Abstract
We analysed the soil nematode community within three different montados (agricultural, pastoral and forestry uses), focusing on temporal variation. Nematodes were classified into trophic groups (bacterivores, fungivores, omnivores, predators and plant-parasitic nematodes (PPN)) and we calculated the maturity index for free-living taxa (MI), maturity index for plant-parasitic taxa (PPI) and the nematode channel ratio (NCR). Temporal variations were most evident during winter when there was a rise in the abundance of the five functional groups. Concordantly, there was a simultaneous increase of soil moisture and organic matter, due to litter decomposition. Fungivore abundance was highest in the forest and the temporal occurrence and abundance of many PPN genera was largely determined by land use. Land management was responsible for differences in plant community structure and composition, thus plant diversity increased from the agricultural to the forestry use. Because the montado shows great temporal variability in vegetation structure our results of MI, PPI and PPI/MI ratio explain the significant changes in the nutritional status over time, with the highest values recorded in the spring. NCR is a good indicator of energy efficiency in the soil decomposition process. Its lowest values were attained in the forest, where the slow-growing plant species favoured a lower activity of the bacterial energy channel and a prevalence of the fungal-based decomposition energy channel. Nematode composition reflected plant succession, changes in decomposition in the soil food web and temporal variations in the structure of soil.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In the southern Iberian Peninsula, Mediterranean forests were dominated by cork oak (Quercus suber) and holm oak (Q. ilex) trees. However, since the late Middle Ages and especially between 1850 and 1950, many of these forests have been lost by human activities such as shrub clearing, ploughing, tree thinning and conversion to agricultural land (Díaz et al. 1997). Presently, these ecosystems, known as montados in Portugal, consist of an open tree layer with a shrub or annual herbaceous understory and are characterized by a systematic combination of agricultural, pastoral, and forestry uses (Pinto-Correia 1993; Joffre et al. 1999; Costa et al. 2009). Cork oaks trees are of major economical importance in montados as their bark (i.e., cork) can be harvested every 9 years, a process that does not harm the trees because the cork layer regenerates after extraction (Pausas et al. 2009). Moreover, montados are protected habitats within the EU Habitats Directive (92/43/EEC) and are considered high nature value farming systems, according to the classification proposed by the European Environmental Agency (Paracchini et al. 2008). This ecosystem has evolved over centuries as a consequence of management activities but, at present, montados are undergoing an unprecedented rate of change. The intensification of grazing and ploughing activities in the undercover hampers tree regeneration and at the same time it disrupts the physical properties of soils, putting at risk the maintenance of this ecosystem in the long-term (Plieninger and Wilbrand 2001; Plieninger 2007; Bugalho et al. 2011a, b). Therefore, the preservation of the montado depends on the consistent application of a management strategy that promotes tree regeneration and soil protection (Pulido and Díaz 2005; Bugalho et al. 2011a; Arosa et al. 2014 submitted).
Nematodes constitute the most abundant group within the soil mesofauna, with free-living nematodes playing an indispensable role in decomposing processes occurring in soil ecosystems (Yeates 1979). The cycling of nutrients above ground is controlled by herbivores, including nematodes, mainly by stimulating soil microbial activity, nutrient recycling and increased nutrient acquisition by plants from soil (McNaughton et al. 1997; Bardgett et al. 1998). Trophic interactions can be assigned to nematodes in the majority of soils (e.g., Moorhead et al. 2002) and these can be classified into five functional groups: bacterivores, fungivores, plant-feeders, predators and omnivores. Bacterivores and fungivores are indirectly involved in decomposition and nitrogen and carbon mineralization by feeding on bacteria and fungi, including mycorrhiza (Ferris et al. 2004; Yeates and Coleman 1982). Plant-parasitic nematodes (hereafter referred to using the abbreviation PPN) interact with flora by feeding on plant roots or shoots (Ingham et al. 1985). Predator nematodes feed upon soil invertebrates including other nematodes, enchytraeids, tardigrades and protozoa (Moore and de Ruiter 1991). Omnivores do not hold a separate position in the food chain but may add connectedness to the food web (Coleman et al. 1993) by feeding on more than one food source, including bacteria, fungi, algae, protozoa and rotifers. Nematode analysis is currently gaining interest in ecological studies in