Abstract
The substantial variations in the anatomy, physiology and life-history trait of wetland plants tend to limit their ability to tolerate environmental stressors and can consequently affect their community composition and distribution. Comparative studies of wetland plants among water bodies of varying limnological characteristics are useful in understanding the different wetland plant communities’ responses to different environmental drivers. This study examined how community structural assemblages in six different tropical wetlands responded to environmental disturbances over a 1-year period (January–December 2017). They included three standing marshes (Kukobila, Tugu and Wuntori marshlands); two riparian systems (Adayili and Nabogo); and one artificial wetland (Bunglung). The prevalence index method was used to categorize plants as wetland or non-wetland species. Geometric series, individual-based rarefaction and Renyi diversity ordering models were applied to quantify community structural assemblages, while a direct ordination technique (CCA) was used to determine the how they respond to the influence of environmental factors. A total of 3034 individuals, belonging to 46 species from 18 families, were registered across the six wetlands. Grasses, herbs and woody species constituted 42.2%, 42.2% and 15.5%, respectively. Obligate species constituted 30.4%, while facultative wetland and obligate upland species were 47.8% and 26.1%, respectively. Wuntori marshland (n = 768) recorded the highest species per plot (18.73 ± 2.49), while Adayili riparian wetland (n = 260) was the least recorded (6.34 ± 1.80). Chrysopogon zizanioides, Echinochloa stagnina and Pennisetum polystachion were the most abundant species. Species assemblages were influenced by grazing, farming, fire, phosphorus, potassium and soil pH. These variables explained 61.29% of total variances in species abundance distribution, richness and diversity. The results highlight the threats on the wetlands and the need to protect them from further degradation.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aquatic plants are vital components of many freshwater ecosystems (Lacoul and Freedman 2006a, b, c). They vary substantially in their anatomy, physiology and life-history traits, and as such, their ability to tolerate inorganic and biological stressors like extreme regimes of flow velocity, irradiance, salinity, ice cover, temperature, nutrients, pollutants, competition, herbivory and disease tend to affect their community composition and distribution (Lacoul and Freedman 2006a, b, c). For instance, the structural assemblages of wetland vegetation have largely been linked to major hydrological factors like duration and depth of flooding (Gunderson 1994) and soil physical characteristics (Kirkman et al. 2000). Also, phosphate derived from agriculture, sewage and industry has been regarded as a key driver of the loss of aquatic plants, because it promotes a transition from clear water in which macrophytes can flourish to highly adverse conditions dominated by phytoplankton and filamentous algae (Lambert and Davy 2010). These ecological factors can be used to identify species and communities that are key indicators of important changes in their ecosystem, including those that may serve as gauges of ecological integrity (Fennessy et al. 1998; Aznar et al. 2003). Comparative studies of aquatic plants among water bodies differing in limnological characteristics (Hutchinson 1975) are useful in understanding the different wetland plant communities’ responses to different environmental drivers of change.
Many scientists have recognized the complex interactions between aquatic plants and environmental factors, as key determinants of community structural distribution and diversity at the local to regional level (van Geest et al. 2003; Ot’ahel’ová and Valachovič 2006; Janauer and Dokulil 2006; Pinto et al. 2006). One analytical technique that has been widely used to evaluate the influence of suites of physical–chemical/environmental factors on macrophytes is the multivariate ordination approach (e.g. canonical correspondence analysis, detrended correspondence analysis and redundancy analysis) (Heegaard et al. 2001; Mackay et al. 2003; Murphy et al. 2003; Mäkelä et al. 2004; Lacoul and Freedman 2006a). Thus, the application of this technique in this study is most appropriate and could provide a better explanation of how key environmental drivers influence the plant community assemblages across the six wetlands that serve as a major source of livelihood to rural communities.
Wetlands in Ghana are generally categorized under both lotic and lentic systems and include coastal lagoons (estuaries)—along the ocean front, inland rivers, marshes and man-made lakes (Biney 1990). These wetlands are threatened by industrial and domestic activities, turbid inflows and eutrophication (Biney 1990). Wetlands from the Northern Region of Ghana play a critical ecological and economic role in the livelihoods of rural dwellers, such as fish supply, water for irrigation, source of thatch materials for roofing, herbal extraction and flood attenuation (Nsor and Alhassan 2015). However, their functional status has been of high conservation concern in recent times (e.g. Nsor and Obodai 2014, 2016; Nsor et al. 2016). Scientific studies on plant structural assemblages among wetlands in Northern Savannah Region of Ghana are scanty or poorly investigated (e.g. Nsor and Obodai 2014, 2016). In this study, we examine natural and anthropogenic influences on the distribution and abundance of aquatic plants and develop a conceptual model of those diverse interactions. The aim of this study was to investigate how environmental factors such as bushfire, grazing, farming activities, erosion and soil nutrient status influenced community structural assemblages and diversity. We hypothesized that: (a) community structural assemblages following their response to environmental factors will not vary across the six wetlands and (b) processes that structure assemblages in the various habitats will not change. We also seek to find out which major environmental factors are driving changes in community assemblages.
Methods
Study area
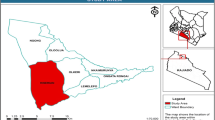
The study area is located in the Northern Region of Ghana, between 8° and 11°N. The co-ordinates of the six wetlands are as follows: Kukobila 10°08.723′N; 0°48.179′W; Tugu 09° 22.550′N; 0°35.004′W; Bunglung 09°35.576′N; 0°47.443′W; Adayili 09°41.391′N; 0°41.480′W; Nabogo 09°49.941′N; 0°51.942′W; and Wuntori 09°08.335′N; 0°109.685′W (Fig. 1). These wetlands were selected for this study because they represent the different types of wetlands found in Northern Ghana, following the description by Ramsar Convention on wetlands classification (2014). Kukobila, Wuntori and Tugu wetlands constituted standing permanent marshes; Adayili and Nabogo represented riparian wetlands, while Bunglung is an artificial or man-made wetland. There is an extensive floodplain along the course of the Volta and Nasia Rivers, which has overtime become incised and modified through meandering and aligning along various topographic features (Slaymaker and Blench 2002). This has led to the development of streams that have diverted from the main White Volta (Slaymaker and Blench 2002). The landscape is gently undulating, with broad and poorly drained valleys and a crest of the scarp forms the northern boundary of the Nasia River. The fine sandy loam soils are from Upper Voltaian sandstone, while the iron pan concretion and the yellow sandy loams soils are from the Lower Voltaian shales and alluvial floodplains and sloughs (i.e. swamps or shallow lakes, mostly from backwater to a larger water body: Mitsch and Gosselink (1993).
The vegetation cover is a mixture of grassland dominated by Chrysopogon zizanioides and Echinochloa pyramidalis and woodland (e.g. Vitellaria paradoxa) interspersed with shrubby communities of Mitragyna inermis and Vitex crysocarpa. The trees are short (av. 3–5 m), with thick bark and occlusions, indicating their adaptation to the cyclical dry season with bush fires. Altitude is 108–138 m above mean sea level. Hydrological regimes of the six wetlands under study were characterized by permanent water all year round, and whose depth did not exceed 2 m on average in the dry season. All the wetlands were within the catchment of the main White Volta River or its tributaries. Wetland areas were measured on spot and Landsat images using Google Earth Pro Software. They were as follows: (a) Wuntori = 7.7 ha; (b) Kukobila = 8.9 ha; (c) Tugu = 2.7 ha; (d) Nabogo = 7.9 ha; (e) Adayili = 6.7 ha; and (f) Bunglung = 11.05 ha (Fig. 1).
