Abstract
Patterns of distribution are influenced by species environmental requirements and limits, but experimental tests are needed to discern whether correlates of abundance directly affect survival and success. Springs in Australia’s arid interior support a wide diversity of gastropods only found in springs, and these species show dichotomous patterns of distribution. “Amphibious” species are broadly distributed across many springs and microhabitats, and “aquatic” species confined to the deepest pool areas within large springs. This pattern appears to be driven by the interaction between different environmental conditions in different microhabitats and the environmental tolerances of each endemic snail species. Factorial experiments were used to test whether conditions in the environmentally extreme and variable tail area of springs (considering pH, conductivity, temperature and desiccation potential, alone and in synergistic scenarios) elicited lethal or sub-lethal responses in spring snails endemic to springs on opposite sides of the Australian arid zone. All species restricted to spring pools were able to endure 24 h exposed to the average tail conditions, alone and in combination, but most suffered mortalities when subjected to extremes, and mortalities occurred sooner in the most restricted species when elevated pH and conductivity were experienced in combination. Responses of species from different locations are similar, but pattern of distribution in the field were not correlated with tolerance of environmental extremes—with the “amphibious” species from the sub-tropics being far more sensitive than its arid counterpart. These findings suggest that environmental variance within springs can influence patterns of distribution and abundance, particularly when extremes are experienced simultaneously over sustained time periods. But despite similarities in responses across species from these two spring complexes, no simple generalisations linking distribution and tolerance were discernible.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Species with more specific environmental requirements are thought to be more restricted in distribution than those that can persist across a broad range of conditions (Gaston et al. 1997; Slatyer et al. 2013). Environmental requirements and limits, and therefore the suitability of conditions within a species’ range, are often inferred from patterns in abundance across naturally occurring gradients (Hengeveld and Haeck 1981, 1982; Guisan and Thuiller 2005). However, experiments are needed to determine whether environmental variables directly affect processes that dictate abundance and distribution (Vanhorne 1983; Mitchell 2005; Kearney 2006). Such mechanistic connections between organism and environment can be subtle, because the environment is multipartite and variable—we must venture to discern which factors, or combinations of factors, as their averages or extremes, influence each species of interest (Underwood et al. 2000; Piggott et al. 2012; Dowd et al. 2015).
A rich and productive history of experimental research has used the environmental tolerances and limits of freshwater species to explain patterns of distribution and diversity (Hart et al. 1991; Stomp et al. 2011; Stendera et al. 2012). However, few experimental tests address spring systems (Wiegert and Mitchell 1973; Ponder et al. 1989; Klockmann et al. 2016). This is unfortunate, as a wealth of descriptive studies suggests environmental matching plays a strong role in spring systems (Fleishman et al. 2006; Zullini et al. 2011; Spitale et al. 2012; Rosati et al. 2014), and springs often present unique physiological challenges (e.g. extreme temperatures (Mitchell 1974; Pritchard 1991), low oxygen or high dissolved CO2 and CaCO3 (Blinn 2008) and unique water chemistry (LeBlanc et al. 2015)). Gradients in diversity and abundance in springs have been related to the strong environmental gradient that exists between a spring’s groundwater source and its outflow (Cantonati et al. 2012). Generally, species specialised to the stable conditions of the upper reaches and spring pools (“crenobionts”) are replaced by species with broader environmental tolerances (“crenophiles”) as water flows out into spring brooks (Cantonati et al. 2012). Springs in arid contexts present a unique system, because environmental gradients between spring outflows and the surrounding desert are stark and, in these regions, springs often provide the only permanent aquatic habitat in a landscape characterised by water impermanence and variability (Shepard 1993; Davis et al. 2017).
