For this reason they are the ideal shade trees for coffee plantations. However, an increasing number of Peruvian coffee farmers have changed the species composition of their plantations in recent years, replacing traditional legume shade trees with native or exotic timber species. This trend has been previously observed for all of Central America (Galloway and Beer 1997). In Peru, the most common introduced tree species used for shading in coffee plantations are Pinus spp., Eucalyptus spp. and Acrocarpus fraxinifolius. Until now, only a few studies have investigated the impact of the aforementioned introduced tree species on coffee plantations. Shaller et al. (2003), in his study from Costa Rica, found that Eucalypthus deglupta is a suitable shade tree for coffee on sites with high precipitation. With regard to studies dealing with Pinus spp. as agroforestry system species, there are only papers about outplantings of pines on pasturelands (Yeates et al. 2000; Sequeira and Gholz 1991).
On optimal sites, coffee can also be grown without shade (Campanha et al. 2004) but using high agrochemical inputs (Beer et al. 1998). It is estimated that in Mexico, Colombia, Central America and the Caribbean, approximately 40 % of agroforestry coffee plantations were converted to sun coffee in the 1990s (Rice and Ward 1996). Monoculture coffee plantations could be a threat to tropical rainforests because of land degradation and poor land management (Fernandez 2001). The quantity of shade influences the coffee yield: according to Soto-Pinto et al. (2000) shade tree cover had a positive effect in a range between 23 and 38 % shade cover while Muschler (1997) found the best yields at 40 % shade cover. Between 38 and 48 % shade, yield is maintained and it decreases with a shade cover greater than 50 % (Soto-Pinto et al. 2000).
It is important to assess carbon stocks provided by agroforestry ecosystems under different shade trees as an additional ecosystem service. The goal of this study was to compare the ecosystem carbon storage ability of agroforestry coffee plantations with different shade trees and without shading, in both aboveground and belowground carbon pools. The results of this study may contribute to the preservation and planting of shade trees for the benefit of carbon sequestration. Evaluating the carbon storage capacity of coffee agroforestry systems with different shade tree species will contribute to a better understanding of the role that these ecosystems can play in REDD + programs because, as mentioned by Schmitt-Harsh et al. (2012), quantifying and understanding carbon budgets of shade-grown coffee systems is needed for the development of sound climate change mitigation strategies.
Materials and methods
Study area description
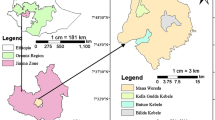
This study was conducted in the Villa Rica district, in the Pasco region of Peru (Fig. 1). The average annual rainfall in this tropical humid mountain forest zone is 1,590 mm, and the average annual temperature is 17.8 °C (Ponce et al. 2008). The rainy season lasts from November to May, while a dry season occurs during July and August (Hamling and Salick 2003). According to the FAO (Food and Agriculture Organization), soils in this region are classified as dystric Cambisols (Egg 2012; Michéli et al. 2006) of low structural stability. The main economic activity in the district is agriculture, mostly from coffee production. Villa Rica is one of the most important districts for coffee production and export in Peru; its landscape is characterised by shaded coffee plantations with some cattle farming.
The following agroforestry coffee plantations were selected for study: Ave Fénix, which was divided into two parts: a first part shaded by Inga spp. (further termed as Inga site) and a non-shaded part (Sun coffee site); Santa Rosa shaded by Pinus spp. (Pinus site) and Gabriela shaded by Eucalyptus spp. (Eucalyptus site). In the past, these sites were used as pastureland. Currently, they are maintained as typical agroforestry systems (except the Sun coffee site) with Coffea arabica as the principal crop, with sparse stands of shading trees.
The coffee plantation Ave Fénix is located in Alto Palomar, near the town of Villa Rica (Table 1; Fig. 1). The Inga site forms one part of this plantation shaded predominantly by Inga spp. It represents a typical shaded coffee plantation in this region as regards management and tree species composition. The other part of the Ave Fénix plantation, where shade trees were absent, is the Sun coffee site serving as a reference coffee plantation without shading. The field has rough-broken topography with an average slope angle of 18.2°. The upper 30 cm of soil at the Inga and Sun coffee sites has its texture classified as loam (Inga site: clay 25.4 %, silt 33.6 %; Sun coffee site: clay 24.4 %, silt 31.3 %, respectively), with extremely acidic soil reaction (pH/KCl 4.0 and 3.7, respectively), and a high amount of organic carbon (Table 5). In the Ave Fénix plantation, we identified the following coffee varieties: Typica, Catimor, Caturra and Catuai.