Vegetation sampling procedure
Sampling of aquatic plants was carried out in each of the 24 modified-Whittaker plots (Stohlgren et al. 1995) over a 1-year period. Sampling was done in the long dry season (i.e. January–June and November–December). The modified-Whittaker plot is a vegetation sampling design that is used to assess plant communities at multiple scales (Stohlgren et al. 1995). The use of 24 Whittaker plots of 1000 m2 (50 × 20 m) was largely influenced by the presence of ground cover within the delineated zone of continuous wetness, relative to the area occupied by open water. We used this plot type at various scales of 1 m2, 10 m2, 100 m2 and 1000 m2. Four Whittaker plots were randomly laid in each of the six wetlands, and along an environmental gradient of the vegetation type being sampled, in order to record majority of species heterogeneity. Sampling gradients for species–environmental factors were site specific. Plot dimensions were 20 m × 50 m (1000 m2) containing three different sizes of nested subplots. A 5 m × 20 m (100 m2) subplot was placed at the centre of the plot, while two 2 m × 5 m (10 m2) subplots were placed in opposite corners of the plot. The remaining ten of 0.5 m × 2 m (1 m2) subplots were placed at the edges of the main plot. Plots were laid perpendicular on the environmental gradient of the vegetation type being sampled, in order to register majority of species heterogeneity and to determine how species assemblages were influenced by the identified disturbance or responded to the environmental factors. Modified-Whittaker plots have the ability to detect more species than round or square quadrats, at multiple scales, and as such, its shape is kept consistent for the plot and its nested subplots (Stohlgren et al. 1995). Sampling was done twice, covering the seven-month period in the dry season. Only new species that were detected in the repeated sampling were recorded. The Domin–Krajina cover abundance scale was used to estimate ground cover (see Mueller-Dombois and Ellenberg 1974). Plants were identified up to species level, with the aid of keys developed by Johnson (1997), Okezie and Agyakwa (1998) and Arbonnier (2004). Identified species were further classified as wetland and non-wetland plants, following the proposed criteria developed by Keddy (2002). The rationale for this classification was to quantify species that are typical hydrophytes and those that are not, but have over time shifted or migrated from their ranges (i.e. dryland areas) and subsequently adapted to continuous wetness, following physiological and morphological modifications of their structure. The prevalence index method (Cronk and Siobhan-Fennessy 2001) was employed to classify the weighted average of indicator status of sampled species as follows: obligate plants (OBL) = 1.0; facultative wetland plants (FACW) = 2.0; facultative plants (FAC) = 3.0; facultative upland plants (FACU) = 4.0; and obligate upland plants (UPL) = 5.0. These categories are described as follows: obligate wetland plants (i.e. hydrophytes with > 99% probability of occurring in wetlands); facultative wetland plants (usually found in wetlands with an estimated probability of 67–99% occurrence, but occasionally found in uplands); facultative plants (having 34–66% equal chance of occurring in wetlands); facultative upland plants (usually occur outside wetlands, but occasionally found in wetlands); and obligate upland (occur only in uplands) (Tiner 1999). In addition to the indicator status categories, positive (+) sign was used to indicate all facultative species categories with a frequency towards wetter ends (more frequently found in wetlands) and the negative (−) sign with a frequency towards drier ends (less frequently found in wetlands) (Tiner 1999).
Plant species in each plot were identified, counted and classified under the different species indicator status, to determine their relative abundance. Total number of species in each indicator status category was subsequently divided by the total number of plots on which they were sampled, in order to obtain the average for each plot. Plots that scored < 3.0 were considered to be obligate wetland plants (OBL) and those that scored > 3.0 were designated as upland plants (FACW; FAC; FACU; and UPL categories), which may have migrated into the wetlands over time (Cronk and Siobhan-Fennessy 2001). We further counted species from each of the indicator status category and expressed it as a percentage of the total species sampled, in order to determine whether the wetland plant communities are predominantly hydrophytes.
Assessment of environmental variables
Random soil samples were taken with a soil augur at a depth of 15 cm, using the zigzag sampling method (Carter and Gregorich 2007) on each of the 24 modified-Whittaker plots. This multiple random soil sampling method was aimed at increasing the sampling effort by covering large areas of the near-level and slopping landscape signature of the six study sites. Three composite samples were taken from three different locations of 25 cores, in a grid design form on each plot (50 m × 20 m2). Eight cores each from two locations and nine cores from one location were sampled, bringing the total to 25 cores across the three locations on each plot. Samples were put in transparent polyethylene bags and labelled according to the code assigned to each plot and taken to the laboratory to analyse the presence of nitrogen, phosphorus, potassium, magnesium, calcium and soil pH, using atomic absorption spectroscopy (AAS) techniques (Murphy and Riley 1962; van der Merwe et al. 1984). Organic carbon was determined using the Walkley–Black method (Walkley and Black 1934; Walkley 1947). All analyses were carried out at the Savanna Agricultural Research Institute (SARI) at Nyankpala in the Northern Region.
Human-led factors such as farming activities, grazing intensity, erosion and bush fire, were measured, by adopting the model approach of Salafsky et al. (2008) and Battisti et al. (2009). Identifying these threats and their regime is important when assessing the status of wetlands of high conservation concern for efficient management. These environmental factors were identified and recorded, following ground truthing assessment. The hierarchical classification of these threats (based on their relative severity) was comprehensive (contains all possible items, at least at higher levels of the hierarchy), consistent (ensures that entries at a given level of the classification are of the same type), expandable (enables new items to be added to the classification if they are discovered) and exclusive (allows any given item to only be placed in one cell within the hierarchy) (Salafsky et al. 2008). A score ranging from 1 to 4 (1 being the lesser impact and 4 the highest impact) was used to assess scope and severity of every threat. More precisely, for ‘scope’ we referred to the percentage ratio of the study area affected by a specific threat within the last 5 years (where 100% correspond to total site area: χ ha) (Battisti et al. 2009). The scores were assigned as follows: 4: the threat is found throughout (50%) the site area; 3: the threat is spread in 15–50% of the study area; 2: the threat is scattered (5–15%); and 1: the threat is much localized (< 5%). The assessment of all identified threats was carried out within 1.2 km radius around each of the wetland, since all land use activities that we measured were observed within the stated radius following preliminary survey.