order to assess the functioning of soils (Wilson and Kakouli-Duarte 2009). Nematode community indices are a useful tool for monitoring environmental conditions and soil ecosystem function (Bongers and Ferris 1999; Ferris et al. 2001; Neher 2001; Berkelmans et al. 2003). The nematode community can be characterized by the maturity index for free-living taxa (MI) and the maturity index for plant-parasitic taxa (PPI) (Bongers 1990; Bongers and Ferris 1999). For the calculation of MI, soil nematodes are categorized into a 1–5 coloniser (r-strategists) and persister (k-strategists) ranking, termed as cp value, following the assumption of a shift toward opportunistic species under stress conditions (Odum 1985; Bongers 1990). The MI is the weighted mean cp value of the number of individuals in a representative soil sample; index values increase for low levels of disturbance. The index values vary according to the levels of disturbance, from less than 2.0 in nutrient-enriched disturbed systems to ±4.0 in pristine environments. Nematodes that feed on higher plants are omitted from the calculation of the MI because their occurrence and abundance is largely determined by the community structure, host status and vigour of plants growing in the soil. Consequently, the equivalent of the MI for plant-feeding nematodes (the plant-parasite index, PPI) is calculated separately (Bongers 1990, Bongers and Ferris 1999). The ratio PPI/MI (Bongers et al. 1997) is a sensitive indicator of enrichment. Moreover, the nematode channel ratio (NCR) expresses the energy efficiency in soil decomposition processes, providing information on the relative dominance of the bacterial or fungal energy channel based on the abundance of bacterial and fungal feeding nematodes in the soil ecosystem (Yeates 2003). These energy channels represent two lines of dead biomass consumption: the fungal energy channels predominate when organic material is of high C/N ratio, when organic material is of low C/N ratio bacterial decomposition channels predominate (Moore and Hunt 1988; Ferris and Bongers 2006; Costa et al. 2011).
The main objective of this study is to provide a functional interpretation of the soil nematode community structure in montados in three sites due to land management practices and through time. We assess if differences in the nematode communities reflect differences in management of montados regarding agricultural, pastoral and forestry uses. Sites with high levels of grazing and soil ploughing for agriculture, are believed to be occupied by organisms that are less able to colonize and become established presenting lower values of maturity indices. On the other hand, we consider the effect of time which, by modifying soil physical–chemical properties, can also affect nematode densities (Yeates et al. 1997; Bardgett and Cook 1998; Costa et al. 2011). Additionally, we will evaluate the potential burden of PPN in the different types of montado to estimate whether these can affect cork oak seedlings, therefore representing a limitation to regeneration.
Methods
Experimental sites
Our study area was set in Montemor-o-Novo, Portugal, most specifically in the Herdade do Freixo do Meio (38°42′N, 8°19′W), an organic farm that manages 1,140 ha of cork-holm oak montado. Within the study area, we selected three sites of approximately 30 ha each representing different management precedents concerning agricultural, pastoral and forestry uses. Cork harvesting and tree pruning were the main forestry activities undertaken in the three sites, despite the fact that they did not take place every year.
Site A was a grassland with scattered cork and holm oaks (25 trees/ha). This site was mechanically disc ploughed in autumn for the sowing of clover (Trifolium spp.) and intensive sheep grazing occurred during part of the year (winter, spring and autumn).
Site B was a dense cork-holm oak montado (40 trees/ha) with shrub undergrowth of Cistus spp. and Asparagus acutifolius. There were no agricultural uses in this site, although it was moderately used by livestock (pigs in winter and sheep in autumn).
Site C was a dense cork oak montado (45 trees/ha), including a minor number of holm oaks and stone pines (Pinus pinea), and a high density heterogeneous understory composed by shrub formations of Cistus spp., A. acutifolius, Ulex australis subsp. welwitschianus, Pistacia lentiscus, Arbutus unedo, Phillyrea angustifolia, Crataegus monogyna, Quercus coccifera and Lavandula pedunculata. This site management comprised only forestry uses.
To account for climatic differences among sites, temperature and relative humidity measures were obtained with a thermohygrometer (HOBO Pro v2 logger, Onset Computer Corporation, USA) at each site. These thermohygrometers were placed 1.5 m above the ground under tree canopy and data were hourly recorded for 1 year starting in January 2012. Soil chemical properties and moisture content were also examined in the three sites twice, coinciding with spring and autumn.