The Santa Rosa plantation (Pinus site) is located in Oconal, 4 km south of the town of Villa Rica (Table 1; Fig. 1). The average slope angle is 16.4°. In the first 30 cm, soil texture is classified as loam (clay 15.0 %; silt 37.0 %), soil reaction is extremely acidic (pH/KCl 4.1), and the amount of organic carbon is very high (Table 5). The Santa Rosa plantation was chosen because of the Pinus spp. dominance. It is the first generation of coffee shaded by Pinus spp. after a change from pastureland and is also one of the first coffee agroforestry plantations shaded by Pinus spp. in the region. The following coffee varieties were found: Typica, Catimor and Caturra.
The plantation Gabriela (Eucalyptus site) is located 4 km north of the town of Villa Rica (Table 1; Fig. 1). The Gabriela plantation is the smallest, and Eucalyptus spp. are the dominant shade tree species there. This is the first generation of coffee shaded by Eucalyptus spp. after conversion from pastureland, and it is also one of the first coffee plantations shaded by Eucalyptus spp. in the region. The site is more homogenous with an average slope angle of 18.5°. The soil by its texture is classified as loam (clay 19.0 %; silt 33.3 %), with strongly acidic soil reaction (pH/KCl 4.4), and a high amount of organic carbon (Table 5). The following coffee varieties were present: Typica, Catimor and Caturra.
All plantations are certified as organic with similar management and inputs. In the past, the selective thinning of shade trees and coffee shrubs has been used on all plantations. Currently, the shade trees are planted at the same time as coffee shrubs, and after 15–20 years, they are removed and replaced by new ones. Coffee is pruned regularly in systematic way (one line of coffee shrubs is pruned every year) at a height of 0.3–0.5 m, and all farmers left the organic material from pruning on their farm to decompose. Management is relatively simple and applied identically across all coffee plantations at each location.
The actual climate at the studied sites is documented by the vapour pressure deficit (VPD) measured at the Inga and Sun coffee sites during 2011 and 2012. The mean monthly values reach approximately 500 Pa during the dry season, while the VPD is markedly lower during the rainy season. This trend is identical for shaded and open locations, but the VPD in tree shading locations (in this case, in an area dominated by Inga spp.) is generally lower compared to open space coffee plantations.
Tree measurement, biomass and carbon stock estimation
Field measurements were conducted in 2011 and 2012. Diameters at breast height (DBH; measured at 1.3 m) of all tree species ≥10 cm were measured at each study site. In total 1368 trees were measured in the Inga site, 492 in the Pinus site and 511 in the Eucalyptus site. Tree heights were measured by Impulse Forest Pro for almost all trees in the study plots. The missing tree heights for the remaining trees, representing 3.7 % of the entire dataset, were estimated based on the approximated height model according to Eq. 1,
where DBH is the diameter at breast height and p1 and p2 are the parameters that were fitted. This exponential model (Ratkowsky 1990) was parameterised for each tree species individually.
The dry aboveground biomass of shade trees was estimated using available allometric models applicable for the species present at the studied plantations. In the case of Inga spp., Pinus spp. and Eucalyptus spp., the corresponding specific allometric models were used. For all other trees, the generic allometric model developed by Chave et al. (2005) for moist forest stands was used (Table 2), as it was found to be best suited for our study. For this model, the values of tree density from different sources were employed (Nogueira et al. 2005; Barker et al. 2004; Brown 1997; Silva et al. 1994). If a species was included in different databases, then the lowest published wood density was applied. Adjusted values for tree aboveground biomass means and confidence intervals for each plot were obtained by a bootstrapping procedure, using SYSTAT 13.1 statistical software (SYSTAT Software. Inc. USA). The belowground tree biomass component was estimated using the regression equations for predicting root biomass density used by Cairns et al. (1997) (Eq. 2),
where ABD is the aboveground biomass density.
For calculations with errors the method of standard deviation was used (Eq. 3),
where x and y are measured quantities and Δx and Δy their uncertainties.
The quantity of carbon was measured as 50 % of the estimated biomass (IPCC 2003; Roy et al. 2001).
Coffee plant measurement and biomass and carbon stock estimation
Field measurements of coffee shrubs were conducted in 2011. In total, 64 rectangular sample plots of 100 m2 were established to monitor coffee plants. Plots were located randomly in the systematic grid of squares, and the heights of all coffee shrubs were measured. The dry aboveground biomass of coffee plants (BC) was estimated based on the model developed in Segura et al. (2006). We used this equation (Eq. 4) because it was applicable for coffee plants using plant height as the sole independent variable in the logarithmic form
where H is the height of coffee shrubs.
The carbon quantity was measured using the same formula used for trees, which was 50 % of the estimated biomass of coffee shrubs (IPCC 2003; Roy et al. 2001).