Statistical analysis
An initial test was undertaken to determine whether the data collected were normally distributed, using Shapiro–Wilk test. This prior test was meant to determine the appropriate statistical test to subject data to (i.e. parametric or nonparametric data analysis approach) (Kent and Coker 1992). Canonical correspondence analysis (a multivariate direct ordination method) was performed to elucidate the relationship between environmental drivers of change and species assemblages across the six wetlands, using Environmental Community Analysis version 1.3 (ECOM.exe) 1.4 package (Henderson and Seaby 1999). The method is designed to extract synthetic environmental gradients from ecological data sets (ter Braak and Verdonschot 1995). To remove multicollinearity (i.e. perfect correlation with other predictors, which tend to inflate variances of the parameter estimates), we used the ridge regression method (which is a variant to least squares regression that ensure a smaller variance in resulting parameter estimates (Schreiber-Gregory and Jackson 2017), by initially examined the variance inflation factor (VIF) and tolerance. Triplots relating to species, environmental factors and sample plots, in all six wetlands, were created to visually interpret the CCA outcomes. Arrows indicate the direction and maximum strength of each of the environmental factors on the species assemblages across the wetlands. A Monte Carlo permutation test (9999 iterations) was used to test for significance of the eigenvalues, generated by the first two axes in the analyses of species to environmental variables. Kruskal–Wallis test (a nonparametric test statistics) was used to determine whether environmental variables differed significantly among the six wetlands, using SPSS version 20. A Student t test was applied to determine whether there was a significant difference in species categories (i.e. FACW, FAC, FACU and UPL) shifting towards wetter areas than drier areas.
Species abundance distribution (SAD)
Species abundance as a measure of diversity was quantified using rank abundance model (Magurran 2004). In each site, we listed the number of plant species say S1 represented by one individual and the number of species, say SK, represented by K individuals, where K denotes the abundance of the most abundant species and S1 + ··· + SK = S (Fattorini 2013). Accordingly, the sequence of relative frequencies fr = Sr/S (r = 1…K) constitutes a frequency distribution for the number of individuals per species which is usually referred to as the species–abundance curve (Fattorini 2013). We then fitted the geometric model (GS) in the species data (raw abundance) using the regression model approach (Fattorini 2005), to determine how the species communities are assembled in each habitat. This model approach was used in order to test against the null hypothesis (Ho) that species abundance distribution and richness did not differ in each of the six sites. All the species in each of the four Whittaker plots per wetland site were ranked from the most to the least abundant on the rank abundant curve (Fattorini et al. 2016). Each species rank is plotted on the x-axis and the abundance plotted on the y-axis. With the geometric series, if a log scale is used for abundance, the species exactly fall along a straight line, according to the model equation \(\log A = b_{0} + b_{1 } R\), where A is the species abundance, R is the respective rank and b0 and b1 are optimized fitting parameters (Fattorini et al. 2016). Analysis of covariance (ANCOVA) was applied to test for the significant difference of the slope of the species abundance distributions (SADs) for the six habitats, while Pearson’s Chi-square test (χ2) was applied to determine whether an observed distribution along the goodness of fit statistically differed in the GS model. The SAD model is mostly used to measure the impact of disturbance on community structure (Gray and Mirza 1979), while the geometric series (a proposed SAD model) represents species distribution with lower evenness and provides a good fit to simple communities characterized by the high dominance of a few species (Magurran 2004). The shape of the frequency distribution gives an insight into the species abundance distribution of the communities under study.
Individual-based rarefaction techniques (Gotelli and Colwell 2011) were applied to compare plant richness across the six sites (rarefaction curves). Rarefaction curves are created by randomly re-sampling the pool of N samples multiple times and then plotting the average number of species found in each sample (1, 2… N) (Gotelli and Colwell 2001). Thus, rarefaction generates the expected number of species in a small collection of n individuals (or n samples) drawn at random from the large pool of N samples. The rarefaction curve \(f_{n}\) is defined as (Gotelli and Colwell 2011):
where \(X_{n}\) = the number of groups still present in the subsample of ‘n’ less than \(K\) whenever at least one group is missing from this subsample, \(N = {\text{total }}\,{\text{number}}\, {\text{of }}\,{\text{items}}\), \(K{\kern 1pt} \, = \,{\text{total}}\, {\text{number}}\, {\text{of }}\,{\text{groups}},\)\(Ni = {\text{total}}\, {\text{number}}\, {\text{of }}\,{\text{items}}\, {\text{in }}\,{\text{group}}\, i \left( {i = 1, \ldots k} \right)\) (Gotelli and Colwell 2001; Siegel 2006). Thus, the linear model for the GS was fitted for each rarefied run in order to build the 95% confidence limits for the slopes of all six sites. Rarefaction methods—both sample based and individual based—allowing for meaningful standardization and comparison of data sets (Gotelli and Colwell 2001) have been used on vegetation community structure analysis. Finally, Renyi diversity ordering approach (Renyi 1961) was applied to quantify and compare current species diversity status among the six sites, following initial analysis that resulted in various diversity indices. Thus, Renyi diversity ordering has the ability to harmonize the different techniques and indices developed for biodiversity analysis (e.g. Berger–Parker, Shannon–Weiner, Simpson’s 1_D, diversity indices, Pielou evenness) that makes it complex to select the right tool for comparing biodiversity measurements (Magurran 2004). Renyi (1961) extended the concept of Shannon’s entropy (Shannon 1948), by defining the entropy of order α (α ≥ 0, α ≠ 1) of a probability distribution (p1, p2….ps). Diversity profile values (H-alpha) were calculated from the frequencies of each component species (proportional abundances pi = abundance of species i/total abundance) and a scale parameter (α) ranging from zero to infinity as (Tóthmérész 1995):
Plant species were subjected to Kruskal–Wallis H test to determine whether overall species abundance significantly differed among the sample blocks in the two sites. Homogeneity of species variance among the six sample blocks was evaluated, using Levene test (Levene 1960), defined as \(W = \left({\frac{N - k}{k - 1}} \right) \frac{{\mathop \sum \nolimits_{i = 1}^{k} Ni\left({{\acute{Z}}i - {\acute{Z}}i} \right)^{2}}}{{\mathop \sum \nolimits_{i = 1}^{k} \mathop \sum \nolimits_{J = 1}^{NI} \left({Zij - {\acute{Z}}i} \right)^{2}}}\) where \(Zij\) can have one of the following three definitions:
\(Zij = \left| {Y_{IJ} - {\acute{Y}}_{i}} \right|\) where \({\acute{Y}}_{i}\) is mean of the ith subgroup; \({\acute{Y}}_{i}\) is the median of the ith subgroup; and finally, \(Zij = \left| { Y_{IJ} -{\acute{Y}}^{\imath} i} \right|\), where \({\acute{Y}}^{\imath} i\) is the 10% trimmed mean of the ith subgroup. \({\acute{Z}} {i}\) are the group means of the \(Zij\), and \({\acute{Z}}\) is the overall mean of the \(Zij\). Kruskal–Wallis H test (a rank-based nonparametric test approach) was then used to determine whether overall species abundance significantly differed among the six wetlands. This was followed by the application of Dunn’s post hoc multiple comparison test (Dunn 1961), to determine the differences in species abundance and richness between each of the six wetlands. All analyses of species abundance, richness and diversity ordering were performed using PAST ver. 3.06 software package (Hammer et al. 2001), which contains robust algorithm (Krebs 1989).