Nematode sampling, extraction and identification
Sampling was carried out in the three study sites four times during 2012. As each sampling coincided with a season, to simplify the text sampled time will be named as winter, autumn, spring and summer. Samples of rhizosphere soil (10 samples/site/season) were collected at a depth of 10–20 cm, placed in individual plastic bags (1,000 cm3) and kept in cold storage at 4 °C until processed. Soil samples were collected within sites at random but the presence of a cork oak seedling was mandatory in order to use its root for the extraction of endoparasitic nematodes. We used an adaptation of the generalist Tray Method for nematode extraction: living mobile nematodes were extracted from 100 cm3 sieved soil samples for 72 h and endoparasitic nematodes were extracted from sieved root samples for 240 h (Whitehead and Hemming 1965). Preparations were observed under an inverted microscope at 100–400× magnification. We counted all extracted nematodes in the sample. For the determination of the feeding group we counted a subsample of 100 nematodes that were classified into functional groups (bacterivores, fungivores, PPN, predators and omnivores) according to their digestive system structure (Tarjan and Hopper 1974; Bongers and Bongers 1998). With these results both maturity indices (MI and PPI) and NCR were calculated. In order to describe PPN community and to determine if these should have an effect on cork oaks, we further identified PPN to genus level (Mai and Mullin 1996).
Statistical analysis
Analyses of variance (ANOVA), followed by post hoc Tukey tests (α = 0.05), were used to test for temporal differences among sites in the number of individuals of each functional group per preparation. Data were log-transformed for normality and homogeneity of variances. To test for differences in the number of bacterivores, PPN and total number of nematodes per preparation we used Kruskal–Wallis tests, followed by multiple comparisons of mean ranks for all groups, because variance was not homogeneous, even after data transformation. ANOVAs were also performed to test for differences among sites in mean daily temperature and relative humidity. All analyses were conducted using Statistica version 8.0 (StatSoft Inc. 2007).
Principal components analyses (PCA) were performed using CANOCO for Windows version 4.5 (ter Braak and Smilauer 2002) to describe temporal patterns in PPN genera composition among sites. This analyses reduces the dimensionality of the PPN genera while retaining most of the variation in the data set. Samples can be plotted allowing us to visually assess similarities and differences between PPN genera. The number of individuals of each PPN genera per preparation (log-transformed) were used as dependent variables through time.
Generalized Linear Models (GLM) were used to test the temporal and site effects for the indices of ecosystem function (MI, PPI, PPI/MI and NCR). Significant differences among groups were further elucidated through the post hoc Tukey test at the 0.05 level. GLM were performed with Statistica version 8.0 (StatSoft Inc. 2007).
Results
Soil nematodes
A mean of 751 ± 660 nematodes were counted per preparation extracted from the soil (100 cm3). The most abundant nematodes were bacterivores followed by fungivores, especially in site A and B while in site C the abundance of fungivore was higher than the other functional groups (bacterivores, PPN, predators and omnivores). Predators and omnivores were considerably less abundant in all sites (Fig. 1). There were no significant differences in the total number of nematodes among sites (H2,117 = 0.84, p = 0.656), however, it was significantly highest in winter (H3,116 = 68.35, p < 0.001). Significant differences in nematodes abundance among sites were only found in fungivores (F2,117 = 4.15, p = 0.018), although these did not occur through time. Nevertheless, an increasing gradient of fungivore abundance was found every sampled time from site A to site C. All functional groups showed significant or nearly significant differences over time (bacterivores: H3,116 = 59.37, p < 0.001; fungivores: F3,116 = 32.17, p < 0.001; PPN: H3,116 = 40.82, p < 0.001; predators: F3,116 = 43.92, p < 0.001; omnivores: F3,116 = 2.65, p = 0.053) and higher abundances were found in winter. Fungivore abundance was significantly lower in summer (sites A and C) and autumn (sites A, B and C), as was the abundance of predators in the three sites.
Temporal abundance of soil nematodes, classified into functional groups, detected in the three sites (see “Methods” for information on experimental sites). Data correspond to the mean ± SD of ten replicates per site
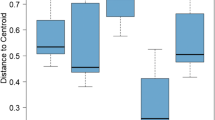
The GLM applied to the indices of ecosystem function showed the highest values of MI (F12, 278.1 = 5.88, p < 0.05), PPI (F12, 278.1 = 7.79, p < 0.01) and PPI/MI (F12, 278.1 = 6.06, p < 0.01) in spring. Within sites, only the NCR was significantly lower in site C (F8, 210 = 4.32, p < 0.05) (Table 1).
Endoparasitic and living mobile PPN
Endoparasitic nematodes were not found in any of the preparations extracted from sieved roots of cork oak seedlings (n = 120). On the contrary, PPN extracted from soil samples consisted of 11 genera (number of samples where present): Criconemella (31), Gracilacus (60), Helicotylenchus (98), Hemicycliophora (2), Heterodera (16), Longidorus (42), Pratylenchus (92), Rotylenchus (74), Trichodorus (5), Tylenchorhynchus (70) and Xiphinema (41).