Soil properties
Soil samples were collected from six rectangular plots in each site (24 in total) with similar slopes (approximately 15°) which were selected from 64 coffee survey plots. In each square plot, soil samples were collected from three randomly selected sample locations. At each sample location, the soil samples were collected as (1) undisturbed soil samples (physical ring with volume 100 cm3), which were collected from three soil pits in four sampling depths (0–3.5; 3.5–12.5; 12.5–21.5; and 21.5–30 cm), and (2) litter from a circle with a diameter of 0.336 m. Samples were weighed as fresh, as well as oven-dried at 105 °C. The bulk density was calculated using the following formula (Eq. 5),
where r d is the bulk density, m od is the weight of the oven-dried soil sample in g, and V is the volume of the soil sample (100 cm3).
Because the presumed prevailing form of carbon in the investigated soils was the carbon present in soil organic matter (SOM), the carbon content was assessed as the oxidative carbon (Cox) percentage (%), according to Walkley and Black (1934). Soil carbon in Mg ha−1 (SC) was calculated using Eq. 6,
where r d is bulk density in g × cm3, s t is the depth of the sampling zone in cm and Cox is oxidative carbon content in %.
SC was expressed (1) as the cumulative SC content (CSC) within the 30 cm depth to obtain the total SC storage and (2) as the relative value of soil carbon content in 1 cm of each sampling depth (relative soil carbon—RSC). To quantify litter amount, litter samples were oven dried at 105 °C and weighed. The results were recalculated to Mg ha−1. Soil reaction was measured as pH/H2O and pH/1 M KCl, both in a suspension soil sample: extractant 1:1 (w:v), soil texture was assessed using a sedimentary method.
Results
Dendrological and mensurational data
Some of the dominant species in the Inga site based on basal area (BA) were as follows: Inga spp. (Inga adenophylla Pittier, I. densiflora Bentham, I. edulis C. Marius, I. feuillei DC., I. velutina Willdenow), Pinus spp (Pinus oocarpa D. Don., P. tecunumanii (Schwd) Equiluz & Perry), Retrophyllum rospigliosii (Pilger) C. Page (Table 3) and other trees that were less represented, including Euphorbia heterophylla L. and Ficus spp. The Pinus site was dominated by Pinus spp. (Pinus oocarpa, P. tecunumanii) and the most represented tree species were Acrocarpus fraxinifolius Wt. & Arn., Eucalyptus spp., Inga spp., Pinus spp. and Retrophyllum rospigliosii (Table 3). Eucalyptus spp. was the dominant tree species at the Eucalyptus site; other tree species included Acrocarpus fraxinifolius, Inga spp., Pinus spp. and Retrophyllum rospigliosii (Table 3). The hectare indices of counts and the stand basal area of trees and coffee shrubs in the plantations are documented in Table 4. As for tree density, it was highest at the Eucalyptus site and lowest at the Pinus site. The Eucalyptus site also had the largest stand basal area. Inversely to above, the highest density of coffee shrubs was present at the Inga site.
Biomass and carbon stock
The aboveground dry biomass of shade trees varied between 9.9 kg (Erythrina edulis Triana ex Micheli) and 18,400 kg (Cariniana decandra Ducke) per tree (DBH ≥ 10 cm). The biomass of coffee plants was approximately two orders of magnitude smaller, with a mean of 0.5 ± 0.2 kg per coffee plant. The estimated total aboveground biomass was 60.6 ± 6.4 Mg ha−1 for the Inga site, 124 ± 9.4 Mg ha−1 for the Pinus site, 107 ± 6.2 Mg ha−1 for the Eucalyptus site and approximately 1.9 ± 0.2 Mg ha−1 for the Sun coffee site.
A comparison of the tree carbon stock in dominant tree species on the plantations studied yielded approximately 115 ± 51 kg/tree for Inga spp., 387 ± 152 kg/tree for Pinus spp. and 270 ± 197 kg/tree for Eucalyptus spp. The distribution of carbon stocks for dominant tree species by 10-cm diameter classes is shown in Fig. 2.
As expected, the relative soil carbon (RSC) values are the highest in all cases in the upper layers of soil and decrease with depth (see Table 5; Fig. 3). Variability is relatively large at the surface layers of soil at the Inga, Pinus and Eucalyptus sites, where higher heterogeneity due to more intensive interaction with external carbon sources can be expected, which corresponds to variation in litter content (Table 7). The values of RSC, as well as Cox and cumulative SC (Table 7), are highest at the Pinus site, which is mainly due to a slower decrease in carbon content as depth increased. However, at the Sun coffee site, the carbon storage characteristics are not as low as expected due to the absence of trees, but they are higher than at the Inga site. This may be caused by the young age of the plantations (Hergoulac´h et al. 2012) because the effect of shading trees in the agroforestry system is not yet evident on the level of the soil environment. For the cumulative SC values (Fig. 4), polynomial equations were used (Table 6) to compare our values with the results of other studies.
