Abstract
Matrix Gla protein (MGP) is an antagonist of bone morphogenetic proteins and expressed in vascular endothelial cells. Lack of MGP causes vascular abnormalities in multiple organs in mice. The objective of this study is to define the role of MGP in early endothelial differentiation. We find that expression of endothelial markers is highly induced in Mgp null organs, which, in wild type, contain high MGP expression. Furthermore, Mgp null embryonic stem cells express higher levels of endothelial markers than wild-type controls and an abnormal temporal pattern of expression. We also find that the Mgp-deficient endothelial cells adopt characteristics of mesenchymal stem cells. We conclude that loss of MGP causes dysregulation of early endothelial differentiation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Normal differentiation of vascular endothelial cells (ECs) is essential for maintaining vascular stability and integrity. EC differentiation requires tight regulation of both intrinsic genetic determinants and microenvironmental factors, which provide extracellular signals for ECs to adapt to the vascular bed. Many extracellular factors have been shown to regulate EC differentiation, such as vascular endothelial growth factor (VEGF), Wnt, activin, fibroblast growth factors (FGFs) and bone morphogenetic proteins (BMPs) [1]. In cross talk with both Wnt and Notch signaling, BMPs regulate EC proliferation, specification and cell motility [1]. In particular, BMP-4 has been shown to be critical for determining the EC fate [2].
Matrix Gla protein (MGP) is an efficient inhibitor of BMP-2, 4 and 7 [3–6]. MGP is best known for its ability to prevent calcification [7, 8], but is highly expressed in tube-forming ECs [9, 10] and acts as an inhibitor of angiogenesis [6, 11]. We previously demonstrated that excessive MGP in mice inhibits pulmonary vascular growth by limiting BMP-4 activity and VEGF expression [6, 12]. Mgp gene mutations in humans cause multiple developmental defects (the so-called Keutel syndrome) [13], which include abnormal growth of vasculature [14]. Several studies have shown that deletion of the Mgp gene in mice causes vascular abnormalities, including arterial calcification and arteriovenous malformations (AVMs) in lungs, kidneys and brain [6, 15]. In addition, the studies suggest altered EC differentiation and the presence of endothelial–mesenchymal transitions (EndMTs) in Mgp null mice [8].
However, the role of MGP in early endothelial differentiation is unclear. This study examines whether lack of MGP disrupts endothelial differentiation in endothelial progenitor cells derived from embryonic stem cells (ESCs).
Methods
Animals
Mgp +/− mice on C57BL/6J background were obtained from the Jackson laboratory (strain: B6.129S7-Mgptm1Kry/KbosJ). Genotypes were confirmed by PCR [16], and experiments were performed with generation F4–F6. Littermates were used as wild-type controls. All mice were fed a standard chow diet (Diet 8604, HarlanTeklad Laboratory). The studies were reviewed and approved by the Institutional Review Board and conducted in accordance with the animal care guideline set by the University of California, Los Angeles. The investigation conformed to the National Research Council, Guide for the Care and Use of Laboratory Animals, Eighth Edition (Washington, DC: The National Academies Press, 2011).
Tissue culture and EC differentiation
Wild-type ESCs (C57BL/6 J background) were obtained from ATCC (SCRC-1002). Mouse ESCs were cultured and maintained as previous described [17]. The derivation of ECs from ESCs was performed by a previously published protocol [18]. BMP-4, activin A, FGF-2, Noggin and VEGF (all from R&D Systems), anti-Jagged 1 antibodies (Abcam) and anti-Jagged 2 antibodies (Abgent) were added to StemPro-34® medium prior to use. The process of derivation lasted 14 days.
RNA analysis
Real-time PCR analysis was performed as previously described [19]. Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) was used as a control gene [19]. Primers and probes for mouse MGP, fetal liver kinase 1 (Flk1), fms-like tyrosine kinase 1 (Flt1), cluster of differentiation (CD) 31, CD34, VE-cadherin, von Willebrand factor (vWF), tyrosine-protein kinase Kit (c-kit), CD90, Snail, N-cadherin, octamer-binding transcription factor 3/4 (Oct3/4), Nanog homeobox (Nanog), Sry-box2 (Sox2) and stage-specific embryonic antigen (SSEA) 1 and 3 were obtained from Applied Biosystems as part of Taqman® Gene Expression Assays.