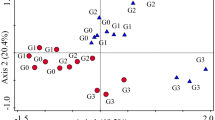
The PCA (Fig. 2) reduced the original 30 × 11 matrices, one for each sampling, to two independent principal components that explained 24.2 % and 21.8 % (winter), 30.2 % and 18.7 % (spring), 24.5 % and 18.1 % (summer), and 24.8 % and 18.3 % (autumn) of the variation, respectively.
Biplots with PPN genera temporal variability and sample scores, according to site (A X-mark, B empty square, C filled square). Codes for genera are Cri Criconemella, Gra Gracilacus, Hel Helicotylenchus, Hem Hemicycliophora, Het Heterodera, Lon Longidorus, Pra Pratylenchus, Rot Rotylenchus, Tri Trichodorus, Tyl Tylenchorhynchus, and Xip Xiphinema
Every sampling showed a regular discrimination between site A and samples from the two other sites, according to PPN variability. The genera that most contributed for this segregation were Gracilacus, Helicotylenchus, and to a lesser extent Criconemella and Rotylenchus. In spring, the variability of the genus Rotylenchus did not stand for that distinction, supporting instead the separation of samples from site B. Samples from site B were generally discriminated by the variability of the genus Xiphinema, although most noticeably in spring. Moreover, in summer, samples from site B showed an inverse relationship with the variability of Longidorus and Tylenchorhynchus. Overall, no particular PPN genus fomented the segregation of samples from site C, except in autumn when it was determined by the variability of Criconemella.
Soil and climatic data
Temperature and relative humidity data recorded during this study agree with the Mediterranean climate of long, hot, dry summer and a mild, humid winter (mean annual temperature = 15.5 °C, mean annual relative humidity = 73.6 %). Among sites mean daily temperature was lowest in B and relative humidity was highest in B (temperature: F2,1,095 = 2.96, p > 0.05; relative humidity: F2,1,095 = 5.06, p < 0.01). Concerning the two samplings, the three sites showed no differences with respect to soil chemical properties and soil moisture content (Table 2). All tested soils were dry and acidic, which compromises the uptake of macronutrients, and had low levels of stable organic matter. These types of soils are prone to erosion and plant cover and soil biota may play crucial roles in the maintenance of a functioning soil layer. The organic matter content increased in the autumn, possibly due to the accumulation of dead plant material in the previous summer, without a correspondent decrease in soil pH. The increased levels of stable organic matter in the autumn may improve soil structure and provide nutrients, after the water and temperature stresses of the previous summer.
Discussion
Temporal variations in nematode communities were most evident during winter when there was a rise in the abundance of the five functional groups in every experimental site. The high availability of food sources, e.g., fungi, plant roots or shoots and microbial communities, during that period may explain these differences (Ferris et al. 2004). Concordantly, there is a simultaneous increase of soil moisture and organic matter, due to litter decomposition, in winter (Sohlenius et al. 2011). Although temperatures are more favourable to decomposition processes in the spring for montado ecosystems, microbial activity peaks in the rainy season (autumn and winter), after the litter inputs resulting from the summer drought (Costa et al. 2013). The increased organic matter content of soil between the spring and the autumn may result from litter inputs from the understory shrub vegetation (Correia et al. 2014) however in site C, which had the most dense and diverse shrub understory, an increase in organic matter and macronutrients was not observed. In fact, the herbaceous vegetation of site A may have a larger contribution to the soil organic matter pool after the summer senescence than the dense shrub understory of site C (Otieno et al. 2011). Litter origin (shrub vs. herbaceous) and hence litter quality is also known to affect decomposition rates, with the former being decomposed at slower rates than the latter (Castro et al. 2010). The soil C/N ratio and soil pH increased with site disturbance (from A to C) in the spring, which agrees with results obtained elsewhere for areas under increasing cattle grazing regimes (Bardgett et al. 2001), but not in the autumn. None of the measured soil parameters could be related to populations of any of the nematode trophic groups or genera. Although it is widely assumed that soil properties influence nematode distribution and damage to crops in agricultural systems, a direct relationship cannot often be found between presence and/or abundance of given genera and soil physic-chemical parameters (Chen et al. 2012). However, the cycling of nutrients in the soil is controlled mainly by nematodes, by stimulating microbial growth when feeding on them, based on the comparative estimates of other studies (e.g., Ingham et al. 1985; Hunt et al. 1987; Ferris et al. 1998). Bacterivorous and fungivorous nematodes appear to be the major contributors to N mineralisation. Increases in mineral N may follow the irrigation of dry soil (Sparling and Ross 1988; Lundquist et al. 1999) and consequently N mineralisation is probably offset by the considerable leaching of N that occurs during winter months (Poudel et al. 2001). However, grazing by aboveground herbivores can interfere over rates of vegetation succession, altering the quantity and quality of litter inputs to soil, which in turn affects soil biota and rates of soil nutrient cycling (Bardgett and Wardle 2003; Wardle et al. 2004).