Results
Composition of species indicator status, classified as wetlands and non-wetlands, across the six sites
Initial test for normality showed that plant data were not normally distributed (p = 0.841, Shapiro–Wilk test). A total of 3034 individuals, belonging to 45 plant species from 18 families, were registered across the six wetlands. Grasses, herbaceous and woody cover constituted 42.2%, 42.2% and 15.5%, respectively (Table 1). We recorded 14 obligate species (OBL) (e.g. Cyperus distans, Nymphaea micrantha and Ipomea aquatica) in ten out of the 24 Whittaker plots, with an average of 1.3 species/plot and a cumulative weighted score < 3.0 (Table 2). Overall, OBL species (i.e. typical hydrophytes) were substantially less abundant (p > 0.05) compared with the remaining indicator categories (FACW, FAC, FACU and UPL) and constituted 28.8% of the total species sampled (Table 2). OBL species mostly grasses and herbs were mainly from Wuntori, Tugu and Kukobila marshlands. Facultative wetland species (FACW) such as L. hexandra, Echinochloa stagnina and Kyllinga pumila were the most abundant (46.6%), while obligate upland species (UPL) (e.g. Helioptropium indicum and Imperata cylindrica) of dryland origin were the least abundant and constituted 26.6% of the total registered (Table 1).
Species categorized as FACW, FAC, FACU and UPL, with similar physiognomic characteristics and frequently grow on dryland areas or derived savannah, appear to adapt or grow in continuous wetter conditions, although their overall shift towards these wetlands was not significant (t test = 1.87, p = 0.11) (Fig. 2). Average species/plot from these four indicator categories (FACW, FAC, FACU and UPL) ranged between 3.25 and 7.5, with a cumulative weighted score > 3.0. Woody species (e.g. Syzygium guineense, Ziziphus abyssinica, Vitex crysocarpa and Khaya senegalensis) were relatively dominant in Adayili and Nabogo riparian wetlands (Table 2).
Species abundance distribution (SAD) and community structural assemblages
Species abundance differed significantly across the six sites (Hc = 30.59, p < 0.0001, Kruskal–Wallis test). Dunn’s post hoc test showed that species differed between Kukobila and Adayili (p < 0.001), Kukobila and Nabogo (p < 0.011), Tugu and Adayili (p < 0.0004) and Tugu and Nabogo (p < 0.003). Species from Wuntori marshland also differed substantially from that of Bunglung (p < 0.03), Adayili (p < 0.0005) and Nabogo (p < 0.0009) wetlands. Wuntori marshland (n = 768) recorded the highest mean number of species (18.73 ± SE 2.49), while the least was registered in Adayili riparian wetland (n = 260, 6.34 ± SE 1.80) (Table 3). Homogeneity of variance in species composition substantially differed in each of the six sites (p < 0.04, Levene’s test) (Table 4).
Geometric series model was well fitted in species abundance distribution (SAD) and showed overall significant difference (slope of SAD: F−test = 4.58, p (regr): 0.012, ANCOVA interactions × species rank) (Fig. 3; Table 4). But from individual wetlands, we observed a significant variation in plant abundance distribution along the slopes of five SAD curves, with the exception of Bunglung constructed wetland, which showed no difference in species distribution (slope [k] = − 0.09 ± 0.16, R2 = 0.008, χ2p = 0.56) (Fig. 2; Table 4). Adayili riparian wetland (k = − 0.28 ± 0.11, R2 = 0.15, χ2p = 0.01) and Nabogo riparian wetlands (k = 0.72 ± 0.09, R2 = 0.62, χ2p = 0.0002) showed higher significant difference in species abundance distribution compared with the three marsh systems. Comparison of SADs for the six sites helps in distinguishing the wetlands species abundance in relation to environmental influence in their distribution. Thus, sites that recorded less species abundance like Adayili riparian system (n = 260, 6.3 ± SE 1.8) were more spatially distributed as shown in their shallow rank abundance curve, while sites like Wuntori marshes with the highest species abundance (n = 768, 18.73 ± SE 2.49) were less spatially distributed, as indicated in the steep rank SAD curve (Fig. 3; Table 4). These distribution patterns generally revealed differences in plant dominance from individual wetlands, which indicate their relative success at competing for soil resources within their niche space.
Plant assemblages in the six wetlands showed that species were ranked from highest to least abundance (Fig. 3). Fewer than four species from the Poaceae family, namely C. zizanioides, E. stagnina, Imperata cylindrica and Pennisetum polystachion, were the most abundant that occurred in all six wetlands and constituted 8.88% of the total sampled (n = 45). The least abundant species was a tree (Mahogany: Khaya senegalensis, 2.22%) which was found only in the two riparian wetlands. Nearly all woody species were mostly found along the fringes of the riparian zone, while grasses and herbs were more widespread in the three natural marshes and the constructed wetland. Rarer species, with the same proportional representation, in the three marshes, were the least ranked on the SAD curve and included: Crotalaria retusa, Syzygium guineense and vitex crysocarpa (Kukobila wetland); Salacia recticulata and Mimosa pigra (Tugu wetland); and Ludwigia hyssopifolia, Mitragyna inermis and Syzygium guineense (Wuntori marshland) (Fig. 3). Cyperus distans and Ludwigia hyssopifolia were the least ranked in Nabogo riparian and Bunglung constructed wetlands, respectively. The presence of rarer woody species was well adapted to continuous wet condition and functionally coexisted with the dominant grasses and herbaceous cover.
Generally, species richness did not show any substantial variations among the sites (Hc = 5, p = 0.451, Kruskal–Wallis test) (Fig. 4). But comparing individual sites, we observed that the three marshes were much richer in species than the riparian wetlands and the constructed wetland (p < 0.05). Adayili riparian wetland was the most species poor, with the lowest turnover. Observed variations in species structural assemblages (i.e. abundance and richness) are reflected in their spatially even distribution as shown in the Renyi diversity ordering (Fig. 5). Renyi diversity curves showed a clear tight bend from higher to lower diversity index, along alpha (α) scale values. Although species diversity generally did not differ significantly (Hc = 1.557, p = 0.91, Kruskal–Wallis test) among the six sites, individual sites revealed Adayili riparian wetland, to be the most diverse (α scale = 0.04, Renyi index (r) = 4.90 to α scale = 3.96, r = 2.89), in spite of its lowest species abundance and richness. The high diversity in this site was linked to its shallower SAD curve (i.e. lowest species abundance) (Fig. 4). Nabogo riparian wetland was the second most diverse (α = 0.04, r = 4.9 to α = 3.96, r = 3.03), while the least species diverse was observed in Tugu (α = 0.04, r = 4.89 to α = 3.96, r = 2.85) and Wuntori (α = 0.04, r = 4.88 to α = 3.96, r = 2.83) marshes, even though they recorded highest species abundance and richness (individual-based rarefaction, Fig. 3). The diversity curve of these two sites appeared similar at the bottom of the Renyi index curve and was the least diverse (Fig. 4). The relationship between α scale values and diversity indices, especially at high infinities (α > ∞), had low proportion of diversity indices and dominant species.
