Abstract
Nitellopsis obtusa is a non-native charophyte introduced to the Laurentian Great Lakes in the 1970s. Over the last decade, there have been increased reports of N. obtusa invasion of inland lakes in the Great Lakes basin. Typically, the star-shaped bulbil associated with N. obtusa can aid in identification; however, bulbils can only be found in established communities. To evaluate the impacts of N. obtusa on macrophyte communities in invaded ecosystems, we sampled 60 lakes across a large geologically diverse area of south-central Ontario. Interestingly, all populations of N. obtusa detected had bulbils present, thus we conclude that N. obtusa has likely been present in inland lakes within the Great Lakes basin well beyond the last decade. High N. obtusa abundance was associated with significantly lower macrophyte diversity (p value < 0.05). Redundancy analysis revealed that increased developed and agricultural land-use, in addition to the cations: sodium, potassium, magnesium, and calcium, positively co-varied with macrophyte communities with detectable N. obtusa presence. A generalized linear latent variable model (gllvm) demonstrated that N. obtusa had significant (p value < 0.05) positive co-occurrences with several native Characeae species. The gllvm model also revealed that N. obtusa had significant (p value < 0.05) negative co-occurrences with most other macrophytes, including the non-native Myriophyllum spicatum. Although N. obtusa was only found in a third of our study lakes, our results infer that increased expansion and establishment of N. obtusa throughout the region may result in significant changes to macrophyte communities, posing a threat to native fish habitat.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Nitellopsis obtusa (Desv.) J. Groves, 1919, commonly referred to as starry stonewort, is a non-native species in North America belonging to the Characeae family. The native distribution of N. obtusa extends from Britain through most of Europe and Asia to Japan. Over 80% of Characeae taxa, including N. obtusa, are now considered endangered in their native ranges due to climate change and persistent anthropogenic stressors (Blaženčić et al. 2006). Throughout its native range, N. obtusa is classified as threatened or endangered (Hamann and Garniel 2002; Blaženčić et al. 2006; Caisová and Gabka 2009; Johansson et al. 2010; Kabus and Mauersberger 2011; Auderset Joye and Schwarzer 2012; Korsch et al. 2013; Kato et al. 2014). Although no systematic studies have been done to compare native and non-native habitats, Escobar et al. (2016) noted that the environmental characteristics of North American and Eurasian distribution ranges were similar. Thus, the invasive tendency of N. obtusa within North America may reflect release from native habitat constraints such as competition and predation.
How N. obtusa came to North America is not known; however, it has been conjectured that accidental introduction occurred via the ornamental gardening trade and trans-oceanic shipping (Kay and Hoyle 2001; Padilla and Williams 2004). The first record of N. obtusa in North America is a specimen collected from the St. Lawrence River cataloged at the New York Botanical Garden, New York, USA, in 1974 (Karol and Sleith 2017). Over the intervening period, N. obtusa did not emerge as an invasive species threat in inland waters of the Great Lakes basin until the mid-2000s (Larkin et al. 2018). In Ontario, N. obtusa has been documented in Lake Simcoe as early as 2008 (Ginn et al. 2021), Presqu’ile Bay of Lake Ontario since 2015 (Midwood et al. 2016), and Lake Scugog as early as 2015 (Harrow-Lyle and Kirkwood 2020a, b). Recently, we confirmed N. obtusa establishment in 19 lakes across south-central Ontario (Harrow-Lyle and Kirkwood 2021a).
The common name of starry stonewort originates from the conspicuous white star-shaped bulbils. These bulbils arise from the rhizoid nodes and monofilament, and serve as hibernation cells and asexual reproductive structures (Bharathan 1987). Despite the presence of bulbils being an important diacritical feature for distinguishing between Characeae, identification solely on the presence of bulbils may prove difficult, as bulbils are only found within established beds of N. obtusa (Bonis and Grillas 2002). When bulbils are absent, N. obtusa can be identified by its long and slender thalli that are typically encrusted in a thick marl, composed of calcium carbonate and phosphorus (Blindow 1992). Branchlets are arranged in 5–8 per whorl, and grow upward of 9 cm in length (Karol and Sleith 2017). Furthermore, in spite of other Characeae such as Chara spp., giving off a characteristic sulfurous odor, N. obtusa does not exude a prominent smell (Pullman and Crawford 2010; Hackett et al. 2014). Generally, N. obtusa resembles Nitella spp. and Chara spp. due to the whorled branches that arise from stem nodes. The high degrees of similarity between N. obtusa and native Characeae make detection difficult, leading to repeated misidentification and underreporting of this invasive taxon.