Contrary to our expectations, distinct land uses had a small effect on nematode communities in montados while concerning functional assemblages. Nevertheless, there were noteworthy differences concerning fungivore abundance. This result is in agreement with the prominent role of fungi in ecosystem processes under low grazing, or completely unmanaged systems (Bardgett et al. 2001) and could be related to the input of lignin-rich, fungal-decomposition based litter originating from the understory shrubs (Castro et al. 2010). The temporal occurrence and abundance of many PPN genera was largely determined by land use. The genera Gracilacus and Helicotylenchus were associated to agriculture and livestock grazing (site A). Management was responsible for differences in plant community structure and composition among land uses, thus plant diversity increased from A to C. Given that PPN genera may have different levels of specificity to their hosts, it would be predictable that C site was the one with higher PPN diversity (Wardle et al. 2003; De Deyn et al. 2004; Viketoft et al. 2005). In our study, the number of PPN genera was identical in the three land uses; nonetheless, their variability was more homogeneous in site C.
Despite PPN being one of the most representative groups in the soil, endoparasitic nematodes were not found in roots of cork oak seedlings. As a significant number of root samples were sampled in the three land uses, we assume that PPN are not likely a limitation to cork oak regeneration since they did not affect seedling establishment.
Whilst data of nematode abundance was useful, the indices of maturity and trophic diversity are less variable than populations of individual trophic groups and thus may be more useful to detect trends in the ecological condition of soils (Neher and Darby 2009), providing a more structured ordination. Our results of MI, PPI and PPI/MI ratio explained the significant changes in the nutritional status over time following modifications in the dynamics of vegetation (Bongers et al. 1997), because the montado shows great variability in vegetation structure through time. The highest values of MI, PPI and PPI/MI appeared in spring. According to Bongers (1999), the MI varies from 1 under enriched conditions to 3 or 4 under undisturbed conditions. An increasing population of bacterial feeders, with high reproduction rates, results in a decreasing MI. The persister nematodes (with higher cp values) are more resistant to disturbances and with higher values MI represents a less disturbed site. The PPI is comparable to MI but based on plant parasitic nematodes and may increase with increasing soil fertility, whereas the MI decreases (Bongers 1990; Bongers and Ferris 1999). This inverse relationship is not shown by our data, which supports the data suggesting organic farming systems are more sustainable through the lack of synthetic pesticide and fertilizer use, and better closed nutrient cycles (Mulder et al. 2005). As the MI and the PPI may show diverging trends, a merging of the information provided by the life strategy (MI) and the functional information given by the PPI is the and PPI/MI ratio (Bongers et al. 1997). We used this ratio as a useful additional parameter to detect differences through time. This ratio significantly varied with the highest values reached in spring. These ratios were higher than 1.6 in several of our observations, indicating that resource utilization by the plants may be far from optimal, according to Bongers et al. (1997) that suggests such high ratios are indicative of severely nutrient enriched habitats. In relation to site only the NCR showed significant differences. NCR is a good indicator to express the energy efficiency in soil decomposition process (Moore et al. 1988; Yeates 2003), providing information on the dominant way in which the breakdown of organic matter proceeds with the participation of bacteria or fungi. Values for this ratio are constrained between 0 (totally fungi mediated) and 1 (totally bacteria mediated). The highest values were attained in A and B, suggesting a higher activity of the bacterial energy channel in these sites with fast-growing plant species, which dominate in early succession and produce high quality litter (i.e., N-rich) promoting bacterial–dominated food webs. NCR values were lowest in autumn and in C (0.32 ± 0.20) evidencing a prevalence of the fungal-based decomposition energy channel, where slow-growing plants dominate in late succession and produce low-quality, phenolic–rich litter that favours fungal dominated food webs (Wardle 2002; Bardgett 2005). In addition, sites A and B are areas where the soil is regularly mechanized and grazed, and the detected differences among sites could indicate that soil conditions in C are more stable (Viketoft et al. 2011).