Standardized comparison of species richness for two individual-based rarefaction curves. The data represent summary counts of plant species that were recorded from the six wetlands. The various colour lines are the rarefaction curves, calculated from Eq. 3 (Mendelssohn et al. 1988), with a 95% confidence interval. The dotted vertical lines illustrate a species richness comparison standardized to 260 individuals, which was the observed species abundance in the Adayili riparian system out of the six wetlands data
Renyi diversity ordering that compares tree evenness and richness in each of the six blocks found in the intact and logged sites. Note that the shape of the curve for a site is an indication of its evenness profile. Thus, shallower shape reflects high diversity and found on top of the curve, while steeper shape curve indicates less diversity and found at the bottom. Notice that Adayili riparian wetland (green colour) is the shallowest curve and spatially evenly distributed, while the steeper curves were observed from Tugu and Wuntori marshes, with the least evenness distribution. (Color figure online)
Environmental influence on community abundance distribution and diversity across the six wetlands
The matrices of the species-site biplot generated by CCA revealed magnesium (r = 0.77), bushfire (r = − 0.47) and soil pH (r = − 0.37) on axis I and farming activities (r = − 0.44), potassium (r = 0.41), phosphorus (r = 0.35 and nitrogen (r = 0.41) on axis II, to be the key environmental drivers of community abundance distribution and diversity among the wetlands (Fig. 6; Table 5). The first two axes accounted for 61.29% of the variation in the weighted averages of the 45 species in relation to 11 environmental factors (Table 5). Since cumulative percentage variances for axes I and II together accounted for more than 50% of species variations, axes III were not considered. Canonical coefficients of the environmental variables correlated significantly (t = − 0.6024; p < 0.05) for axis I and axis II (approximate t test, ter Braak 1986) and varied substantially, in all six sites (Kruskal–Wallis test, p < 0.05). Monte Carlo test further indicated a significant difference (p < 0.01) between eigenvalues generated for the two axes, as shown in the varying responses of species to environmental factors (Fig. 5; Table 5).
Canonical correspondence analysis (CCA) ordination diagram, showing the influence of environmental factors on species range shift, explained by the first two axes (axis I = 24.84 and axis II = 36.45) and accounted for 61.29% cumulative percentage variance across the six wetlands (R2 = 0.61, p < 0.05). The gray squares represent abbreviated plant species (e.g. Ceratophyllum demersum = Cera deme and Setaria pumila = Seta pumi), the black triangles represent sample sites, and the arrows represent each of the environmental variables plotted pointing in the direction of maximum change of explanatory variables across the six wetlands. The abbreviations denote different sample plots in the six wetlands. WUA-WUD = Wuntori wetland at Yapei; TUA-TUD = Tugu wetland; KUA-KUD = Kukobila wetland; BUA-BUD = Bunglung wetland; ADA-ADD = Adayilli wetland; and NAA-NAD = Nabogo wetland
Obligate wetland species (OBL), namely Pistia stratiotes Linn, N. micrantha Perr. & Guill and I. aquatica Forsk and FACW species (e.g. E. alba (L.) Hassk, Ludwigia abyssinica A. Rich. and Alternanthera sessilis (L.) R.Br. ex DC) in Tugu, Kukobila and Wuntori marshlands, correlated negatively with plots that had high levels of nitrogen, phosphorus, potassium and positively with farming activities and grazing intensity on the same axis (Fig. 6). Farming activities were found to correlate well with major soil nutrients—nitrogen (rp = − 0.54, p < 0.05) and phosphorus (rp = − 0.55, p < 0.05) (Table 6). Grazing activities in the marshes did not significantly impact (p > 0.05) on species assemblages, which were largely dominated by OBL and FACW species. Plots that were affected by fire occurrence in the marshes had low forage for grazing (rp = − 0.66). Woody species like S. guineense (Willd.) DC. Z. abyssinica Hochst. and V. crysocarpa Planch. ex Benth., from Adayilli and Nabogo riparian wetlands (plots ADA, ADB, NAA, NAB), tended to show resilience to fire (axis I, r = − 0.47) and were well adapted to prolonged wet condition, while the presence of grasses and herbaceous cover was affected by fire. Woody species resistant to frequent fire were characterized by their coarse bark and had buttresses that help anchor them on sloping ground along the edges of the riparian wetlands. Erosion (axis I, r = − 0.13 and axis II, r = − 0.10) occurred in places where bushfire was severe (rp = − 0.53, p < 0.01) along the riparian zone and grazed areas (rp = 0.70, p < 0.01) in the three marsh wetlands (Table 6). Soil pH was weak acidic to basic and correlated well with sites characterized by erosion (rp = 0.83, p < 0.01), grazing activities (rp = 0.55, p < 0.01) and organic carbon (rp = 0.76, p < 0.01). Patchiness was common in some severely burnt sites, in the two riparian systems.
Bunglung constructed wetland (lower right of CCA diagram—plots BUA, BU, BUC) was typically dominated by grasses and herbaceous community of derived savannah (facultative upland species) (e.g. Heliotropium indicum, Leersia hexandra and Crotalaria retusa), where farming activities were widespread (Fig. 5). Persicaria decipiens and Neptunia oleracea were the few obligate species sampled in this wetland. We did not observe grazing activities in many of the plots, where farming activities occurred, and this is reflected in the weak correlation (rp = − 0.14, p = 0.63) between these two factors (Table 6). The majority of species not represented in the ordination diagram grew in habitats with average conditions of the environmental factors investigated, whereas ubiquitous species that were near the centre of the CCA diagram (e.g. L. hexandra, Mimosa pigra and Mitragyna inermis) showed their broad responses to almost all of the ecological variables considered in the analysis.
Discussion
Changes in the structural assemblages of wetland vegetation have largely been linked to major hydrological factors like duration and depth of flooding (Gunderson 1994; Patten 1998), soil physical characteristics (Kirkman et al. 2000), altitude and hydroperiod (Rolon and Maltchik 2006) and phosphate derived from agriculture, sewage and industrial waste (Lambert and Davy 2010). But, in this study, we found that plant community structure, diversity and distribution were influenced by grazing, farming activities, bushfires and soil nutrient levels, following canonical correspondence analysis of the species–environment data. The findings in this study relative to previous studies could be due to different environmental drivers prevailing at the different biogeographical zones. The identified threats in this study have the tendency to impair the functional status of wetland, by altering its hydrological regime, reduction in plant gene pool and wildlife and introduction of alien invasive species to take a foothold on wetland systems (through endozoochorous and exozochorous means). Anning and Yeboah-Gyan (2007) reported the presence of alien species like Chromolaena odorata, Centrosema pubescens and Rottboellia cochinchinensis, in disturbed aquatic systems in the humid forest zone of Ghana. These ecological factors can be used to identify a suite of species and communities that are key indicators of important changes in their ecosystem and ecological integrity (Fennessy et al. 1998; Aznar et al. 2002). One criterion of detecting functional state of wetlands is the abundance of hydrophytes. Thus, their lesser abundance compared to the remaining species group categories (i.e. FACW, FAC, UPL) in this study was indicative of the sheer scale of human-led and animal disturbances. For instance, widespread grazing pressure in the Kukobila, Tugu and Wuntori marshes affected species diversity, in spite of increased species abundance and richness recorded. This was so because of the uneven distribution of species, leading to increased competition for resource utilization in the niche space.
During the long dry season (6–7 months), characterized by high temperatures and drought, forage availability and other seasonal pools of water in the study area tend to become scarce, leaving the permanent wetlands as the only alternative source of quality forage and watering points for both domestic and wild animals. Due to their broad forage preference, extensive range movements during grazing and long complex gut retention times, cattle in particular (common in pastoral farming in Northern Region of Ghana), have the potential to disperse plant propagules that are either alien or ruderal from terrestrial origin. They do this over a vast area in wetland systems through dung droppings and from their skin. Disturbances on the wetland vegetation zone are broadly classified as those induced by man, livestock and wildlife (Mathooko and Kariuki 2000). The multiple effects of these disturbance included loss of vegetation vertical strata, increase/decrease in species diversity, introduction of alien plant species and reduction in plant sizes and vegetation hectarage (Mathooko and Kariuki 2000). Similarly, Rachich and Reader (2000) observed that physical disturbances from livestock activities allowed non-native species like Lythrum salicaria L. a Eurasian plant to get a foothold in North American wetlands and, in the process, replace native species. Grazing and browsing by wildlife have been reported as the main disturbances of the vegetation near the Njoro River estuary at the Lake Nakuru National Park (Mathooko and Kariuki 2000). The authors concluded that grazing on the vegetation was severe around livestock watering points. In agricultural landscapes, the stress posed by grazing pressure on wetlands is of particular concern as domestic stock and feral grazing herds usually congregate in and around water areas (Jansen and Robertson 2001).