A comparison of the total carbon stock in the different types of coffee plantations is shown in Table 7 and in Fig. 5. The highest amount of carbon in tree biomass was fixed at the Pinus site. The amount of carbon held in the coffee shrubs was also highest at the same site. The amount of carbon held in tree biomass was smaller at the Eucalyptus site and smallest in the Inga site. Although Inga spp. trees have large crowns, their stem diameter and planting densities are low, which explains the lower values of carbon held in tree biomass compared to other plantations with different tree species used for shading. The carbon stock in coffee shrubs was about the same at the Inga and Eucalyptus sites. The smallest amount of carbon held in coffee shrubs was observed in the Sun coffee site.
The total carbon stock at the Inga site was 119.9 ± 19.5 Mg ha−1, of which 69 % was located in the soil and 29 % was located in the trees. The Sun coffee site contained 99.7 ± 17.2 Mg ha−1, which was mainly located in the soil (99 %), while litter and coffee shrubs represented the remaining fraction, estimated at 1 and 0.2 %, respectively. The total carbon stock at the Pinus site was 177.5 ± 14.1 Mg ha−1, where the majority of carbon was fixed in the soil (57 %) and trees (40 %). The total carbon stock at the Eucalyptus site was 162.3 ± 18.2 Mg ha−1, which was mainly located in the soil (59 %) and trees (39.5 %).
The Eucalyptus site fixed the most carbon per year into tree biomass (7.3 Mg ha−1 per year), while slightly less was fixed at the Pinus site (3.8 Mg ha−1 per year); the lowest amount of carbon was fixed at the Inga site (1.8 Mg ha−1 per year).
Discussion
The disappearance of a large proportion of tropical forests at all latitudes could lead to an increase in GHG emissions if sustainable management and conservation policies are not employed (Dixon 1995). In Peru, this problem is evident due to the fast rate of deforestation (Stephen 2005, Oliveira et al. 2007), and it is obvious that agroforestry systems are one of the possibilities that can mitigate GHG emissions from crop production. Our study from the Peruvian Amazon finds that agroforestry systems are important for carbon mitigation. As with other authors (Avila et al. 2001; Hergoulac´h et al. 2012; Soto-Pinto et al. 2010; Dossa et al. 2008), our results demonstrate that carbon stocks are greater in agroforestry coffee plantations than in the coffee plantations without shade. The difference in carbon stocks between agroforestry and sun plantations range from 20.4 Mg ha−1 (the Sun coffee site compared with the Inga site) to 77.8 Mg ha−1 (the Sun coffee site compared with the Pinus site). Other authors reported the following differences between carbon stocks in agroforestry plantations and sun coffee plantations: Hergoulac´h et al. (2012) found a difference of 15.4 Mg ha−1 (for coffee–Inga association) and Dossa et al. (2008) 123.6 Mg ha−1 (for coffee–Albizia association).
It is clear that agroforestry systems store more carbon than open space plantations, but it is also important to identify the distribution of carbon in the ecosystem. In our study, the amount of carbon in tree biomass (which ranges from 27.5 ± 3.2 to 57.5 ± 4.5 Mg ha−1) is greater than the amount published in other studies. In Häger’s study (2012), shade tree carbon storage in an organic agroforestry coffee plantation was 23.2 Mg ha−1 (shaded by Dracaena fragrans and Yucca guatemalensis) and in the study of Häger (2012) for an Inga shaded agroforestry system, it was only 13.9 Mg ha−1. It should be noted that the biomass estimates do not include error components. For example, only the standard error associated with the allometric model of Chave et al. (2005) is 12.5 % and adding sampling and measurement errors would further increase the total uncertainty bounds. But this is inherent in all similar studies.
The carbon stock in coffee biomass by the same authors is 2.3 Mg ha−1 (Häger 2012) and 9.1 Mg ha−1 (Hergoulac´h et al. 2012) with a coffee density of 6045 plants ha−1 and 4722 plants ha−1, respectively. If we compare these data with our results, we see that only the Pinus site has carbon stocks (2.8 Mg ha−1) comparable to the Häger (2012) study, and in the rest of sites, our values are smaller. The coffee density in the Häger (2012) study is comparable with the Inga site (6830 plants ha−1) and that of the Hergoulac´h et al. (2012) study with the Pinus site (4840 plants ha−1), as seen in Table 4. The carbon stock in the litter in studied sites (0.7–1.7 Mg ha−1, Table 7) is smaller than in the studies of Häger (2012) (4.8 Mg ha−1) and Hergoulac´h et al. (2012) (2.2 Mg ha−1). Avila et al. (2001) studied the carbon stock in agroforestry systems in Costa Rica and found that the carbon stock in aboveground pools of 8-year-old Coffea-Eucalyptus plantations was 12.3 Mg ha−1 which is less than in the Eucalyptus site.