The threat that N. obtusa poses toward North American freshwater ecosystems is thought to be similar to other non-native macrophytes (Pullman and Crawford 2010; Hackett et al. 2014; Brainard and Schulz 2017). Pullman and Crawford (2010) concluded after several years of lake monitoring that N. obtusa was the most aggressive macrophyte ever observed within Michigan, United States, yet the invasion biology of N. obtusa in North America is largely unknown. Few studies have documented community- to ecosystem-level impacts of N. obtusa, from early colonization to full establishment. However, some studies have observed displacement of other macroalgae and vascular aquatic plants upon N. obtusa establishment (Pullman and Crawford 2010; Brainard and Schulz 2017; Ginn et al. 2021; Harrow-Lyle and Kirkwood 2020b, 2021c).
To improve our understanding of N. obtusa impacts on macrophyte communities, we surveyed 60 lakes across Ontario, Canada, to (1) Document the occurrence and abundance of N. obtusa and (2) Evaluate macrophyte community composition and diversity as a function of environmental conditions and N. obtusa biomass. In previous work (Harrow-Lyle and Kirkwood 2021a) we were able to elucidate the key abiotic habitat conditions constraining and promoting N. obtusa presence in invaded lakes. Here, we not only wanted to characterize the macrophyte community composition in invaded lakes, but we also wanted to tease apart the relative influence of N. obtusa on community structure when abiotic variables could be accounted for using a generalized linear latent variable modeling (gllvm) approach. The study area stretches across a notable geological mosaic, where the limestone-dominated bedrock of the St. Lawrence lowlands shifts to the granite-dominated bedrock of the Precambrian shield. By sampling over such a geologically diverse landscape, we were able to capture a wide range of habitat conditions reflecting broad environmental gradients.
Materials and methods
Study design
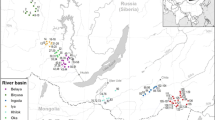
A survey of 60 lakes spanning a large geographic region (~ 65,000-km2) in Ontario, Canada, was conducted within a narrow time frame (August 1, 2019, to August 22, 2019) to minimize seasonal effects. Candidate lakes that were investigated are outlined in Harrow-Lyle and Kirkwood (2021a) (Fig. 1). Briefly, lake selection was based on capturing a broad water hardness gradient using historical calcium data collected from a variety of sources (The Land Between Charity, Kawartha Conservation, and the Ontario Ministry of Environment, Conservation, and Parks), while also minimizing surface connectivity through Geographic Information System (GIS) analysis (QGIS Development Team 2016). A transect which ran perpendicular to the shoreline was identified using bathymetric maps (e.g., Anglers Atlas https://www.anglersatlas.com) and was sampled within each of the candidate lakes. Four sampling sites were distributed along each transect to capture a range of depths (0.5 to 15 m) in each lake. At each location along the depth transect, water and macrophyte communities were sampled.
Map of candidate lakes with underlying geology. Candidate lakes are shown as circles, where black circles denote lakes with N. obtusa, and gray circles are lakes where N. obtusa was not documented. Underlying geology shape files were obtained from the Ontario Geological Survey (2011), which represent a 1: 250 000 scale in bedrock geology
Sample collection and field measurements
Depth at each site was verified using a Hawkeye handheld depth finder (DT1H, Hawkeye Electronics, Stuart, Florida, USA). Water clarity was measured using a standard 20-cm diameter Secchi disk. Conductivity, dissolved oxygen, temperature, and pH field measurements were recorded at each site using a YSI 6-series multiparameter probe (YSI Inc., Yellow springs, Ohio, USA). For consistency, water samples were collected at 0.5 m using a Van Dorn Sampler. Samples were decanted into four 50-mL conical tubes that were previously acid washed to remove trace nutrients and contaminants. Following collection, samples were immediately placed on ice until they were transferred to a cold room facility at Ontario Tech University at the end of each sampling day. Macrophyte collection followed the lake-rake method of Ginn (2011). At each site, three rake throws that extended to the lakebed were performed by the same person in following a consistent protocol. All material captured was placed in extra-large Ziplock bags and placed in a cooler on ice.
Sample processing
Samples were processed within 24 h of collection. Total phosphorus (TP) concentrations were determined following the modified ascorbic acid method of Murphy and Riley (1962) that was developed by the Ontario Ministry of Environment (1983). Total organic carbon (TOC) and total nitrogen (TN) were measured using a combustion catalytic oxidation method at York University, Toronto, Canada (TOC5000 series TOC analyzer, Shimadzu, Kyoto, Japan). Samples were sent to an accredited laboratory (SGS Canada, Lakefield, Ontario) for cation suite analysis that included: calcium, magnesium, sodium, potassium, iron, and manganese. Macrophytes were washed with reverse osmosis water, sorted, and identified using a QZE stereomicroscope (Walter Products, Windsor, Ontario). Samples were dried in a convection oven at 80 °C for 24 h following Carr et al. (2003). Biomass of each macrophyte species was determined by dry weight of collected material (g).