This work is the first to use the soil nematodes as indicators of disturbance in the montado ecosystem. Nematode composition provided information on plant succession and changes in decomposition pathways in the soil food web. Our results stressed temporal variations in the structure of soil nematodes communities despite the effect of disturbance caused by land use. Future studies should be carried out in other areas of montado in order to confirm these patterns on a broad scale. Also, routine analysis of nematode fauna in different agroforestry systems affords rapid assessment of response to soil management practices and levels of stressors, so that sustainable optimal yields can be attained.
References
Arosa ML, Ceia RS, Costa SR, Freitas H (2014) Factors affecting cork oak (Quercus suber) regeneration: acorn sowing success and seedling survival under field conditions. Plant Ecol Divers (in press)
Bardgett RD (2005) The biology of soil: a community and ecosystem approach. Oxford University Press, UK
Bardgett RD, Cook R (1998) Functional aspects of soil animal diversity in agricultural grasslands. Appl Soil Ecol 10:263–276
Bardgett RD, Wardle DA (2003) Herbivore mediated linkages between aboveground and belowground communities. Ecology 84:2258–2268
Bardgett RD, Wardle DA, Yeates GW (1998) Linking above-ground and below-ground interactions: how plant responses to foliar herbivory influence soil organisms. Soil Biol Biochem 30:1867–1878
Bardgett RD, Jones AC, Jones DL, Kemmitt SJ, Cook R, Hobbs PJ (2001) Soil microbial community patterns related to the history and intensity of grazing in sub-montane ecosystems. Soil Biol Biochem 33:1653–1664
Berkelmans R, Ferris H, Tenuta M, van Bruggen AHC (2003) Effects of long-term crop management on nematode trophic levels other than plant feeders disappear after 1 year of disruptive soil management. Appl Soil Ecol 23:223–235
Bongers T (1990) Oecologia disturbance based on nematode species composition. Freshw Biol 83:14–19
Bongers T (1999) The maturity index, the evolution of nematode life history traits, adaptive radiation and cp-scaling. Plant Soil 212:13–22
Bongers T, Bongers M (1998) Functional diversity of nematodes. Appl Soil Ecol 10:239–259
Bongers T, Ferris H (1999) Nematode community structure as a bioindicator in environmental monitoring. Trends Ecol Evol 14:224–228
Bongers T, van der Meulen H, Korthals G (1997) Inverse relationship between the nematode maturity index and plant parasite index under enriched nutrient conditions. Appl Soil Ecol 6:195–199
Bugalho MN, Caldeira MC, Pereira JS, Aronson J, Pausas JG (2011a) Mediterranean cork oak savannas require human use to sustain biodiversity and ecosystem services. Front Ecol Environ 9:278–286
Bugalho MN, Lecomte X, Gonçalves M, Caldeira MC, Branco M (2011b) Establishing grazing and grazing-excluded patches increases plant and invertebrate diversity in a Mediterranean oak woodland. For Ecol Manag 261:2133–2139
Castro H, Fortunel C, Freitas H (2010) Effects of land abandonment on plant litter decomposition in a montado ecosystem: relation to litter chemistry and community functional parameters. Plant Soil 333:181–190
Chen SY, Sheaffer CC, Wyse DL, Nickel P, Kandel H (2012) Plant-parasitic nematode communities and their associations with soil factors in organically farmed fields in Minnesota. J Nematol 44:361–369
Coleman DC, Hendrix PF, Beare MH, Cheng W, Crossley DA Jr (1993) Microbial and faunal dynamics as they affect soil organic matter dynamics in subtropical agroecosystems. In: Paoletti MG, Foissner W, Coleman DC (eds) Soil biota and nutrient cycling farming systems. Lewis Publishing Company, Chelsea, pp 1–14
Correia AC, Costa e Silva AV, Correia AV, Hussain MZ, Rodrigues AD, David JS, Pereira JS (2014) Carbon sink strength of a Mediterranean cork oak understory: how do semi-deciduous and evergreen shrubs face summer drought? J Veg Sci 25:411–426
Costa A, Pereira H, Madeira M (2009) Landscape dynamics in endangered cork oak woodlands in Southwestern Portugal (1958–2005). Agrofor Syst 77:83–96