High levels of soil nitrogen, available phosphorus and potassium in these marshes were probably due to frequent dung deposits, transport of leached fertilizer application/crop residue from nearby farmlands during flood pulses and the deposition of ash, following bushfire occurrence. These mediated factors may be the reason why Cyperus difformis Linn., Cyperus spacelatus Rottb. and Fimbristylis ferruginea (L.) Vahl, across the three marshlands, responded to phosphorus, potassium and nitrogen availability. Phosphorus and potassium have been shown to support the growth of other Cyperus species and include: Cyperus papyrus-dominated vegetation community (Nalubega and Nakawunde 1995; Ssegawa et al. 2004) and C. backii (Vellend et al. 2000). Ash deposition following frequent bushfires in Adayili and Nabogo riparian wetlands is thought to have contributed to low levels of soil pH, compared to the remaining four wetlands. Bushfire is known to play a major role in soil nutrient enrichment, though this will depend on the intensity and frequency of the fire and type of vegetation. Despite the fact that surface fires increase soil pH and other major nutrients, this does not last long (Kotze 2013) because pH increases and availability of Ca, Mg, K and phosphates increases briefly following a fire and then returns to pre-burn levels (Wilbur and Christensen 1983; Schmalzer and Hinkle 1992).
Apart from the effects of fire on soil nutrients, impacts of fire occurrence were evident from the low species turnover, especially in the riparian wetlands, notwithstanding the high diversity recorded. This was due to the fact that low species abundance tended to be spatially evenly distributed, which facilitates efficient utilization of resources for growth. Seasonal variations in extreme weather events compel nomadic herdsmen to undertake early burning at the periphery of the marshes and riparian systems, in the peak of the dry season, in order to induce early vegetation sprout. Species that constitute the undergrowth (e.g. Schizachyrium sanguineum) in these sites were the most affected, since they were easily prone to fire occurrence, compared with the woody species (e.g. Vitex crysocarpa and Ziziphus abyssinica). Species in the three marshes were also affected by isolated fire occurrence, and this equally contributed in the less abundance of obligate species like Cyperus sphacelatus and Cyperus distans that are very sensitive to fire disturbance. Similar species like Cyperus papyrus in the Okavango Delta have been reported to be susceptible to fire (Ellery et al. 2003). In other parts of Africa, a number of authors (e.g. Bond et al. 1984; Mathooko and Kariuki 2000; Mulatu et al. 2004; Heinl et al. 2007a, b) have also reported on different plant species response to incidence of fire disturbance on wetlands. But according to Kotze (2013), the varying response of plant species to fire depends upon a wetland’s characteristics, including its climatic and hydrological context, as well as interactions with other disturbances such as grazing. Thompson and Hamilton (1983) and Kotze (2013) thus concluded that similar fire regimes may have dramatically different outcomes. The deliberate setting of fire through illegal hunting or poaching activities (e.g. African savannah—Mwazvita et al. 2017) is believed to help stimulate new growth by opening up the canopy, increasing nutrient availability to plants and removing litter (Heinl et al. 2007a, b). For example, the floodplain grasslands of the Lualaba River, Upper Congo, are annually burnt in order to improve the value of the forage for livestock (Thompson and Hamilton 1983). Plants poorly adapted to survive fire in the adult stage show two main strategies for recovering. These include: (1) ‘reseeding’, in which the plant is killed by fire and regeneration occurs from seed and (2) ‘resprouting’, in which the perennating buds of a plant generally survive fire and regeneration takes place by the sprouting of new shoots from protected structural features such as rhizomes or stolons (Bond and van Wilgen 1996). It can therefore be argued in this study that apart from fire occurrence as a factor, the structural distribution and assemblages of species probably reflect the type of species and the level of wetness of the sites. Hence, species recruitment, following post-fire occurrence, will depend on these factors as a measure of the wetlands resilience.
Widespread dominance of facultative species (FAC) in Bunglung constructed wetland was largely due to farming activities. Being the major livelihood activity among rural dwellers, farming is widely practiced all year round. Species that are sensitive to this form of disturbance go extinct in the process, thus paving the way for the invasion of alien species that have the competitive advantage of exploiting disturbed scenarios over native species. Ruderal species of derived savannah tend to regularly live in disturbed habitats, through their ability to develop masses of phytomaterial that have a high generative reproductive rate (Müllar 1995). Crop farming in Ugandan wetlands is noted as key determinants in explaining species composition and structural distribution (Ssegawa et al. 2004), while in some wetlands in Ethiopia farming activities have been attributed to losses of wetland plants vital for ecosystem services (Mulatu et al. 2004).
Conclusions
Generally, farming activities, fire, grazing and soil nutrient status were the main factors affecting species range shift and structural assemblages. The overall low obligate species abundance (hydrophytes) compared with the facultative wetland plants (FACW), facultative upland species (FACU), suggests the impact of disturbance altering community composition. Although the environmental disturbances documented in this study created some heterogeneous community assemblages in wetlands of Northern Ghana, its overall negative effect far outweighed its positive effect. This is because the majority of the species recorded were typically of derived savannah that have the ability to establish on disturbed habitats, by modifying their morphological and physiological features. It will therefore be useful to have a suit of hydrophytes, as indicators of wetlands functional status. The findings of this study highlight the current threats that these wetlands are subjected to. Thus, wetland managers and environmental policy makers should consider these threats as early warning signals on the overall functional status of wetlands in the study area and elsewhere. This will lead to the implementation of conservation measures for the sustainable exploitation of resources from these wetlands, which serve as a major source of livelihood for the rural communities.