Statistical analysis
Statistical analyses were done using the open-sourced platform R version 4.0.3 (R Core Team 2019). Trophic status was inferred by calculating a trophic state index using TP as previously described (https://www.nalms.org/secchidipin/monitoring-methods/trophic-state-equations/). Macrophyte taxa abundance and environmental parameters were averaged for each lake (n = 4). Macrophyte Simpson’s diversity (1-D) was calculated for each lake using the R package vegan (Oksanen et al. 2013). Nitellopsis obtusa biomass was removed from the macrophyte community matrix prior to Simpson diversity calculations. Diversity for each candidate lake with confirmed N. obtusa populations was split into two categorical classifications; low N. obtusa presence (< 50% of relative abundance) and high N. obtusa presence (> 50% of relative abundance). A Mann–Whitney-Wilcoxon test was used to determine whether Simpson’s diversity significantly differed between the categorical classifications.
Redundancy analysis (RDA) was used to visualize the variation in macrophyte community structure using log-transformed macrophyte dry-weights. RDA was chosen based on the results of a detrended correspondence analysis, which found the longest gradient to be < 3. Biplot scores are representative of the weighted sums of species, as this measure is robust to noise within environmental variables. RDA analysis was performed using a Bray–Curtis dissimilarity matrix. The resulting RDA was prepared using the R packages stringr (Wickham 2019), dplyr (Wickham and Francois 2016), tidyr (Wickham and Henry 2019), vegan (Oksanen et al. 2013), and plotted in ggord (Beck 2016). A permutational analysis of variance (PERMANOVA) was used to test if there was a statistical difference between communities with (low and high) and without N. obtusa present (Oksanen et al. 2013).
Although an assessment of macrophyte species diversity revealed general community patterns, diversity alone does not address individual relationships. Understanding interactions between taxa within a community, especially in response to an invading taxon, are often difficult and complex to quantify. A relatively new multivariate approach that was developed to address this issue is generalized linear latent variable modeling (gllvm). An important advantage of this modeling technique is its ability to handle many species at once compared to other methods, and because the covariance model scales linearly (Warton et al. 2015).
The resulting gllvm model was constructed following Niku et al. (2019). Poisson, Tweedie, zero-inflated Poisson, and negative binomial distributions were fit to macrophyte abundance data (dry weight g). Information criterion, Dunn-Smyth residual plots, and normal quantile–quantile plots with 95% confidence intervals were used to assess the fit for macrophyte community distributions (Niku et al. 2019). A negative binomial distribution was identified to be the best fit for the data and was used in the following analysis. Environmental parameters were added into the model, to account for the effects on species interactions. A model that used five of the water quality parameters (calcium, potassium, pH, manganese, and depth) was chosen based on residual analysis indicating the most suitable mean–variance relationship for the responses. Covariance stored in the residual matrix that is not explained by the water quality parameters were calculated and adjusted to account for the variation explained by the negative binomial distribution, as outlined by Niku et al. (2019). Correlation patterns for species-specific relationships were plotted in R using the corrplot, RColorBrewer, and gclus packages (Hurley 2005; Neuwirth 2014; Wei et al. 2017).
Results
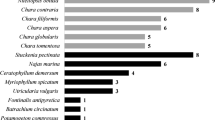
General lake characteristics, dominant submerged vegetation, and trophic status are displayed in supplementary table 1 (Table S1). Nitellopsis obtusa was found in 19 of the 60 study lakes (Fig. 2). The community proportion that N. obtusa comprised in each of the invaded lakes varied from < 1 to 90% (Fig. 2). Lakes Scugog (90%), Simcoe (86%), and Couchiching (86%) had the highest proportion of N. obtusa biomass (Fig. 2). In contrast, lakes Canal (0.89%), Rice (2.1%), and Opinicon (4.3%) had the lowest proportions of N. obtusa biomass in this study (Fig. 2). The remaining thirteen study lakes had N. obtusa at variable proportions (5.7 to 67%) of the total macrophyte community (Fig. 2). In the low N. obtusa presence classification, macrophyte diversity was demonstrated to be significantly higher than the high N. obtusa (p value = 0.023, W = 16.00, Mann–Whitney-Wilcoxon) classification (Fig. 2).
Redundancy analysis allowed us to evaluate whether there were distinct communities associated with N. obtusa presence across Ontario. The RDA biplot shows that axis one explained 34.67% of the variation observed and axis two explained 22.82% of the variation observed (Fig. 3). Macrophyte communities tended to have distinct community profiles when N. obtusa was present/absent (Fig. 3). Developed and agricultural land-use in the watershed positively co-varied with macrophyte communities that had N. obtusa present (Fig. 3). Additionally, concentrations of sodium, potassium, magnesium, calcium, and total nitrogen were positively associated with communities that had N. obtusa present (Fig. 3). In contrast, averaged depth, Secchi depth, iron, and TP concentrations were positively associated with macrophyte communities that did not have N. obtusa present (Fig. 3). A PERMANOVA confirmed that the macrophyte communities had distinct profiles when N. obtusa was present or absent in those communities (Fig. 3) (F1,47 = 4.84, p value = 0.001).