Comparing our results from the Sun coffee site with other studies, we conclude that the carbon stock in coffee shrubs and litter is less in our studied site. Hergoulac´h et al. (2012) published the value 8.5 and 1.3 Mg ha−1 for the amount of carbon in aboveground coffee biomass and litter.
Our results of carbon fixed in the deepest 10 cm of soil using equations from Table 6 indicate that the average carbon contents were as follows: 42.3 Mg ha−1 at the Inga site; 43.9 Mg ha−1 at the Sun coffee site; 52.2 Mg ha−1 at the Pinus site, and 46.3 Mg ha−1 at the Eucalyptus site. Schmitt-Harsh et al. (2012) found that the soil in a coffee agroforestry system had a carbon content of 38.2 Mg ha−1, while the value in the secondary forest was 45.1 Mg ha−1. Using equations from studies on carbon stocks in the topsoil (0–25 cm, Table 6), we see the following values: Inga site: 77.5 Mg ha−1, Sun coffee site: 88.9 Mg ha−1, Pinus site: 96.4 Mg ha−1, and Eucalyptus site: 89.2 Mg ha−1, which are comparable with the results of Avila et al. (2001) (108.6 Mg ha−1) and Häger (2012) (73 Mg ha−1). Soto-Pinto et al. (2010) studied the soil to a depth of 30 cm and obtained the value 151.0 Mg ha−1, which is greater than our results for all studied plantations. Another study from Indonesia (Noordwijk et al. 2002) found that the total carbon stock (above 30 cm in the soil) for shade coffee was 82 Mg ha−1, which is comparable with the value from the Inga site.
The published values of total carbon stocks for coffee agroforestry plantations range from 82 to 198 Mg ha−1, while soil carbon might reach over 89 % the total (Avila et al. 2001; Schmitt-Harsh et al. 2012; Noordwijk et al. 2002; Häger 2012; Soto-Pinto et al. 2010; Vásquez et al. 2007; Roncal-García et al. 2008; Dossa et al. 2008). Our results for all studied types of agroforestry plantations are inside this range. However, in our study, the percentage of the entire carbon stock comprised by SC (soil carbon) was 75 % for the Inga site, 65 % for the Pinus site and 67 % for the Eucalyptus site. With no trees present at coffee plantations, the ecosystem carbon stock is basically determined by soil compartment, whereas the carbon held in aboveground pools is not significant: it represented only 1 % of the total in the Sun coffee site.
However, comparing soil carbon stock from the available literature is not easy. This is due to inconsistencies in methodological approaches for soil carbon assessment (Nair 1993) and several factors conditioning carbon sequestration potential. Factors in soil carbon sequestration potential and carbon turnover include soil properties like biological activity, microbial community composition, the molecular recalcitrance of organic matter, soil mineralogy, structure and texture, continuous temperature and humidity. In the case of loam texture with clay content <20 % in soils containing low activity clay (Feller and Beare 1997), the stability of organo-mineral complexes is not obvious, just like resistance to soil erosion. Hence soil carbon needs to be enhanced by suitable management, which might be based on suitable soil cover care, coarser and more stable soil structure and organic matter supply.
Besides natural conditions (climate, topography, soil-forming substrate etc.) the type of agroforestry management also significantly affects the soil carbon sequestration process (von Lützow et al. 2006; Six et al. 2000; Schimel 1994; Parton et al. 1987; Feller and Beare 1997). It includes the type, density and distribution of shading trees providing differently decomposing litter, as well as litter utilization either as mulch retained on the plantation or as a base material for compost production, compost management etc. In addition, management is often changed over time. Hence, time-sequence studies on soil carbon are rare in agroforestry systems (Nair et al. 2009b). Therefore, the data on carbon stock might be estimated as surrogates or indicators of carbon sequestration potential.
The benefit of agroforestry systems for reducing the CO2 in the atmosphere is not only the direct near-term C storage in trees and soils but also the potential to offset immediate GHG emissions associated with deforestation and subsequent shifting agriculture (Dixon 1995). For growers, it is important to know how much wood they could produce in an agroforestry system and what they will do with it. In our study, we studied plantations shaded by introduced and local tree species. The wood of Inga trees is usually used as firewood by the plantation owners, and the wood of Eucalyptus spp. and Pinus spp. is sold as construction material. Both uses are very important because they reduce pressure on the rainforests from the local community.