Immunofluorescence
Immunofluorescence was performed as previously described in detail [8]. We used specific antibodies for CD34 and Flk1 (both from Cell Signaling Technology). The nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich).
Flow cytometric analysis
Fluorescence-activated cell sorting analysis was done as previously described [8]. The cells were stained with fluorescein isothiocyanate (FITC)-, phycoerythrin (PE)- or Alexa Fluor 488 (AF-488)-conjugated antibodies against CD34, Flk1 and VE-cadherin. Nonspecific fluorochrome- and isotype-matched IgGs (BD Pharmingen) served as controls.
Statistical analysis
Data were analyzed for statistical significance by ANOVA with post hoc Tukey’s analysis. The analyses were performed using GraphPad Instat®, version 3.0 (GraphPad Software). Data represent mean ± SD. P values less than 0.05 were considered significant, and experiments were repeated a minimum of three times.
Results
Enhanced expression of EC markers in multiple organs of Mgp−/− mice
To assess how MGP expression relates to endothelial differentiation, we examined the expression of MGP and the endothelial-specific markers Flk1, VE-cadherin and vWF in Mgp +/+ and Mgp −/− organs, including lungs, kidneys, brain, aorta, heart, stomach, intestine, bone, muscle and liver by real-time PCR. MGP was highly expressed in lungs, kidneys, aorta, heart, stomach and intestine (Fig. 1a). In Mgp −/− mice, EC markers were strongly induced in several of these organs, including lungs, kidneys and aorta (Fig. 1b–d). These are the same organs where AVMs or vascular calcification has been documented [6, 8]. The EC markers were also highly induced in Mgp −/− brain (Fig. 1b–d), although the total MGP expression in wild-type brain was relative low (Fig. 1a). The data were consistent with the previous findings of AVMs in Mgp −/− brains [15] and suggested that MGP expression may be limited to the brain ECs. The endothelial markers were mildly increased in heart, stomach, intestine, bone and muscle of Mgp −/− mice, which had relatively lower levels of MGP expression (Fig. 1a–d). There were no significant changes in endothelial markers in Mgp −/− liver (Fig. 1b–d), which had very low MGP expression (Fig. 1a) and no detectable phenotype in Mgp −/− mice [7].
Expression of endothelial markers in tissues and organs of Mgp−/− mice. a Expression of MGP in the lungs, kidneys, brain, aorta, heart, stomach, intestine, bone, muscle and liver of wild-type mice. Expression of MGP was determined by real-time PCR with normalization to GAPDH expression. The MGP expression in the different organs is calculated as fold change compared to the MGP expression in the lungs of wild-type mice. b, c Expression of VE-cadherin (b), Flk1 (a) and von Willebrand factor (vWF) (c) in the lungs, kidneys, brain, aorta, heart, stomach, intestine, bone, muscle and liver of wild-type (Mgp +/+) and Mgp −/− mice. ***P < 0.0001; **P < 0.01; *P < 0.05
Derivation of Mgp−/− embryonic stem cells
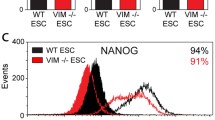
To determine whether MGP regulates early endothelial differentiation, we generated Mgp −/− mouse ESCs by using a previously published protocol [17]. We isolated Mgp −/− embryoblast at E3.5 days and cultured them on Mgp −/− mouse embryonic fibroblast feeders (Fig. 2a, top). After 7–9 days of culture, the ESC colonies were disaggregated and expanded (Fig. 2a, bottom). We compared the expression of pluripotency markers to wild-type ESCs by real-time PCR and found that the expression of Oct3/4, Nanog, Sox2, and SSEA 1 and 3 was unchanged in Mgp −/− ESCs (Fig. 2b).
We also performed karyotypic analysis of Mgp −/− ESCs, which showed that 49 of 50 spreads had 40 chromosomes and the cell line was 98 % euploid. The Mgp −/− ESCs were then expanded and used for further experiments.