Redundancy analysis (RDA) biplot presenting macrophyte community compositions where N. obtusa is present or absent. Blue dots in the biplot represent the mean of each individual species where NO = N. obtusa, CV = Chara vulgaris, MB = Myriophyllum sibricum, MS = Myriophyllum spicatum, EC = Elodea canadensis, UV = Utricularia vulgaris, VA = Vallisneria americana, MV = Myriophyllum verticillatum, TI = Tolypella intricata, NL = Nuphar lutea, CD = Ceratophyllum demersum, PN = Potamogeton natans, PC = Potamogeton crispus, PF = Potamogeton foliosus, PA = Potamogeton amplifolius, PZ = Potamogeton zosteriformis, LM = Limnea minor, NF = Najas flexilis, NM = Nitella mucronata, NA = Nitella acuminata, and PP = Potamogeton pusillus. Arrows pointing in the same direction reflect positive correlations, and arrows pointing in opposite directions indicate negative correlations. The length of the arrow is a direct representation of the variance explained by the environmental variable. The first axis explained 34.67% of the total canonical eigenvalues and the second axis explained 22.82% of the total canonical eigenvalues
The gllvm model demonstrated statistically significant (p value < 0.05) positive co-occurrences between N. obtusa and native Characeae such as Nitella acuminata, Nitella mucronata, Chara vulgaris, and Tolypella intricata (Fig. 4). Statistically significant (p value < 0.05) positive associations between N. obtusa and other macrophytes were also found including Elodea canadensis, Potamogeton foliosus, and Nuphar lutea (Fig. 4). In contrast, N. obtusa had statistically significant (p value < 0.05) negative co-occurrence patterns with the majority of other macrophyte taxa in the study lake communities, and most notably with Myriophyllum spicatum (Fig. 4).
General linear latent variable model (gllvm) output matrix to visualize the species-specific interactions between macrophyte assemblages present within the candidate lakes (n = 49) when influencing environmental covariates have been accounted for. The gllvm model represents a negative binomial distribution, incorporating influences from five of the water quality parameters; calcium, potassium, pH, manganese, and depth. A heatmap depicting the strength of the correlation between the species covariates is presented. Non-native species encountered across this study have been presented in bold
Discussion
In three of the nineteen study lakes where N. obtusa was found, it represented over 80% of macrophyte biomass for the sampled transect. This is the first time that near monoculture dominance of N. obtusa in the macrophyte community of several Canadian lakes has been reported. In Ontario, aquatic ecosystems are not systematically monitored for non-native species, until dramatic community-level shifts are observed. Since most of the study lakes, including those with ideal growth conditions (i.e., calcium concentrations > 15 mg · L−1) (Harrow-Lyle and Kirkwood 2021a), had no N. obtusa or less than 50% relative abundance, it is possible that the N. obtusa invasion front in Ontario lakes is still in its early stages. Although increased sampling intensity in each lake may have increased the detection of N. obtusa populations, the dataset compiled in this study was robust enough to statistically detect macrophyte community interactions that may be conducive to successful establishment of N. obtusa in invaded regions.
Interestingly, the earliest record of N. obtusa within North America was first misidentified as a Chara spp., which was later corrected through herbarium records (Karol and Sleith 2017). In this study, N. obtusa had strong positive co-occurrences with Chara vulgaris, Nitella accuminata, and Nitella mucronata. These species are among the genera that are often mistaken as N. obtusa, and are frequently found in tangled masses of N. obtusa. This intermingling can thwart identification in the early stages of invasion by lake users and practitioners alike (Pullman and Crawford 2010). Due to common misidentification, it is also plausible that N. obtusa has been inhabiting Ontario lakes for decades as sleeper populations. A sleeper population is defined as a non-native species persisting at low abundances until environmental changes have triggered subsequent population increases (Spear et al. 2021).
Generally, when a population explosion of a new non-native species occurs, it is assumed that the introduction occurred recently (Spear et al. 2021). The premise of non-native species persisting within habitats for lengthy periods of time prior to population eruption is often overlooked (Spear et al. 2021). Within our study, we observed 11 instances where N. obtusa populations were at low abundance (< 50% relative abundance), often mixed within beds of native Characeae. Given a history of misidentification, it is likely that N. obtusa has been present in inland lakes within the Great Lakes basin well beyond the last decade, and was missed due to inadequate macrophyte community monitoring and skilled identification. Populations of N. obtusa discovered across the study period all had the presence of the star-shaped bulbils. Typically, bulbils can only be found within established beds, thus we conclude that N. obtusa has established populations within our study lakes (Bonis and Grillas 2002). Therefore, future paleolimnological studies may be able to ascertain the time period of initial colonization in invaded lakes via fossilized tissues, including bulbils.