Costa SR, Van Der Putten WH, Kerry BR (2011) Microbial ecology and nematode control in natural ecosystems. In: Davies Keith G, Spiegel Yitzhak (eds) Biological control of plant-parasitic nematodes. Springer, The Netherlands
Costa D, Freitas H, Sousa SP (2013) Influence of seasons and land-use practices on soil microbial activity and metabolic diversity in the ‘Montado ecosystem’. Eur J Soil Biol 59:22–30
De Deyn GB, Raaijmakers CE, VanRuijven J, Berendse F, Van Der Putten WH (2004) Plant species identity and diversity effects on different trophic levels of nematodes in the soil food web. Oikos 106:576–586
Díaz M, Campos P, Pulido FJ (1997) The Spanish dehesas: a diversity of land use and wildlife. In: Pain D, Pienkowski M (eds) Farming and birds in Europe. Academic Press, London
Ferris H, Bongers T (2006) Nematode indicators of organic enrichment. J Nematol 3:3–12
Ferris H, Venette RC, van der Meulen HR, Lau SS (1998) Nitrogen mineralization by bacterial-feeding nematodes: verification and measurement. Plant Soil 203:159–171
Ferris H, Bongers T, de Goede RGM (2001) A framework for soil food web diagnostics: extension of nematode faunal analysis concept. Appl Soil Ecol 18:13–29
Ferris H, Venette RC, Scow KM (2004) Soil management to enhance bacterivore and fungivore nematode populations and their nitrogen mineralization function. Appl Soil Ecol 24:19–35
Hunt HW, Coleman DC, Ingham ER, Ingham RE, Elliott ET, Moore JC, Rose SL, Reid CPP, Morley CR (1987) The detrital food web in a shortgrass prairie. Biol Fertil Soils 3:57–68
Ingham RE, Trofymow JA, Ingham ER, Coleman DC (1985) Interactions of bacteria, fungi, and their nematode grazers: effects on nutrient cycling and plant growth. Ecol Monogr 55:19–140
Joffre R, Rambal S, Ratte JP (1999) The dehesa system of southern Spain and Portugal as a natural ecosystem mimic. Agrofor Syst 45:57–79
Lundquist EJ, Scow KM, Jackson LE, Uesugi SL, Johnson CR (1999) Rapid response of soil microbial communities from conventional, low input, and organic farming systems to a wet/dry cycle. Soil Biol Biochem 31:1661–1675
Mai WF, Mullin PG (1996) Plant-parasitic nematodes: a pictorial key to genera. Comstock Publishing Associates, USA
McNaughton SJ, Banyikwa FF, McNaughton MM (1997) Promotion of the cycling of diet-enhancing nutrients by African grazers. Science 278:1798–1800
Moore JC, de Ruiter PC (1991) Temporal and spatial heterogeneity of trophic interactions within below-ground food webs. Agric Ecosyst Environ 34:371–397
Moore JC, Hunt HW (1988) Resource compartmentation and the stability of real ecosystems. Nature 333:261–263
Moore JC, Walter DE, Hunt HW (1988) Arthropod regulation of micro- and mesobiota in below-ground detrital food webs. Annu Rev Entomol 33:419–439
Moorhead DL, Wall DH, Virginia RA, Parsons AN (2002) Distribution and life-cycle of Scottnema lindsayae (Nematoda) in Antarctic soils: a modeling analysis of temperature responses. Polar Biol 25:118–125
Mulder C, Schouten AJ, Hund-rinke K, Breure AM (2005) The use of nematodes in ecological soil classification and assessment concepts. Ecotoxicol Environ Saf 62:278–289
Neher D (2001) Role of nematodes in soil health and their use as indicators. J Nematol 33:161–168
Neher DA, Darby BJ (2009) General community indices that used for analysis of nematode assemblages. In: Wilson MJ, Kakouli-Duarte T (eds) Nematodes as environmental indicators. CABI, Wallingford, pp 107–123
Odum EP (1985) Trends expected in stressed ecosystems. BioScience 35:419–422
Otieno DO, Mirzaei H, Hussain MZ, Li YL, Schmidt MWT, Wartinger M, Jung E, Ribeiro N, Pereira JS, Tenhunen J (2011) Herbaceous layer development during Spring does not deplete soil nitrogen in the Portuguese montado. J Arid Environ 75:231–238
Paracchini ML, Petersen JE, Hoogeveen Y, Bamps C, Burfield I, van Swaay C (2008) High nature value farmland in Europe. An estimate of the distribution patterns on the basis of land cover and biodiversity data, Luxemburg: Office for Official Publications of the European Communities