Enhanced EC induction in Mgp −/− ESCs
To assess the effect of MGP on endothelial differentiation, Mgp −/− and wild-type ESCs were driven into EC lineage by established protocols [18]. We first examined the expression of MGP at different time points of derivation from day 1 to day 14. In wild type, expression of MGP increased slightly on day 3 and kept increasing from day 6 to day 14 (Fig. 3a). No MGP expression was detected in Mgp −/− ESCs. Then, we examined the expression of endothelial markers Flk1, Flt1, CD31, CD34 and VE-cadherin. In wild-type cells, expression of Flk1 showed an early peak on day 6 and continued high expression from day 6 to day 14 (Fig. 3a). The expression of Flt1, CD31 and VE-cadherin peaked on day 9, whereas expression of CD34 peaked on day 12. The results suggested that, under these conditions of derivation, different EC markers had different patterns of expression, with Flk1 being the earliest marker.
Expression of endothelial markers increase during Mgp+/+ and Mgp−/− EC derivation. a Time course of expression of MGP, Flk1, Flt1, CD31, CD34 and VE-cadherin as determined by real-time PCR during EC derivation from Mgp +/+ and Mgp −/− ESCs. Gene expression is calculated as fold change compared to the expression of Mgp +/+ control cells on day 1. b Comparison of expression pattern of MGP, Flk1, Flt1, CD31, CD34 and VE-cadherin in ECs derived from Mgp +/+ and Mgp −/− ESCs. c Comparison of expression pattern of Flk1, Flt1, CD31, CD34 and VE-cadherin in ECs derived from Mgp −/− ESCs after adding noggin (top), or anti-jagged 1 and 2 antibodies (bottom)
We then examined the expression of EC markers in cells derived from Mgp −/− ESCs. We found that expression of all EC markers was higher in Mgp −/− cells compared to wild-type controls (Fig. 3a). We also observed that the pattern of expression of EC markers was altered. To better visualize this, we compared the expression pattern in cells derived from wild-type ESCs and Mgp −/− ESCs by combining the curves from Fig. 3a in two graphs using arbitrary units (Fig. 3b). In cells derived from Mgp −/− ESCs, expression of Flk1 was increased a second time on day 14 (Fig. 3b, bottom). Furthermore, in Mgp −/− cells, the induction of Flt1, CD31 and VE-cadherin occurred earlier, and the expression of CD34 and VE-cadherin peaked earlier, compared to wild-type controls (Fig. 3b, bottom). Overall, the progression of the EC differentiation was less organized in the Mgp −/− cells than in the wild-type controls.
To determine whether inhibition of BMP improved the Mgp −/− EC differentiation, we added Noggin on day 3 of the EC derivation from Mgp −/− ESCs and examined the expression of EC markers. The results showed that Noggin decreased induction of EC markers in cells derived from Mgp −/−ESCs (Fig. 3c, top), confirming that increased BMP activity contributed to abnormal EC differentiation.
We previously showed that the Notch ligands Jagged 1 and Jagged 2 were abnormally induced in Mgp −/− cerebral vasculature [15]. To determine whether limiting Jagged 1 and 2 affected Mgp −/− EC differentiation, we added anti-Jagged 1 and 2 neutralizing antibodies on day 3 in the EC derivation from Mgp −/− ESCs. We examined the expression of EC markers and found that inhibition of Jagged 1 and 2 reduced induction of EC markers in cells derived from Mgp −/− ESCs, confirming that Notch signaling is involved in abnormal Mgp −/− EC differentiation.
We confirmed the enhanced expression of EC markers on day 9 during the EC derivation by flow cytometric analysis using specific antibodies to CD34, Flk1 and VE-cadherin. The analysis showed that the populations of CD34, Flk1 or VE-cadherin positive cells in cells derived from Mgp −/− ESCs were about double the size of those from wild-type ESCs (Fig. 4a). We also costained ECs derived from Mgp −/− ESCs with antibodies to CD34 and Flk1 on day 9. The CD34 and Flk1 staining was more intense in ECs derived from Mgp −/− ESCs than in wild-type controls (Fig. 4b), consistent with the flow cytometric analysis.