Within N. obtusa’s native range, reproduction occurs primarily through vegetative fragments representing asexual and sexual propagules deposited in the sediments (Migula 1897). Sediments can host transient propagule banks that reproduce within one year of deposition (Thompson and Grime 1979), or persistent propagule banks that can last up to 89 years or more (Bonis and Grillas 2002). In contrast, a shift in reproduction has been observed when Characeae colonize shallow environments (Krause 1985). The development of gametangia and fertility within such environments has been linked to warmer water temperatures, and sunny growing seasons (Willén 1960; Boissezon et al. 2018).
Initial reports surmised that both male and female specimens of N. obtusa were present in North America; however, examination of suspected oogonia revealed that they were all male antheridia (Sleith et al. 2015). Currently, only specimens that do not produce female sexual organs have been documented in North America (Mann et al. 1999; Sleith et al. 2015). Within our study, all samples of N. obtusa had distinct, visible male antheridia present. Despite sexual reproduction being typically beneficial in dynamic shallow environments, as well as facilitating viable long-ranged dispersal, low sexual fertility of N. obtusa has been reported as early as the 1800s (Migula 1897).
Though dispersal of N. obtusa within invaded regions is not well understood, it is thought to occur through distribution of propagules and vegetative fragments (Reynolds et al. 2015; Green 2016). Propagules are known to spread through epizoochory (Bonis and Grillas 2002); however, there is growing evidence that the bulk of N. obtusa transfer is occurring by the movement of watercraft. For example, Midwood et al. (2016) noted that N. obtusa was found in areas with higher dock densities, and docks are strongly tied to recreational watercraft activity. Masses of N. obtusa found on watercraft and trailers remain viable after several days, suggesting that proper clean and drain protocols are not being followed (Glisson et al. 2019). In addition, we found that populations of N. obtusa tended to have the highest biomass at sites nearest boat launches (Harrow-Lyle and Kirkwood 2021a). Given the close proximity of invaded lakes documented in this study to the Trent-Severn Waterway, it is probable that boats serve as an important vector of N. obtusa spread. The Trent-Severn Waterway is a lake-canal navigation system that allows free movement of watercraft between Lake Huron and Lake Ontario, and has been implicated as an important conveyance system for invasive species spread (Kelly et al. 2013; Masson et al. 2016).
As more studies are published, it is becoming apparent that N. obtusa can alter habitat structure and function, as well as displace other macroalgae and vascular macrophytes (Pullman and Crawford 2010; Brainard and Schulz 2017; Ginn et al. 2021; Harrow-Lyle and Kirkwood 2021b). Total and native macrophyte biomass decreased in response to N. obtusa in invaded regions within the United States (Brainard and Schulz 2017). In Ontario, Ginn et al. (2021) also described the displacement of native and invasive macrophytes upon N. obtusa establishment within Lake Simcoe. Similarly, the initial identification and subsequent increase in N. obtusa within Lake Scugog, Ontario, has resulted in the displacement of native and invasive macrophytes (Harrow-Lyle and Kirkwood 2021c).
In other invaded regions, Ceratophyllum demersum and Utricularia vulgaris were found to reach nuisance levels in conjunction with N. obtusa growth (Pullman and Crawford 2010). Furthermore, N. obtusa has also been found in stands of C. demersum within its native range (Pełechaty et al. 2014). In Ontario, our results demonstrate N. obtusa negatively co-occurs with these taxa, along with most other members of the macrophyte community. Notably, this study highlights a negative relationship between Myriophyllum spicatum, a normally aggressive non-native macrophyte long established in many Ontario lakes over the last 60 years. Within two invaded systems in Ontario, Lake Scugog and Lake Simcoe, similar negative relationships have been observed between these two non-native macrophytes (Ginn et al. 2021; Harrow-Lyle and Kirkwood 2021c).
In our region-wide study across south-central Ontario, we observed a reduction in macrophyte diversity within lakes invaded by N. obtusa; however, there was still considerable variation. When examining the species-specific interactions revealed by gllvm, N. obtusa had negative co-occurrences with most members of the macrophyte community, but also had significant positive associations with native Characeae across Ontario. Based on these findings, and the possibility that N. obtusa is still in its early stages of an invasion front, it is too early to conclude that N. obtusa has caused diversity loss and species displacement across Ontario lakes. However, more comprehensive in-depth lake studies such as those done in other invaded systems (Brainard and Schulz 2017; Ginn et al. 2021; Harrow-Lyle and Kirkwood 2021c) do offer supporting evidence of N. obtusa’s negative impacts to macrophyte community diversity. To understand this disparity between native and invaded regions requires further study, but it may be due to differences in biotic interactions, such as competitive exclusion, that drives this divergence in environmental tolerance.