References
Anning AK, Yeboah-Gyan K (2007) Diversity and distribution of invasive weeds in Ashanti Region, Ghana. Afr J Ecol 45:355–360
Arbonnier M (2004) Trees, shrubs and liannes of West African dry zones. CIRAD, Magraf Mnhn, CTA-The Netherlands, Wageningen
Aznar J, Dervieux A, Grillas P (2002) Association between aquatic vegetation and landscape indicators of human pressure. Wetlands 23:149–160
Aznar J, Dervieux A, Grillas P (2003) Association between aquatic vegetation and landscape indicators of human pressure. Wetlands 23:149–160
Battisti C, Luiselli L, Teofili C (2009) Quantifying threats in a Mediterranean wetland: are there any changes in their evaluation during a training course? J Biol Conserv 18(11):3053–3060
Biney C (1990) A review of some characteristics of freshwater and coastal ecosystems in Ghana. Hydrobiologia 208(1):45–53. https://doi.org/10.1007/bf00008442
Bond WJ, Van Wilgen BW (1996) Fire and plants. Population and community biology series. Chapman and Hall, London, p 14
Bond WJ, Vlok J, Viviers M (1984) Variation in seedling recruitment of Cape Proteaceae after fire. J Ecol 72:209–221. https://doi.org/10.2307/2260014
Carter MR, Gregorich EG (2007) Soil sampling and methods of analysis. Taylor & Francis Group, Boca Raton, FL, p 11. https://doi.org/10.1201/9781420005271
Cronk JK, Siobhan-Fennessy M (2001) Wetland plants: biology and ecology. Lewis Publishers, CRC Press, London
Dunn OJ (1961) Multiple comparisons among means. JASA 56:54–64
Ellery WN, McCarthy TS, Smith ND (2003) Vegetation, hydrology and sedimentation patterns on the major distributary system of the Okavango fan, Botswana. Wetlands 23:357–375
Fattorini SA (2005) simple method to fit geometric series and broken stick models in community ecology and island biogeography. Acta Oecol 28:199–205. https://doi.org/10.1016/j.actao.2005.04.003
Fattorini S (2013) Species ecological preferences predict extinction risk in urban tenebrionid beetle guilds. J Anim Biol 63:93–106. https://doi.org/10.1163/15707563-00002396
Fattorini S, Rigal F, Cardoso P, Borges PAV (2016) Using species abundance distribution models and diversity indices for biogeographical analyses. Acta Oecol 70:21–28. https://doi.org/10.1016/j.actao.2015.11.003
Fennessy MS, Geho R, Elifritz B, Lopez R (1998) Testing the floristic quality assessment index as an indicator of riparian Wetland quality. Final Report to US Environmental Protection Agency, Columbus
Gotelli NJ, Colwell RK (2001) Quantifying biodiversity: procedures and pitfalls in the measurement and comparison of species richness. Ecol Lett 4:379–391
Gotelli NJ, Colwell RK (2011) Estimating species richness. In: Magurran A, McGill B (eds) Biological diversity: frontiers in measurement and assessment. Oxford University Press, Oxford, pp 39–54
Gray JS, Mirza FBA (1979) Possible method for the detection of pollution-induced disturbance on marine benthic communities. Mar Pollut Bull 10:142–146
Gunderson LH (1994) Vegetation of the everglades: determinants of community composition. In: Davis SM, Ogden JC (eds) Everglades: the ecosystem and its restoration. St. Lucie Press, Boca Raton
Hammer Ö, Harper DAT, Ryan PD (2001) Paleontological statistics software package (PAST) for education and data analysis. Paleo Ecol Electron 4(1):9
Heegaard E, Birks HH, Gibson CE, Smith SJ, Wolfe-Murphy S (2001) Species- environmental relationships of aquatic macrophytes in Northern Ireland. Aquat Bot 70:175–223
Heinl M, Sliva J, Murray-Hudson M, Tacheba B (2007a) Post-fire succession on savanna habitats in the Okavango Delta wetland, Botswana. Trop Ecol 23:705–713
Heinl M, Sliva J, Murray-Hudson M, Tacheba B (2007b) Post-fire succession on savanna habitats in the Okavango Delta wetland, Botswana. Afr J Ecol 6:350–358. https://doi.org/10.2989/16085914.2013.828008
Henderson PA, Seaby RM (1999) Community analysis package 1.14, S041 8GN. Pieces Conservation Ltd, IRC House, Pennington
Hutchinson GE (1975) A treatise on limnology: III. Limnological botany. Wiley, New York
Janauer GA, Dokulil M (2006) Macropyhtes and algae in running waters. In: Ziglio G, Siligardi M, Flaim G (eds) Biological monitoring of rivers. Wiley, New York
Jansen A, Robertson AI (2001) Relationships between livestock management and the ecological condition of riparian habitats along an Australian floodplain river. Appl Ecol 38:63–75
Johnson DE (1997) Weeds of rice in West Africa. ADRAO/WARDA. CTA, DFID. Imprint Design, London
Keddy PA (2002) Wetland ecology: principles and conservation. Cambridge University Press, Cambridge
Kent M, Coker P (1992) Vegetation description and analysis. A practical approach. Wiley, Chichester
Kirkman LK, Goebel PC, West L, Drew MB, Palik BJ (2000) Depressional wetland vegetation types: a question of plant community development. Wetlands 20(2):373–385
Kotze DC (2013) The effects of fire on wetland structure and functioning. Afr J Aquat Sci 38(3):237–247
Krebs CJ (1989) Ecological methodology. Harper & Row, New York
Lacoul P, Freedman B (2006a) Environmental influences on aquatic plants in freshwater ecosystems. Environ Rev 14:89–136. https://doi.org/10.1139/a06-001
Lacoul P, Freedman B (2006b) Relationships between aquatic plants and environmental factors along a steep Himalayan altitudinal gradient. Aquat Bot 84:3–16
Lacoul P, Freedman B (2006c) Recent observation of a proliferation of Ranunculus trichophyllus Chaix. in high-altitude lakes of the Mt. Everest region. Arct Antarct Alp Res 38(3):394–398. https://doi.org/10.1657/1523-0430(2006)38[394:rooapo]2.0.co;2
Lambert SJ, Davy AJ (2010) Water quality as a threat to aquatic plants: discriminating between the effects of nitrate, phosphate, boron and heavy metals on charophytes. J Phytol 189(4):1051–1059. https://doi.org/10.1111/j.1469-8137.2010.03543.x
Levene H (1960) Robust tests for the equality of variances. In: Olkin I, Alto P (eds) Contributions to probability and statistics. Stanford University Press, Calif
Mackay SJ, Arthington AH, Kennard MJ, Pusey BJ (2003) Spatial variation in the distribution and abundance of submerged macrophytes in an Australian subtropical river. Aquat Bot 77:169–186
Magurran AE (2004) Measuring biological diversity. Blackwell Science, Oxford
Mäkelä S, Huitu E, Arvola L (2004) Spatial patterns in aquatic vegetation composition and environmental covariates along chains of lakes in the Kokemäenjoki watershed (S. Finland). Aquat Bot 80:253–269
Mathooko JM, Kariuki ST (2000) Disturbances and species distribution of the riparian vegetation of a Rift Valley stream. Afr J Ecol 38:123–129. https://doi.org/10.1046/j.1365-2028.2000.00225.x
Mendelssohn KIA, McKee L, Chabreck R (1988) The influence of burning on the growth response of Scirpus olneyi to saltwater intrusion and subsidence. Final report to Board of Regents. Louisiana State University, Baton Rouge
Mitsch WJ, Gosselink JG (1993) Wetlands, 2nd edn. Van Nostrand Reinhold Co., New York
Mueller-Dombois D, Ellenberg H (1974) Aims and methods of vegetation ecology. Wiley, New York
Mulatu K, Hunde D, Kissi K (2004) Impacts of wetland cultivation on plant diversity and soil fertility in South-Bench District, Southwest Ethiopia. Afr J Agric Res 9:2936–2947. https://doi.org/10.5897/ajar2013.7986