Enhanced number of EC-like cells derived from Mgp−/− ESCs. a Flow cytometry analysis of ECs derived from Mgp+/+ and Mgp−/− ESCs using specific antibodies to CD34, Flk1 and VE-cadherin ECs at day 9. b Costaining of CD34 and Flk1 in ECs derived from Mgp+/+ and Mgp−/− ES cells on day 9. Scale bars 50 µm
Enhanced mesenchymal stem cell characteristic in ECs derived from Mgp−/− ESCs
We previously showed that lack of MGP caused stem cell characteristic to emerge in the aortic endothelium [8]. To investigate whether ECs derived from Mgp −/− ESCs exhibited stem cell characteristics, we first sorted Flk1-positive ECs derived from Mgp −/− and wild-type ESCs by flow cytometry. We then examined the expression of the stem cell markers c-kit, CD90 and N-cadherin, and the mesenchymal marker Snail by real-time PCR in the Flk1-positive cells. The results showed that the expression of all these markers increased in ECs derived from Mgp −/− ESCs on days 6, 9 and 12, compared to wild-type cells (Fig. 5a). CD90 showed a significant increase already on day 3 (Fig. 5a). We further examined whether lack of MGP affected the expression pattern of stem cell markers in ECs derived from ESCs. We combined the curves from Fig. 5a in graphs using arbitrary units (Fig. 5b). In ECs from wild-type and Mgp −/− ESCs, CD90 peaked on day 3, whereas c-kit peaked on day 6 in Mgp −/− ECs and on day 9 in wild-type ECs. Snail and N-cadherin increased progressively from day 3 to day 12 in the Mgp −/− ECs. The results suggested that ECs derived from Mgp −/− ESCs maintain expression of stem cell markers and may start to undergo EndMTs.
Expression of mesenchymal stem cell markers in Mgp−/− ECs. a Expression of c-kit, CD90, N-cadherin and Snail in ECs derived from Mgp+/+ and Mgp−/− ESCs, which were presorted using specific antibodies to Flk1, as shown by real-time PCR. b Comparison of expression patterns of c-kit, CD90, N-cadherin and snail in ECs derived from Mgp+/+ and Mgp−/− ESCs
Together, our results suggested that lack of MGP caused dysregulation of early EC differentiation
Discussion
In this study, we found enhanced expression of endothelial markers in multiple organs of Mgp −/− mice. The induction of endothelial markers in MGP deficiency was often strong in organs that normally expressed a lot of MGP. Moreover, lack of MGP dysregulated differentiation of ECs when derived from ESCs and caused characteristics of mesenchymal stem cells to emerge.
MGP is normally highly expressed in ECs [20, 21], and deletion of the Mgp gene in mice causes a number of vascular abnormalities including AVMs and irregular vessel caliber in the lungs, kidneys and brain, an increased number of glomeruli in the kidneys, and calcification in the elastic arteries [6, 8, 15, 22]. The dysregulation of endothelial differentiation and appearance of stem cell characteristics when MGP is reduced, nonfunctioning or missing are likely to form the basis for such vascular abnormalities.
Our results showed that Mgp −/− and wild-type ESCs had identical pluripotency while still undifferentiated and that differentiation of Mgp −/− ECs turned abnormal between day 3 to day 6 when MGP usually begins to express. It is consistent with previous reports that did not detect expression of MGP in the mesenchymal epithelial interphase until E10.5 in mice [23], and supports a role for MGP once the initial vasculature has been established.
Our results further suggested that MGP regulates EC differentiation by inhibiting BMP activity. Lack of MGP increases BMP activity in ECs of multiple organs, such as aorta, lung, brain and kidneys [6, 15, 20]. Here, we show that lack of MGP promotes EC differentiation, whereas Noggin reduces the EC induction. We argue that MGP regulates EC differentiation, both the level of expression and the timing of induction, in ESCs by controlling the BMP activity.
BMP-4 is known to induce expression of MGP, which provides negative feedback inhibition by binding and inhibiting BMP-4 [12]. BMP activity is important for both maintaining stem cell characteristics and promoting EC differentiation [24, 25] and has been shown to induce EndMTs in ECs [8, 26]. In published protocol and our experiments, BMP-4 acts as a critical exogenous factor when ECs are derived from ESCs [18]. The loss of MGP is likely to dysregulate the activity of BMP-4 and potentially other BMPs, triggering the abnormal progression of the endothelial differentiation.