The Peruvian state should improve the monitoring of agroforestry plantations, assessing their area and quality. This information would help to better understand the role of agroforestry in the landscape and make the related policy decisions more effective. It is important to continue to study introduced species in agroforestry plantations in South America and evaluate more precise equations for local plantations. It is also essential to study the behaviour of Eucalyptus and Pinus tree species, especially in agroforestry systems in South America, because it is a new trend and information on the effects on plantations shaded by these species is insufficient.
Conclusions
Agroforestry systems play an important role in fixing carbon in agricultural landscapes that have lost their original forest cover. They are especially important in tropical areas that have been suffering over the past century from exceptional rates of change as they are degraded by human activities. The amount of sequestered carbon depends on the tree species used for shading. Our study, conducted in Peru, suggests that the coffee agroforestry plantations shaded by introduced tree species (Pinus spp., Ecualyptus spp.) perform better with regard to carbon storage than those shaded by the local tree genus Inga.
The results should be generally applicable to agroforestry coffee plantations with organic certification, where the coffee is grown at a comparable altitude, in a similar climate and with comparable soil conditions. The carbon sequestration potential can also be considered in REDD + programs in which Peru could participate. However, it is also important to consider and evaluate the impact of introduced tree species on biodiversity, soil fertility, hydric function and appearance of coffee diseases in order to make sound management decisions on the tree species used for shading.
References
Avila G et al (2001) Almacenamiento, fijacion de carbono y valoracion de servicios ambientales en sistemas agroforestales en Costa Rica. Agroforestría en las Americas 8:32–35
Barker TR et al (2004) Variation in wood density determines spatial patterns in Amazonian forest biomass. Glob Change Biol 10:545–562
Batjes NH, Sombroek WG (1997) Possibilities for carbon sequestration in tropical and subtropical soils. Glob Change Biol 3:161–173
Beer J, Muschler R, Kass D, Somarriba E (1998) Shade management in coffee and cacao plantation. Agrofor Syst 38:139–164
Brack A (1999) Diccionario enciclopedio de plantas utiles del Peru. CBC—Centro de estudios regionales andino “Bartolome de las casa”, Cusco
Brown S (1997) Estimating biomass and biomass change of tropical forests. FAO
Cairns MA et al (1997) Root biomass allocation in the world’s upland forests. Oecologia 111:1–11
Campanha MM, Henrique R, Santos S et al (2004) Growth and yield of coffee plants in agroforestry and monoculture systems in Minas Gerais, Brazil. Agrofor Syst 63:75–82
Chave J, Andalo C, Brown S, Cairns M et al (2005) Tree allometry and improved estimation of carbon stocks and balance in tropical forests. Oecologia 145:87–99
Dixon RK (1995) Agroforestry systems: sources of sinks of greenhouse gases? Agrofor Syst 31:99–116
Dossa EL, Fernandes ECM, Reid WS, Ezui K (2008) Above and belowground biomass, nutrient and carbon stock contrasting an open-grown and a shaded coffee plantation. Agrofor Syst 72:103–115
Egg AB (2012) Ecología del Perú. Bruno, Lima
Feller C, Beare MH (1997) Physical control of soil organic matter dynamics in the tropics. Geoderma 79:69–116
Fernandez S (2001) Deforestation, coffee cultivation and land degradation: the challenge of developing a sustainable land management strategy in Brazil’s Mata Atlântica rainforest. Thesis, Lund University Master’s Programme in Environmental Science
Galloway G, Beer J (1997) Oportunidades para fomentar la silvicultura en cafetales en América Central. Serie Técnica. Informe Técnico No. 285. CATIE. Turrialba, Costa Rica
Häger A (2012) The effects of management and plant diversity on carbon storage in coffee agroforestry systems in Costa Rica. Agrofor Syst 86:159–174
Hamling CH, Salick J (2003) Yanesha agriculture in the upper Peruvian Amazon: persistence and change fifteen years down. Econ Bot 57:163–180
Hergoulac´h K, Blancart E, Skiba U, Hénault C, Harmand JM (2012) Changes in carbon stock and greenhouse gas balance in coffee (Coffea arabica) monoculture versus an agroforestry system with Inga densiflora, in Costa Rica. Agric Ecosyst Environ 148:102–110