Overall, our results provide further evidence that N. obtusa threatens ecosystem health and productivity in invaded regions. The displacement of native macrophytes shown in this study will in turn have negative impacts on native fish habitat. With the formation of a physical barrier, reduced nesting area, and spawning activities are likely for fish communities (Pullman and Crawford 2010). Pullman and Crawford (2010) have suggested that fish such as Ambloplites rupestris, Lepomis macrochirus, and Lepomis gibbosus avoid areas with N. obtusa growth due to the formation of dense benthic mats. Additionally, other fish species such as Sander vitreus, Esox niger, and Esox masquinongy have displayed altered hunting strategies, due to the impenetrable N. obtusa mat architecture (Pullman and Crawford 2010). Furthermore, we have recently shown that N. obtusa can alter the biogeochemical cycling of oxygen and phosphorus with increasing biomass (Harrow-Lyle and Kirkwood 2021b). Finally, N. obtusa has been shown to have negative impacts on macroinvertebrate communities (Caraco et al. 1990; Murphy et al. 2018).
In conclusion, Nitellopsis obtusa may be the most enigmatic and aggressive, non-native macrophyte species to be introduced into North America. Early identification and mitigation are often foiled when N. obtusa is present within stands of native Characeae (Brainard and Schulz 2017). The reduction in macrophyte diversity shown to occur in this study and others, coupled with the frequent misidentification of N. obtusa, warrant increased monitoring efforts. Early detection, as with most invasive species, will be critical for lake management efforts aimed at mitigating the negative ecological effects of N. obtusa establishment and increasing dominance in inland North American lakes.
Data Availability
All data used within this manuscript is available upon the reader’s request.
References
Auderset Joye D, Schwarzer A (2012) Liste rouge Characées. Espèces menacées en Suisse, état 2010
Beck MW (2016) ggord: Ordination Plots with ggplot2. R package version 011
Bharathan S (1987) Bulbils of some charophytes. Proc Plant Sci 97(3):257–263. https://doi.org/10.1007/BF03053351
Blaženčić J, Stevanović B, Blaženčić Ž, Stevanović V (2006) Red data list of charophytes in the Balkans. Biodivers Conserv. https://doi.org/10.1007/s10531-005-2008-5
Blindow I (1992) Decline of charophytes during eutrophication: comparison with angiosperms. Freshw Biol. https://doi.org/10.1111/j.1365-2427.1992.tb00557.x
Boissezon A, Auderset Joye D, Garcia T (2018) Temporal and spatial changes in population structure of the freshwater macroalga Nitellopsis obtusa (Desv.) J. Groves. Bot Lett 165(1):103–114. https://doi.org/10.1080/23818107.2017.1356239
Bonis A, Grillas P (2002) Deposition, germination and spatio-temporal patterns of charophyte propagule banks: a review. Aquat Bot. https://doi.org/10.1016/S0304-3770(01)00203-0
Brainard AS, Schulz KL (2017) Impacts of the cryptic macroalgal invader, Nitellopsis obtusa, on macrophyte communities. Freshwater Sci. https://doi.org/10.1086/689676
Caisová L, Gabka M (2009) Charophytes (Characeae Charophyta) in the Czech Republic Taxonomy, autecology and distribution. Fottea 9(1):1–43. https://doi.org/10.5507/fot.2009.001
Caraco N, Cole J, Likens GE (1990) A comparison of phosphorus immobilization in sediments of freshwater and coastal marine systems. Biogeochemistry 9:277–290. https://doi.org/10.1007/BF00000602
Carr GM, Bod SAE, Duthie HC, Taylor WD (2003) Macrophyte biomass and water quality in Ontario rivers. J North Am Benthol Soc. https://doi.org/10.2307/1467991
Escobar LE, Qiao H, Phelps NBD et al (2016) Realized niche shift associated with the Eurasian charophyte Nitellopsis obtusa becoming invasive in North America. Sci Rep. https://doi.org/10.1038/srep29037
Ginn BK (2011) Distribution and limnological drivers of submerged aquatic plant communities in Lake Simcoe (Ontario, Canada): utility of macrophytes as bioindicators of lake trophic status. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2010.05.004
Ginn BK, Dias EFS, Fleischaker T (2021) Trends in submersed aquatic plant communities in a large, inland lake: impacts of an invasion by starry stonewort (Nitellopsis obtusa). Lake Reservoir Manage. https://doi.org/10.1080/10402381.2020.1859025