In our experiments, lack of MGP increased both EC and mesenchymal stem cell markers, suggesting that MGP helps differentiate endothelial lineage from early mesenchymal differentiation [27]. The results showed that lack of MGP increased the expression of the mesenchymal stem cell markers CD90 and c-kit as well as the duration of the expression, indicating an enhanced mesenchymal state in MGP-deficient conditions. Also, we showed that Snail and N-cadherin increased simultaneously with EC markers, suggesting that Mgp −/− ECs adopt stem cell characteristics, previously noted in the Mgp −/− aortic ECs [8]. Overall, the results are consistent with our previous results, showing that stem cell and EC markers coexist in Mgp −/− ECs.
References
Le Bras A, Vijayaraj P, Oettgen P (2010) Molecular mechanisms of endothelial differentiation. Vasc Med 15(4):321–331. doi:10.1177/1358863X10371685
Chiang PM, Wong PC (2011) Differentiation of an embryonic stem cell to hemogenic endothelium by defined factors: essential role of bone morphogenetic protein 4. Development 138(13):2833–2843. doi:10.1242/dev.061440
Bostrom K, Tsao D, Shen S, Wang Y, Demer LL (2001) Matrix GLA protein modulates differentiation induced by bone morphogenetic protein-2 in C3H10T1/2 cells. J Biol Chem 276(17):14044–14052. doi:10.1074/jbc.M008103200
Zebboudj AF, Imura M, Bostrom K (2002) Matrix GLA protein, a regulatory protein for bone morphogenetic protein-2. J Biol Chem 277(6):4388–4394. doi:10.1074/jbc.M109683200
Yao Y, Shahbazian A, Bostrom KI (2008) Proline and gamma-carboxylated glutamate residues in matrix Gla protein are critical for binding of bone morphogenetic protein-4. Circ Res 102(9):1065–1074. doi:10.1161/CIRCRESAHA.107.166124
Yao Y, Jumabay M, Wang A, Bostrom KI (2011) Matrix Gla protein deficiency causes arteriovenous malformations in mice. J Clin Investig 121(8):2993–3004. doi:10.1172/JCI57567
Luo G, Ducy P, McKee MD, Pinero GJ, Loyer E, Behringer RR, Karsenty G (1997) Spontaneous calcification of arteries and cartilage in mice lacking matrix GLA protein. Nature 386(6620):78–81. doi:10.1038/386078a0
Yao Y, Jumabay M, Ly A, Radparvar M, Cubberly MR, Bostrom KI (2013) A role for the endothelium in vascular calcification. Circ Res 113(5):495–504. doi:10.1161/CIRCRESAHA.113.301792
Glienke J, Schmitt AO, Pilarsky C, Hinzmann B, Weiss B, Rosenthal A, Thierauch KH (2000) Differential gene expression by endothelial cells in distinct angiogenic states. Eur J Biochem 267(9):2820–2830
Enis DR, Dunmore B, Johnson N, Pober JS, Print CG (2008) Antiapoptotic activities of bcl-2 correlate with vascular maturation and transcriptional modulation of human endothelial cells. Endothelium 15(1):59–71. doi:10.1080/10623320802092393
Yao Y, Nowak S, Yochelis A, Garfinkel A, Bostrom KI (2007) Matrix GLA protein, an inhibitory morphogen in pulmonary vascular development. J Biol Chem 282(41):30131–30142. doi:10.1074/jbc.M704297200
Yao Y, Zebboudj AF, Shao E, Perez M, Bostrom K (2006) Regulation of bone morphogenetic protein-4 by matrix GLA protein in vascular endothelial cells involves activin-like kinase receptor 1. J Biol Chem 281(45):33921–33930. doi:10.1074/jbc.M604239200
Munroe PB, Olgunturk RO, Fryns JP, Van Maldergem L, Ziereisen F, Yuksel B, Gardiner RM, Chung E (1999) Mutations in the gene encoding the human matrix Gla protein cause Keutel syndrome. Nat Genet 21(1):142–144. doi:10.1038/5102
Meier M, Weng LP, Alexandrakis E, Ruschoff J, Goeckenjan G (2001) Tracheobronchial stenosis in Keutel syndrome. Eur Respir J 17(3):566–569