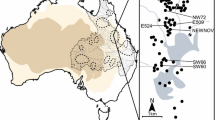
Springs fed by Australia’s Great Artesian Basin (GAB) are maintained by one of the world’s largest arid zone aquifers not in a serious state of decline (Richey et al. 2015), though Australian springs have still experienced severe losses of habitat (Fairfax and Fensham 2002, 2003; Powell et al. 2015). In this system, a phylogenetically diverse range of species persists in springs that are hydrogeologically similar (Fensham and Fairfax 2003; Fensham et al. 2011) and share a common quaternary history (Murphy et al. 2015), despite being clustered into discrete “supergroups” in different climatic and geological contexts (see Habermehl (1982) for more detail) (Fig. 1). Akin to spring systems in the Americas (e.g. Hershler et al. 2014) and Europe (e.g. Benke et al. 2011), GAB springs are rich in endemic gastropods (Ponder 1995; Ponder and Slatyer 2010; Fensham et al. 2011). Each species is restricted to a single spring supergroup (Perez et al. 2005) (and often a small locality within that), and diversity is concentrated in particular regions (Ponder 1995; Ponder and Slatyer 2010)—for example springs surrounding Kati Thanda (Lake Eyre) (McMahon et al. 2005) are particularly rich in Tateidae (Ponder et al. 1989), whilst springs in the Pelican Creek complex house a different radiation of Tateidae (Ponder and Clark 1990) living in sympatry with species of Bithyniidae (Ponder 2003) and Planorbidae (Brown 2001) (Ponder et al. 2010) (Fig. 1).
The spring supergroups of the Great Artesian Basin overlaid on a map of the arid and semi-arid regions of the Australian continent (centre), with examples of a subset of the species of mollusc endemic to two regions [with their family denoted by the letter in brackets—Tateidae (T), Planorbidae (P) and Bithyniidae (B)]: the Kati Thanda (Lake Eyre) region in the arid south-west (which incorporates numerous spring complexes) (left) and the Pelican Creek complex (a single locality) in the Barcaldine region of the basin’s semi-arid north-east (right)
Gastropods endemic to GAB springs on opposite sides of the basin have one of two distinct types of distribution, echoing broader generalisations about the role of environmental gradients in springs. Most species occupy few springs and are restricted to the deepest areas of permanent pool at the spring vent, but some species persist in high abundance in shallow and ephemeral spring outflows (or tails), and these species generally occupy more springs (Tables 1, 2). Strong differences in prevailing environmental conditions, and patterns of variance, across pool and outflow areas have been documented in GAB springs (Table 3). Outflow (aka tail) areas experience higher conductivity, pH and temperature as well as greater variance in these (Rossini et al. 2017). Early studies in one region of the GAB system provided a mechanistic link between patterns of distribution and environmental tolerances of endemic gastropods (Ponder et al. 1989). Species deemed “aquatic” had restricted distributions and suffered severe mortalities in the face of environmental extremes, whilst species deemed “amphibious”, by contrast, could tolerate environmental extremes for up to 48 h and were the only species found in shallow outflows and edges and occupied more springs (Table 2). Therefore, the environmental tolerance of each species predicted its pattern of distribution. An ability to generalise using links between physiology and distribution across the vast and diverse GAB springs system would be beneficial to pure science and management; however, such tests are only available for this single radiation of Tateidae alone.
By extending studies to species and regions yet to be investigated (e.g. those from Pelican Creek, Table 1), we can assess whether the aquatic-amphibious dichotomy proposed for the Kati Thanda system can be used to explain patterns of distribution generally in GAB springs, or instead, whether generalisations can be made using the taxonomic affinity of species [i.e. more closely related species are more similar (Dussart 1979; Losos 2008)] or locality (i.e. species from the same region are more similar). Such research also provides an opportunity to build on previous tests. Environmental extremes coincide spatiotemporally in springs (Rossini et al. 2017). Periods of little rainfall in hotter months bring extremes of desiccation potential, pH and conductivity, and these extremes are more severe in tail areas (as high as pH 10.5, 4000 μS/cm @ 25 °C conductivity and temp ranges of 5–50 °C; Table 1). Therefore, important interactions that occur between variables (Piggott et al. 2015) and their timing (Layman et al. 2000), as well as any physiological costs when non-lethal conditions are endured for long periods (Sultan 2015), have, to date, been overlooked. Springs also differ in their prevailing conditions (Rossini et al. 2017) (Table 4), and populations of the same species from different springs respond to stressors in different ways (Ponder et al. 1989). By extending our tests to consider the responses of multiple populations of each species to factors in isolation and in combination and for extended periods, we can attempt to capture the subtleties by which environmental conditions dictate patterns of distribution and diversity in this spring system.