Müllar N (1995) River dynamics and floodplain vegetation and their alterations due to human impact. Arch Hydrol Suppl 1(101):477–512
Murphy J, Riley JP (1962) A modified single solution method for the determination of phosphate in natural waters. Anal Chem Acta 27:31–36
Murphy KJ, Dickinson G, Thomaz SM, Bini LM, Dick K, Greaves K, Kennedy MP, Livingstone S, Mcferran H, Milne JM, Oldroyd J, Wingfield RA (2003) Aquatic plant communities and predictors of diversity in a subtropical river floodplain: the upper Rio Parana, Brazil. J Aquat Bot 77:257–276
Mwazvita TBD, Wasserman RA, Dalu T (2017) A call to halt destructive, illegal mining in Zimbabwe. S Afr J Sci. https://doi.org/10.17159/sajs.2017/a0242
Nalubega M, Nakawunde R (1995) Phosphorus removal in macrophyte based treatment. In: 21st WEDC conference: sustainability of water and sanitation systems, Kampala, Uganda
Nsor CA, Alhassan EH (2015) Wetlands disturbance in northern region of Ghana: an indigenous conservation approach. J Biol Nat 4(2):77–88
Nsor CA, Obodai EA (2014) Environmental determinants influencing seasonal variations of bird diversity and abundance in wetlands, Northern Region (Ghana). Int J Zool 2014:10. https://doi.org/10.1155/2014/548401
Nsor CA, Obodai EA (2016) Environmental determinants influencing fish community structure and diversity in two distinct seasons among wetlands of northern region (Ghana). Int J Ecol 2016:10. https://doi.org/10.1155/2016/1598701
Nsor CA, Acquah E, Braima CA (2016) Seasonal dynamics of physico-chemical characteristics in Wetlands of Northern Region (Ghana): implications on the functional status. Int J Aquat Sci 7(1):39–49
Okezie IA, Agyakwa CW (eds) (1998) A handbook of West African weeds. International Institute of Tropical Agriculture (IITA) Nigeria, African Book Builders Ltd, Ibadan
Oťaheľová H, Valachovič M (2006) Diversity of macrophytes in aquatic habitats of the Danube River (Bratislava region, Slovakia). Thaiszia. J Bot Košice 16:27–40
Patten DT (1998) Riparian ecosystems of semi-arid North America: diversity and human impacts. Wetlands 18:498–512
Pinto P, Morais M, Ilhéu M, Sandin L (2006) Relationships among biological elements (macrophytes, macroinvertebrates and ichthyofauna) for different core river types across Europe at two different spatial scales. Hydrobiologia 389:73–88
Rachich J, Reader RJ (2000) An experimental study of wetland invisibility by purple loosestrife (Lythrum salicaria). Can J Bot 77(10):1499–1503
Ramsar Convention (2014) The Ramsar convention secretariat|Rue mauverney 28-1196 Gland, Switzerland. https://www.ramsar.org. Accessed 8 Jan 2019
Renyi A (1961) On measures of entropy and information. In: Proceedings of Fourth Berkeley symposium on mathematics, statistics and probability. University of California Press, Berkeley
Rolon AS, Maltchik L (2006) Environmental factors as predictors of aquatic macrophyte richness and composition in wetlands of southern Brazil. Hydrobiologia 556:221–231
Salafsky N, Salzer D, Stattersfield AJ, Hilton-Taylor C, Neugarten R, Butchart SH, Wilkie D (2008) A standard lexicon for biodiversity conservation: unified classifications of threats and actions. Conserv Biol 22(4):897–911
Schmalzer PA, Hinkle CR (1992) Soil dynamics following fire in Juncus and Spartina marshes. Wetlands 2:8–21
Schreiber-Gregory DN, Jackson HM (2017) Multicollinearity: what is it, why should we care, and how can it be controlled? 2017, pp 1–12
Shannon CE (1948) A mathematical theory of communication. J Bell Syst Tech 27: 379–423, 623–656
Siegel AF (2006) Rarefaction curves. In: Samuel K, Read CB, Balakrishnan N, Vidakovic B (eds) Encyclopedia of statistical sciences. Wliey, London. https://doi.org/10.1002/0471667196.ess2195.pub2, ISBN 9780471667193
Slaymaker T, Blench RM (eds) (2002) Rethinking natural resource degradation in Sub-Saharan Africa: policies to support sustainable soil fertility management, soil and water conservation among resource poor farmers in semi-arid areas: country studies, vol 1. University of Development Studies, Ghana, Tamale
Ssegawa P, Kakudidi E, Muasya M, Kalema J (2004) Diversity and distribution of sedges on multivariate environmental gradients. Afr J Ecol 42(1):21–33
Stohlgren TJ, Falkner MB, Schell LD (1995) A modified-whittaker nested vegetation sampling method. Plant Ecol 117(20):113–121
ter Braak CJF (1986) Canonical correspondence analysis: a new eigenvector technique for multivariate direct gradient analysis. Ecology 67(5):1167–1179
ter Braak CJF, Verdonschot PFM (1995) Canonical correspondence analysis and related multivariate methods in aquatic ecology. Aquat Sci 57(3):255–289
Thompson JK, Hamilton AC (1983) Peatlands and swamps of the African continent. In: Gore AJP (ed) Ecosystems of the world, 4B Mires: swamp, bog, fen and moor. Regional studies. Elsevier, Amsterdam
Tiner RW (1999) Wetland indicators: a guide to wetland identification, delineation, classification, and mapping. Lewis publishers, London
Tóthmérész B (1995) Comparison of different methods for diversity ordering. J Veg Sci 6(2):283–290. https://doi.org/10.2307/3236223
Van der Merwe AJ, Johnson, JC, Ras LSK (1984) An NH4HCO3–, NH4F, (NH4)2EDTA method for the determination of extractable P, K, Ca, Mg, Cu, Fe, Mn & Zn in soils. In: Handbook of standard soil testing methods for advisory purposes. Soil Science Society of Southern Africa, Sannyside, Pretoria
Van Geest GJ, Roozen FCJM, Coops H, Roijackers RMM, Buijse AD, Peeters ETHM, Scheffer M (2003) Vegetation abundance in lowland floodplain lakes determined by surface area, age and connectivity. Freshw Biol 48:440–454
Vellend M, Lechowicz MJ, Waterway MJ (2000) Environmental distribution of four Carex species (Cyperaceae) in an old-growth forest. Am J Bot 87(10):1507–1516
Walkley A (1947) A critical examination of a rapid method for determining organic carbon m soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Walkley A, Black IA (1934) An examination of the Degtjareff method for determining organic carbon in soils: effect of variations in digestion conditions and of inorganic soil constituents. Soil Sci 63:251–263
Wilbur RB, Christensen NL (1983) Effects of fire on nutrient availability in a North Carolina coastal plain pocosin. Am Midl Natlist 110:54–61
Acknowledgements
We express our sincere gratitude to the staff of the herbarium at the University for Development Studies, for permitting us to use their laboratory for plant identification.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that there is no conflict or competing interest whatsoever, regarding the publication of this research article, among my co-authors.
Data availability
Data for this study are available and can be accessed upon request by the editor of Journal or by other readers.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Handling Editor: Télesphore Sime-Ngando.
Rights and permissions
About this article
Cite this article
Nsor, C.A., Antobre, O.O., Mohammed, A.S. et al. Modelling the effect of environmental disturbance on community structure and diversity of wetland vegetation in Northern Region of Ghana. Aquat Ecol 53, 119–136 (2019). https://doi.org/10.1007/s10452-019-09677-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-019-09677-5