Yao Y, Yao J, Radparvar M, Blazquez-Medela AM, Guihard PJ, Jumabay M, Bostrom KI (2013) Reducing Jagged 1 and 2 levels prevents cerebral arteriovenous malformations in matrix Gla protein deficiency. Proc Natl Acad Sci USA 110(47):19071–19076. doi:10.1073/pnas.1310905110
Yao Y, Jumabay M, Ly A, Radparvar M, Wang AH, Abdmaulen R, Bostrom KI (2012) Crossveinless 2 regulates bone morphogenetic protein 9 in human and mouse vascular endothelium. Blood 119(21):5037–5047. doi:10.1182/blood-2011-10-385906
Czechanski A, Byers C, Greenstein I, Schrode N, Donahue LR, Hadjantonakis AK, Reinholdt LG (2014) Derivation and characterization of mouse embryonic stem cells from permissive and nonpermissive strains. Nat Protoc 9(3):559–574. doi:10.1038/nprot.2014.030
Israely E, Ginsberg M, Nolan D, Ding BS, James D, Elemento O, Rafii S, Rabbany SY (2014) Akt suppression of TGFbeta signaling contributes to the maintenance of vascular identity in embryonic stem cell-derived endothelial cells. Stem Cells 32(1):177–190. doi:10.1002/stem.1521
Bostrom K, Zebboudj AF, Yao Y, Lin TS, Torres A (2004) Matrix GLA protein stimulates VEGF expression through increased transforming growth factor-beta1 activity in endothelial cells. J Biol Chem 279(51):52904–52913. doi:10.1074/jbc.M406868200
Yao Y, Bennett BJ, Wang X, Rosenfeld ME, Giachelli C, Lusis AJ, Bostrom KI (2010) Inhibition of bone morphogenetic proteins protects against atherosclerosis and vascular calcification. Circ Res 107(4):485–494. doi:10.1161/CIRCRESAHA.110.219071
Bostrom KI, Jumabay M, Matveyenko A, Nicholas SB, Yao Y (2011) Activation of vascular bone morphogenetic protein signaling in diabetes mellitus. Circ Res 108(4):446–457. doi:10.1161/CIRCRESAHA.110.236596
Bostrom KI, Guihard P, Blazquez Medela AM, Yao J, Moon JH, Penton A, Yao Y (2015) Matrix Gla protein limits pulmonary arteriovenous malformations in ALK1 deficiency. Eur Respir J 45(3):849–852. doi:10.1183/09031936.00114714
Luo G, D’Souza R, Hogue D, Karsenty G (1995) The matrix Gla protein gene is a marker of the chondrogenesis cell lineage during mouse development. J Bone Miner Res 10(2):325–334. doi:10.1002/jbmr.5650100221
Vicente Lopez MA, Vazquez Garcia MN, Entrena A, Olmedillas Lopez S, Garcia-Arranz M, Garcia-Olmo D, Zapata A (2011) Low doses of bone morphogenetic protein 4 increase the survival of human adipose-derived stem cells maintaining their stemness and multipotency. Stem Cells Dev 20(6):1011–1019. doi:10.1089/scd.2010.0355
Boyd NL, Dhara SK, Rekaya R, Godbey EA, Hasneen K, Rao RR, West FD 3rd, Gerwe BA, Stice SL (2007) BMP4 promotes formation of primitive vascular networks in human embryonic stem cell-derived embryoid bodies. Exp Biol Med (Maywood) 232(6):833–843
Medici D, Shore EM, Lounev VY, Kaplan FS, Kalluri R, Olsen BR (2010) Conversion of vascular endothelial cells into multipotent stem-like cells. Nat Med 16(12):1400–1406. doi:10.1038/nm.2252
Park C, Kim TM, Malik AB (2013) Transcriptional regulation of EC and vascular development. Circ Res 112(10):1380–1400
Acknowledgments
Funding for this work was provided in part by NIH grants NS79353, HL30568, HL81397 and HL112839, and the American Heart Association (Western Affiliate).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors have declared that no conflict of interest exists.
Rights and permissions
About this article
Cite this article
Yao, J., Guihard, P.J., Blazquez-Medela, A.M. et al. Matrix Gla protein regulates differentiation of endothelial cells derived from mouse embryonic stem cells. Angiogenesis 19, 1–7 (2016). https://doi.org/10.1007/s10456-015-9484-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-015-9484-3