This study had three interrelated aims, each with its own hypotheses (Fig. 2). First, we determined whether six of the previously untested species of gastropod endemic to the Pelican Creek springs complex differed in their short-term (within 24 h) lethal responses to environmental conditions representative of the averages and extremes that prevail in tail areas (elevated desiccation potential, conductivity, pH and temperature), and whether those responses differ across populations or seasons. Second, we extended these analyses evaluating the potential for factors tolerable in short-term tests to interact over short- (within 24 h) and long (over 1 month)-exposure periods to cause lethal or sub-lethal effects. Third, we compared species from Pelican Creek to those previously tested from Kati Thanda (Ponder et al. 1989) to establish whether species with similar patterns of distribution in each region respond to environmental extremes in a similar fashion, or whether similarities lie in their taxonomic affinity or their region.
A schematic showing the three main aims of this study, and their relationship to one another—Aim 2 involves further tests of factors from Aim 1 on species from Pelican Creek, Aim 3 involves assessing similarities between the species assessed in Aim 1 and those from the Kati Thanda springs tested previously by Ponder et al. (1989). Under each Aim are the species tested, the factors considered and the treatments compared, followed by the hypotheses tested for each Aim
Methods
This study compares endemic gastropods from two spring supergroups on opposite sides of the GAB (Fig. 1). All of the experimental results presented here derive from work conducted at the Pelican Creek complex within the Barcaldine supergroup in the northeast of the basin, within the Edgbaston Reserve, which encompasses most of the ~ 145 springs in the Pelican Creek springs complex. Snails were collected from two large springs: one in the centre of the complex (site code E509, Lake Eyre Basin Springs Assessment (LEBSA) ID 651 (DSITIA 2015), lat. long. −22.732 and 145.431) and one in the south (site code SW50, LEBSA ID 635 (DSITIA 2015), lat. long. −22.753, 145.427). These springs were chosen because they are among the few within the complex that contain all species and exist in different landscape contexts, and thus have prevailing differences in their water chemistry (Table 4) (for more detail on environmental variance within E509 see Rossini et al. (2017)).
Data collected from the species endemic to Pelican Creek were compared to the previously collected and published data of Ponder et al. (1989), which concerned species endemic to multiple spring complexes surrounding Kati Thanda (Lake Eyre) in the GAB’s south-west. For both regions, only a subset of species was used. There are nine species of snail endemic to the Pelican Creek complex; however, this study uses six of these: the Tateidae Jardinella acuminata, J. jesswiseae, J. edgbastonensis, the Planorbidae Glyptophysa sp., Gyraulus edgbastonensis and the Bithyniidae Gabbia fontana. Other species were excluded as they are numerically rare (Edgbastonia alanwillsi, J. pallida) or have very similar patterns of distribution to tested species (J. corrugata is very similar to J. edgbastonensis). For the Kati Thanda springs, Fonscochlea variabilis, F. accepta, F. aquatica, F. zeidleri and Trochidrobia punicea (all Tateidae) were selected, despite Ponder et al. (1989) considering 5 additional species (F. billakalina, F. conica, T. smithi, T. minuta and T. inflata), because they were the only species for which all tests were conducted and included in the publication.
Aim 1.1: Short-term tests of lethal responses to conditions in tail areas at Pelican Creek
These experiments considered six species endemic to Pelican Creek and assessed their short-term (24 h) tolerances to the average and maxima (extreme) of conductivity, pH, desiccation and temperature experienced in spring tails as isolated variables. They were designed to test the hypothesis that species differ in their tolerances, and that species found in spring tails will survive longer under average and extreme tail conditions than species restricted to spring pools (for each species distribution pattern see Table 1). The experimental design for tests of pH, conductivity and desiccation differed to those for temperature (see details below). Individuals from the six species from Pelican Creek were selected by haphazardly collecting in the maximum size range in each spring (assumed to be adults, different for each species, see Fig. 1). In all experiments, individuals were transported in spring water to a shaded area within 30 min of collection and held in aerated tanks filled with water from their spring of origin until experiments began.