Glisson WJ, Wagner CK, Verhoeven MR et al (2019) Desiccation tolerance of the invasive alga starry stonewort. J Aquat Plant Manag 58:7–18
Green AJ (2016) The importance of waterbirds as an overlooked pathway of invasion for alien species. Divers Distrib. https://doi.org/10.1111/ddi.12392
Hackett R, Caron J, Monfils A (2014) Status and strategy for starry stonewort (Nitellopsis obtusa (NA Desvaux) J. Groves) management. Michigan Department of Environmental Quality, Lansing, Michigan
Hamann U, Garniel A (2002) Die Armleuchteralgen Schleswig-Holsteins-Rote Liste. Landesamt für Natur und Umwelt des Landes Schleswig Holstein
Harrow-Lyle TJ, Kirkwood AE (2020a) The invasive macrophyte Nitellopsis obtusa may facilitate the invasive mussel Dreissena polymorpha and Microcystis blooms in a large, shallow lake. Can J Fish Aquat Sci. https://doi.org/10.1139/cjfas-2019-0337
Harrow-Lyle TJ, A Kirkwood (2020b) An assessment of Lake Scugog offshore water quality and ecological condition (2017–2019)
Harrow-Lyle TJ, Kirkwood AE (2021a) An ecological niche model based on a broad calcium-gradient reveals additional habitat preferences of the invasive charophyte Nitellopsis obtusa. Aquat Bot. https://doi.org/10.1016/j.aquabot.2021.103397
Harrow-Lyle TJ, Kirkwood AE (2021b) Low benthic oxygen and high internal phosphorus-loading are strongly associated with the invasive Macrophyte Nitellopsis obtusa (starry stonewort) in a large Polymictic Lake. Front Environ Sci. https://doi.org/10.3389/fenvs.2021.735509
Harrow-Lyle TJ, Kirkwood AE (2021c) Pervasive changes to the lower aquatic food web following Nitellopsis obtusa establishment in a large, shallow lake. Freshw Biol. https://doi.org/10.1111/fwb.13860
Hurley C (2005) The gclus package
Johansson G, Aronsson M, Bengtsson R et al (2010) Alger-Algae. Nostocophyceae, Phaeophyceae, Rhodophyta & Chlorophyta. Rödlistade Arter I Sverige 2010:223
Kabus T, Mauersberger R (2011) Liste und Rote Liste der Armleuchteralgen (Characeae) des Landes Brandenburg 2011. Landesumweltamt Brandenburg
Karol KG, Sleith RS (2017) Discovery of the oldest record of Nitellopsis obtusa (Charophyceae, Charophyta) in North America. J Phycol 53(5):1106–1108. https://doi.org/10.1111/jpy.12557
Kato S, Kawai H, Takimoto M et al (2014) Occurrence of the endangered species Nitellopsis obtusa (Charales, Charophyceae) in western Japan and the genetic differences within and among Japanese populations. Phycol Res. https://doi.org/10.1111/pre.12057
Kay SH, Hoyle ST (2001) Mail order, the internet, and invasive aquatic weeds. J Aquat Plant Manag 39(1):88–9
Kelly NE, Wantola K, Weisz E, Yan ND (2013) Recreational boats as a vector of secondary spread for aquatic invasive species and native crustacean zooplankton. Biol Invasions. https://doi.org/10.1007/s10530-012-0303-0
Korsch H, Doege A, Raabe U, van de Weyer K (2013) Rote Liste der Armleuchteralgen (Charophyceae) Deutschlands 3. Fassung, Stand: Dezember 2012. Hausknechtia Beiheft 17:1
Krause W (1985) Über die Standortsansprüche und das Ausbreitungsverhalten der Stern-Armleuchter-alge Nitellopsis obtusa (Desvaux). J Groves Carolinea 42:31–42
Larkin DJ, Monfils AK, Boissezon A et al (2018) Biology, ecology, and management of starry stonewort (Nitellopsis obtusa; Characeae): a red-listed Eurasian green alga invasive in North America. Aquatic Botany. https://doi.org/10.1016/j.aquabot.2018.04.003
Mann H, Proctor VW, Taylor AS (1999) Towards a biogeography of North American charophytes. Aust J Bot. https://doi.org/10.1071/BT97087
Masson L, Brownscombe JW, Fox MG (2016) Fine scale spatio-temporal life history shifts in an invasive species at its expansion front. Biol Invasions. https://doi.org/10.1007/s10530-015-1047-4
Midwood JD, Darwin A, Ho ZY et al (2016) Environmental factors associated with the distribution of non-native starry stonewort (Nitellopsis obtusa) in a Lake Ontario coastal wetland. J Great Lakes Res. https://doi.org/10.1016/j.jglr.2016.01.005
Migula W (1897) Die Characeen Deutschlands, Oesterreichs und der Schweiz; Unter Berücksichtigung aller Arten Europas BT - Kryptogamenflora Von Deutschland, Oesterreich Und Der Schweiz. In: Kryptogamenflora Von Deutschland, Oesterreich Und Der Schweiz
Ministry of Environment (1983) Handbook of analytical methods for environmental samples
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chim Acta. https://doi.org/10.1016/S0003-2670(00)88444-5