IPCC (2003) Good practice guidance for land use, land-use change and forestry. IPCC National Greenhouse Gas Inventories Programme and Institute for Global Environmental Strategies, Kanagawa, Japan. http://www.ipcc-nggip.iges.or.jp/public/gpglulucf/gpglulucf_contents.html. Accessed 11 March 2013
IPCC (2013) Climate change 2013. The Physical science basis. Intergovernmental panel on climate change. Cambridge University Press. http://www.climatechange2013.org/images/report/WG1AR5_ALL_FINAL.pdf. Accessed 23 July 2013
Leff B, Ramankutty N, Foley JA (2004) Geographic distribution of major crops across the world. Global Biochem Cycles 18:1–27
Lewin B, Giovannucci D, Varangis P (2004) Coffee markets new paradigms in global supply and demand. Agriculture and Rural Development Discussion, paper 3, The World Bank
Meza A, Sabogal C, Jong W (2006) Rehabilitación de áreas degradadas en la Amazonia peruana Revisión de experiencias y lecciones aprendidas 1. CIFOR, p. 107
Muschler RG (1997) Efectos de sombra de Erythrina poeppigiana sobre Coffea arabica vars. Caturra y Catimor. Memorias del XVIII Simposium Latinoamericano de Cafeticultura, September 1997. San Jose, Costa Rica pp. 157–162
Michéli E, Schad P, Spaargaren O, Dent D, Nachtergale F (2006) World reference baser for soil resources. World Soil Resources Reports 103. Food and Agricultural Organization of the United Nations, Rome
MINAM (2010) Segunda Comunicación Nacional del Perú a la Convención Marco de las Naciones Unidas sobre Cambio Climático. http://cdam.minam.gob.pe/novedades/peruycambioclimaticoresumen.pdf. Accessed 2 August 2013
Moguel P, Toledo VM (1999) Biodiversity conservation in traditional coffee systems of Mexico. Conserv Biol 13:11–21
Nair PKR (1993) An introduction to agroforestry. Kluwer Academic Publisher, Dordrecht
Nair PKR, Mohan KB, Nair VD (2009a) Agroforestry as a strategy for carbon sequestration. J Plant Nutr Soil Sci 172:10–23
Nair PKR, Nair VD, Kumar BM, Solomon GH (2009b) Soil carbon sequestration in tropical agroforestry systems: a feasibility apprasial. Environ Sci Policy 12:1099–1111
Návar J (2009) Allometric equations for trees species and carbon stock for forests of north-western Mexico. For Ecol Manag 257:427–434
Nogueira EM, Nelson BW, Fearnside PM (2005) Wood density in dense forest in central Amazonia, Brazil. For Ecol Manag 208:261–286
Noordwijk M, Rahayu S, Hairiah K, Wulan YC, Farida A, Verbist B (2002) Carbon stock assessment for a forest-to-coffee conversion landscape in Sumber-Jaya (Lampung, Indonesia): from allometric equations to land use change analysis. Sci China 45:75–86
Noponen MR, Haggar JP, Edwards-Jones G, Healey JR (2013) Intensification of coffee systems can increase the effectiveness of REDD mechanisms. Agric Syst 119:1–9
Oliveira PJC et al (2007) Land-use allocation protects the Peruvian Amazon. Science 317:1233–1236
Parton WJ, Schimel DS, Cole CV, Ojima DS (1987) Analysis of factors controlling soil organic matter levels in great plains grassland. Soil Sci Soc Am J 51:1173–1179
Perfecto I, Rice RA, Greenbergr R, Van der Voort ME (1996) Shade coffee: disappearing refuge for biodiversity. Bioscience 46:596–608
Ponce MG et al. (2008) Plan de Desarollo Concentrado de Villa Rica 2009–2018. Municipalisas distrital de Villa Rica, provincia Oxapampa, Región Pasco
Ratkowsky DA (1990) Handbook of nonlinear regression. Marcel Dekker, New York
Rhoades CC, Eckert GE, Coleman DC (1998) Effect of pasture trees on soil nitrogen and organic matter : implications for Tropical Montane forest restoration. Restor Ecol 6:262–270
Rice RA, Ward J (1996) Coffee, conservation and commerce in the western hemisphere. The Smithsonian Migratory Bird Center and the Natural Resources Defense Council, Washington
Rice RA, Ward J (2008) Agricultural intensification within agroforestry: the case of coffee and wood products. Agric Ecosyst Environ 128:212–218
Roncal-García S, Soto-Pinto L, Castellanos JA, Ramírez NM, Jong B (2008) Sistemas agroforestales y almacenamiento de carbono en comunidades indígenas de Chiapas, México. Interciencia 33:200–206
Roy J, Saugier B, Mooney HA (2001) Terrestrial global productivity. Academic, San Diego
Ruark GA, Schoeneberger MM, Nair PKR (2003) Agroforestry–Helping to Achieve Sustainable Forest Management. UNFF (United Nations Forum for Forests) Intersessional Experts Meeting on the Role of Planted Forests in Sustainable Forest Management, New Zealand. http://maf.govt.nz/unff-planted-forestry-meeting. Accessed 2 August 2013