In water naturally emerging in springs at Edgbaston, the major ions likely to influence pH and conductivity are HCO3, Na and Cl (Table 5). The different proportions of major ions are of critical importance in determining the effect of changes in salinity (Cañedo-Argüelles et al. 2016). Further, in this system, naturally alkaline water (due to its long residence time in the basin, alkalinity 402 mg/L on average at Edgbaston), gradients in pH (between 7 and 10) and the concentration of bicarbonates are likely to interact to drive species responses (Vera et al. 2014). Therefore, to manipulate water chemistry we chose to harvest water from the bore to maintain the alkalinity and trace ions in the water, but mimic tail conditions with the addition of NaCl (measured as an increase in conductivity) and NaHCO3 (measured as an increase in pH, though its addition also influenced conductivity). Three levels of conductivity and two levels of pH were selected to represent the pool-tail gradient (based on Rossini et al. (2017)): average conductivity in pools = 800 μS/cm, the average in tails = 3500 μS/cm and the extreme in tails = 10 mS/cm and average pH in pools = 8 and in tails = 9.5. For treatments focussed on single variables (i.e. tail conductivity or tail pH) either NaCl or NaCO3 was added until the desired treatment levels were reached.
Desiccation tests aimed to replicate the three degrees of emersion these snails experience in nature (Table 3) (Rossini et al. 2017). The extreme tail treatment represented the complete loss of standing water, which occurs when the tail areas of springs dry seasonally. For this treatment, snails were patted dry with filter paper and placed into containers with dry filter paper. Snails are also left without standing water when water evaporates, but groundwater maintains the moisture of the sediment (as occurs for up to 6 h daily in tail areas, or near the vent of shallow springs with no pool (Rossini et al. 2017)). This situation was represented by a moist treatment, in which snails were placed on moistened filter paper without excess water. Paper was checked constantly and re-moistened if required. Finally, pools were represented by a wet treatment, in which snails were held in containers full of bore water. Filter paper was placed on the bottom to control for any possible effect the filter paper may have had.
These tests of conductivity, pH and desiccation were conducted in 150-mL plastic containers filled with water of the chosen treatment. The lethal response was measured as the proportion of individuals alive after being taken from their experimental container and placed in water from their home spring for 1 h. In these experiments, time was treated as an independent factor; thus, a different set of 5 individuals was allocated to each of the 1-, 2-, 6- and 24-h time periods. For each set, individuals were haphazardly allocated to treatments and immersed by 1000 h on the day of collection; then, response was measured after the allocated time had passed (i.e. all four sets were started at 1000 h, and the 1-h treatment was assessed at 1100 h, the 2-h one at 1200 h and the 24-h one at 1000 h the following day). Data are presented as the proportion of individuals surviving in each time treatment.
Tests regarding temperature were conducted using 90-mL containers filled with spring water, placed in water baths that were either heated or cooled. The response variable for temperature tests was different from all other tests, as this experiment employed a repeated-measures design where temperature increments for each species are not independent of one another. The full range of increasing (25–50 °C) or decreasing (25–0 °C) temperatures, changed at a rate of 5 °C per hour, were subjected on each jar containing ten individuals of each species. This rate reflects the rate of temperature change observed in the field (e.g. an increase of 4 °C/h (Rossini et al. 2017) and is conservative compared to other studies of thermal limits (e.g. 18 °C/h in Shah et al. (2017)). At each 5 °C increment (starting at 25 °C, up to 50 °C and down to 0 °C), temperature was maintained for 2 min at the chosen temperature and snails were observed in situ. All individuals were deemed to be active or inactive depending on whether they retained traction with the container and were crawling normally (active) or had lost traction, dropped to the bottom of the container and ceased movement (not active). Results presented are the proportion of individuals active at each 5 °C interval across the full 0–50 °C test range.
Aim 1.2-3: Variance in response across populations and seasons at Pelican Creek
Within this aim, we also tested whether different populations responded in the same manner, and whether those responses were consistent across seasons. These comparisons were restricted to four of the previously tested species—J. jesswiseae, Glyptophysa sp., Gy. edgbastonensis and Ga. fontana, as we struggled to collect sufficient numbers of other species in both seasons from both springs. In these tests, the same methods presented above were used, but populations from each of the two springs were tested simultaneously. These springs differ in their prevailing conditions (Table 4), with E509 being shallow and having higher pH and conductivity on average. These tests were also repeated in July (winter) and November (summer). Conditions in springs in these two times are highly dissimilar (see Rossini et al. 2017 for detail); springs are at their largest in July, average conductivity is at its lowest (800–1500 for pool and tail, respectively), and temperature variance is lower with lower extremes (15–20 and 5–25 °C for pool and tail, respectively), in contrast to November when springs severely retract in size before the onset of summer rains, conductivity is higher and the discrepancy between pool and tail is greater (800–2200 μS/cm for pool and tail, respectively), and temperature is higher and reaches higher extremes (25–30 and 20–45 °C for pool and tail, respectively).