Murphy F, Schmieder K, Baastrup-Spohr L et al (2018) Five decades of dramatic changes in submerged vegetation in Lake Constance. Aquat Bot. https://doi.org/10.1016/j.aquabot.2017.10.006
Neuwirth E (2014) RColorBrewer. R package version1.1–2
Niku J, Hui FKC, Taskinen S, Warton DI (2019) gllvm: Fast analysis of multivariate abundance data with generalized linear latent variable models in r. Methods Ecol Evol. https://doi.org/10.1111/2041-210X.13303
Oksanen J, Blanchet FG, Kindt R et al (2013) Package vegan. Community Ecol Package 2(9):1–295
Padilla DK, Williams SL (2004) Beyond ballast water: aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front Ecol Environ. https://doi.org/10.1890/1540-9295(2004)002[0131:BBWAAO]2.0.CO;2
Pełechaty M, Pronin E, Pukacz A (2014) Charophyte occurrence in Ceratophyllum demersum stands. Hydrobiologia. https://doi.org/10.1007/s10750-013-1622-6
Pullman GD, Crawford G (2010) A decade of starry stonewort in Michigan. Lakeline 52:36–42
QGIS Development Team (2016) QGIS Geographic Information System. Open Source Geospatial Foundation Project
R Core Team (2019) R: A language and environment for statistical computing. R Foundation for Statistical Computing
Reynolds C, Miranda NAF, Cumming GS (2015) The role of waterbirds in the dispersal of aquatic alien and invasive species. Divers Distrib. https://doi.org/10.1111/ddi.12334
Sleith RS, Havens AJ, Stewart RA, Karol KG (2015) Distribution of Nitellopsis obtusa (Characeae) in New York, USA. Brittonia 67(2):166–72. https://doi.org/10.1007/s12228-015-9372-6
Spear MJ, Walsh JR, Ricciardi A, Vander ZMJ (2021) The invasion ecology of sleeper populations: prevalence, persistence, and abrupt shifts. Bioscience. https://doi.org/10.1093/biosci/biaa168
Thompson K, Grime JP (1979) Seasonal variation in the seed banks of herbaceous species in ten contrasting habitats. J Ecol. https://doi.org/10.2307/2259220
Warton DI, Blanchet FG, O’Hara RB et al (2015) So many variables: joint modeling in community ecology. Trends Ecol Evol. https://doi.org/10.1016/j.tree.2015.09.007
Wei T, Simko V, Levy M et al. (2017) R package “corrplot”: Visualization of a correlation matrix. Statistician
Wickham H, Francois R (2016) The dplyr package. R Core Team
Wickham H, Henry L (2019) tidyr: Tidy messy data. R package version 100
Wickham H (2019) stringr: Simple, consistent wrappers for common string operations. R package version 1.4.0. Cran
Willén T (1960) The charophyte Nitellopsis obtusa (Desv.) Groves found fertile in central Sweden. Sven Bot Tidskr 54:360–367
Acknowledgements
We would like to thank and acknowledge the Indigenous first nation communities from whose lands and waters we traversed to collect data. The study area is located on treaty lands that are the traditional territory of the great Anishinaabeg Nation, including Algonquin, Ojibway, Odawa, and Pottawatomi. We also acknowledge funding support from a Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to A. Kirkwood (RGPIN 246150). A big thank-you to Denina Simmons for lending her research laboratory truck, which was instrumental in supporting fieldwork. Special thanks to Jennifer Korosi at York University for providing support to conduct the TN and TOC analyses. Finally, we thank Eric Anderson, Claire Gibbs, Emily Hassal, Jennifer Newman, and Erin Smith for their assistance in the field and laboratory.
Funding
Funding for this research was provided by the Natural Sciences and Engineering Research Council (NSERC) Discovery Grant to A. Kirkwood (RGPIN 246150).
Author information
Authors and Affiliations
Contributions
TJHL contributed to the design, data collection, analysis, and writing of the manuscript with support from AEK. AEK supervised the project, validated results, and acquired funding.
Corresponding author
Ethics declarations
Conflicts of Interest
We have no conflicting interests to declare.
Code Availability
All R code used within this manuscript is available upon the reader’s request.
Additional information
Handling Editor: Asaeda Takashi.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Harrow-Lyle, T.J., Kirkwood, A.E. The non-native charophyte Nitellopsis obtusa (starry stonewort) influences shifts in macrophyte diversity and community structure in lakes across a geologically heterogeneous landscape. Aquat Ecol 56, 829–840 (2022). https://doi.org/10.1007/s10452-022-09950-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-022-09950-0