Saint-André L et al (2005) Age-related equations for above- and below- ground biomass of Eucalyptus hybrid in Congo. For Ecol Manag 205:199–214
Schimel D (1994) Climatic, edaphic, and biotic controls over storage and turnover of carbon in soils. Global Biochem Cycles 8(3):279–293
Schmitt-Harsh M et al (2012) Carbon stock in coffee agroforests and mixed dry tropical forests in the western highlands of Guatemala. Agrofor Syst 86:141–157
Schroth et al (2002) Conversion of secondary forest into agroforestry and monoculture plantations in Amazonia: consequences for biomass, litter and soil carbon stocks after 7 years. For Ecol Manag 163:131–150
Sequeira W, Gholz HL (1991) Canopy structure, light penetration and tree growth in a slash pine (Pinus elliottii) silvo-pastoral system at different stand configurations in Florida. For Chron 67:263–267
Segura M, Kanninen M, Suárez D (2006) Allometric models for estimating aboveground biomass of shade trees and coffee bushes grown together. Agrofor Syst 68:143–150
Shaller M, Götz S, Beer J, Jimenéz J (2003) Species and site characteristics that permit the association of fast-growing trees with crops: the case of Eucalyptus deglupta as coffee shade in Costa Rica. For Ecol Manag 175:205–215
Silva FG, Barrichelo LEG, Shimoyama VRS, Wiecheteck MSS (1994) Avaliação da qualidade da madeira de Pinus patula var. tecunumanii visando a produção de celulose kraft e pasta mecânica. O Papel 55:32–35
Six J, Elliott ET, Paustian K (2000) Soil macroaggregate turnover and microaggregate formation: a mechanism for C sequestration under no-tillage agriculture. Soil Biol Biochem 32:2099–2103
Smith J, Scherr SJ (2003) Capturing the value of forest carbon for local livelihoods. World Dev 31:2143–2160
Soto-Pinto L, Perfecto I, Castillo-Hernandez J, Caballero-Nieto J (2000) Shade effect on coffee production at the northern Tzeltal zone of the state of Chiapas, Mexico
Soto-Pinto L, Anzueto M, Mendoza J, Ferrer GJ, Jong B (2010) Carbon sequestration through agroforestry in indigenous communities of Chiapas, Mexico. Agrofor Syst 78:39–51
Stephen GP (2005) Population land use and deforestation in the pan Amazon basin: a comparison of Brazil, Bolivia, Colombia, Ecuados, Peru and Venezuela. Environ Dev Sustain 7:23–49
The World Bank (2011) Estimating the Opportunity Costs of REDD+
Vásquez EH, Ángeles GVC, del Valle JRE, Ortiz GR, Velasco VAV (2007) Captura des carbono por Inga jinucul Schltdl. en un sistema agroforestal del café bajo sombra. Revista Mexicana de ciencias forestales 3(9):11–20
von Lützow M, Kögel-Knabner I, Ekschmitt K, Matzner E, Guggenberg G, Marschner B, Flessa H (2006) Stabilization of organic matter in temperate soils: mechanisms and their relevance under different soil conditions—a review. Eur J Soil Sci 57:426–445
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining soil organic matter and a proposed modifications of the chromic acid titration method. Soil Sci 37:29–38
Wintgens JN (2004) Coffee: growing, processing, sustainable production: a guidebook for growers, processors, traders, and researchers. Druckhaus Darmstadt GmbH, Darmstad
Yeates GW, Hawke MF, Rijkse WC (2000) Changes in soil fauna and soil conditions under Pinus radiata agroforestry regimes during a 25 year tree rotation. Biol Fertil Soils 31:391–406
Acknowledgments
This study was mainly supported by the Internal Grant Agency (IGA 29/2011) of the Faculty of Forestry and Wood Technology, Mendel University in Brno, Institutional research plan MSM6215648902—Forest and Wood: the support of functionally integrated forest management and use of wood as a renewable raw material, project N29/2011 and TA 02020867 Use of the new organic mineral stimulators and natural organic materials for revitalisation of the forest ecosystems influenced by biotic and abiotic impacts. Additional financial support was obtained by Jorge Mattos Olavarria, General Manager of MapGeosolution and the POPRAR project CZ.1.07/2.2.00/28.0303. We are grateful to the Marín and Carrillo families and Ms. Selena Contreras for their help and for letting us conduct this study on their plantations.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ehrenbergerová, L., Cienciala, E., Kučera, A. et al. Carbon stock in agroforestry coffee plantations with different shade trees in Villa Rica, Peru. Agroforest Syst 90, 433–445 (2016). https://doi.org/10.1007/s10457-015-9865-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-015-9865-z