Pausas J, Pereira J, Aronson J (2009) The tree. In: Aronson J, Pereira JS, Pausas JG (eds) Cork oak woodlands on the edge. Ecology, adaptive management and restoration. Island Press, Washington DC, pp 11–21
Pinto-Correia T (1993) Threatened landscape in Alentejo, Portugal: the ‘montado’ and other ‘agro-silvopastoral’ systems. Landsc Urban Plan 24:43–48
Plieninger T (2007) Compatibility of livestock grazing with stand regeneration in Mediterranean holm oak parklands. J Nat Conserv 15:1–9
Plieninger T, Wilbrand C (2001) Land use, biodiversity conservation, and rural development in the dehesas of Cuatro Lugares, Spain. Agrofor Syst 51:23–34
Poudel DD, Horwath WR, Mitchell JP, Temple SR (2001) Impacts of cropping systems on soil nitrogen storage and loss. Agric Syst 68:253–268
Pulido FJ, Díaz M (2005) Regeneration of a Mediterranean oak: a whole-cycle approach. Écoscience 12:92–102
Sohlenius B, Boström S, Viketoft M (2011) Effects of plant species and plant diversity on soil nematodes e a field experiment on grassland run for seven years. Nematology 13:15–131
Sparling GP, Ross DJ (1988) Microbial contributions to the increased nitrogen mineralization after air-drying of soils. Plant Soil 105:163–167
StatSoft Inc., 2007. STATISTICA (data analysis software system), version 8.0. http://www.statsoft.com
Tarjan AC, Hopper BE (1974) Nomenclatorial compilation of plant and soil nematodes. Society of Nematologists Inc., E. O. Painter Printing Co., DeLeon Springs, FL
ter Braak CJF, Smilauer P (2002) CANOCO reference manual and canodraw for windows user’s guide: software for canonical community ordination (version 4.5). Biometris, Wageningen
Viketoft M, Palmborg C, Sohlenius B, Huss-Danell K, Bengtsson J (2005) Plant species effects on soil nematode communities in experimental grasslands. Appl Soil Ecol 30:90–103
Viketoft M, Sohlenius B, Boström S, Palmborg C, Bengtsson J, Berg MP, Huss-Danell K (2011) Temporal dynamics of soil nematode communities in a grassland plant diversity experiment. Soil Biol Biochem 43:1063–1070
Wardle DA (2002) Communities and ecosystems: linking the aboveground and belowground components. Princeton University Press, USA
Wardle DA, Yeates GW, Williamson W, Bonner KI (2003) The response of a three trophic level soil food web to the identity and diversity of plant species and functional groups. Oikos 102:45–56
Wardle DA, Bardgett RD, Klironomos JN, Setälä H, Van Der Putten WH, Wall DH (2004) Ecological linkages between aboveground and belowground biota. Science 304:1629–1633
Whitehead AG, Hemming JR (1965) A comparison of some quantitative methods of extracting small vermiform nematodes from soil. Ann Appl Biol 55:25–38
Wilson MJ, Kakouli-Duarte T (2009) Nematodes as environmental indicators. CABI, Wallingford
Yeates GW (1979) Soil nematodes in terrestrial ecosystems. J Nematol 11:213–229
Yeates GW (2003) Nematodes as soil indicators: functional and biodiversity aspects. Biol Fertil Soils 37:199–210
Yeates GW, Coleman DC (1982) Nematodes in decomposition. In: Freckman DW (ed) Nematodes in soil ecosystems. University of Texas, Austin
Yeates GW, Bardgett RD, Cook R, Hobbs PJ, Bowling PJ, Potter JF (1997) Faunal and microbial diversity in three Welsh grassland soils under conventional and organic management regimes. J Appl Ecol 34:453–470
Acknowledgments
The present work was financed by a Ph.D. Grant (SFRH/BD/70708/2010) and a post-doc Grant (SFRH/BPD/26496/2006) from FCT (Portuguese Foundation for Science and Technology) and was partially included within the RESCOE project (PTDC/BIA-BEC/102834/2008) financed by the FCT through the COMPETE program (FCOMP-01-0124-FEDER-008937). We are grateful to Alfredo Sendim from Herdade do Freixo do Meio who kindly allowed us to develop this work at his property. We also appreciated the help of Ricardo Ceia in field work.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Arosa, M.L., Freitas, H. & Costa, S.R. Temporal effects dominate land use as factors affecting soil nematode communities in Mediterranean oak woodlands. Agroforest Syst 90, 127–136 (2016). https://doi.org/10.1007/s10457-014-9760-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10457-014-9760-z