As replication was kept deliberately low to avoid excessive mortality of these threatened species, we assessed whether different populations tested in different seasons were dissimilar using nMDS following the recommendations of Borcard et al. (2011). For each population of each species, the proportion of individuals alive for each treatment level and time were input as a proportion. For factors in which the response of each species across all seasons and populations were the same (e.g. all pool treatments), the factor was excluded from the analysed dataset. This meant that the final data set was composed of 22 input values for each population of each species [one for each time (1, 2, 6 and 24 h) for each of the dry and 10mS and one for each of the ten temperature increments]. As data to be input were quantitative and used the same scale (proportion of individuals alive as a value between 0 and 1), the distance matrix was calculated on non-standardised data using Euclidean distance via the “vedist” function and plotted into nMDS ordinations using the “metaMDS” function with the vegan package (Oksanen et al. 2015) executed in R Studio (R Studio Team 2015).
Aim 2: Sub-lethal and interactive effects on Glyptophysa sp. of tail conditions over short and extended time periods
These experiments aimed to test subtleties in the effect of pH and conductivity concurrent with average tail conditions using a single species, Glyptophysa sp. This species was chosen as it has the most environmentally particular distribution in the field, being found in large springs with deep pools and only within the deepest parts of those springs (Rossini et al. 2017). These tests were conducted as an extension of Aim 1, because Glyptophysa sp. is never found in tail areas, but it was able to endure all average tail conditions for 24 h. Within this aim, we assessed its sub-lethal responses (response time, see below for details) to short-term exposure and lethal responses to long-term exposure by running experiments for 1 month (vs. 1 day for Aim 1). It also included comparisons of the effect of pH and conductivity treatments alone, when elevated pH and conductivity are experienced in unison. For treatments focussed on single variables (i.e. tail conductivity or tail pH), either NaCl or NaCO3 was added until the desired treatment levels were reached (see previous section). For the combined treatment (i.e. tail conductivity and pH), both NaCl and NaCO3 were added to the same end. Snails were collected and tested in September 2016 from the population in SW50.
The potential for pH and conductivity to cause sub-lethal effects (Aim 2, H1) was tested using the same methods as Aim 1.1; however, in addition to the number of individuals surviving at each time in each treatment, the response time of all individuals in each time and treatment combination were assessed. The response variable for these tests was the time (in seconds) that each individual took to fully extend both tentacles beyond the aperture after being placed in a container full of water from their home spring, which is referred to from here as response time. It is assumed that individuals that are slower to resume normal activity are physiologically stressed. Results presented are the average response time of all 6 tested individuals (± standard error). Differences in response time across times and treatments were tested with n = 6 for each treatment (except for 10 mS/cm, where the sample size was reduced by mortalities, so 3 random replicates were removed from the 800 μS/cm treatment to give a final of n = 3 in both treatments) using ANOVA, with time and treatment as factors, and a Tukey’s HSD post hoc test for pairwise comparisons.
Tests of the potential for factors tolerable in the short term to cause cumulative effects that result in death over exposure periods > 24 h employed a different system. Long-term tests on Glyptophysa sp. were conducted in a laboratory in Brisbane using snails and water collected from spring SW50 and transported to Brisbane. Experiments were conducted in a flow-through aquarium system, where each individual was housed in its own sealed 90-mL container with water of the desired treatment circulating continuously from an aerated sump shared between all 6 replicates for each treatment. The conductivity and pH of water within the sumps, mortalities of Glyptophysa sp. and whether food was required were checked daily. Glyptophysa sp. were fed washed pesticide-free lettuce ad libitum, a food source used in other studies of closely related species (Kefford et al. 2007), and one on which a laboratory colony of this species of Glyptophysa sp. have been maintained with individuals reared through multiple generations for over a year (Rossini, pers. obs.). The response variable for these long-term experiments was the time until death for the n = 6 individuals tested in each treatment. These were compared using ANOVA, with treatment as the only factor and individuals that survived at the end of the experiment allocated a time until death of 38 days.
Aim 3: Assess similarities between species with different patterns of distribution from different locations
In order to contextualise the responses of species from Pelican Creek with those previously studied from the Kati Thanda (Lake Eyre) region, we replicated the experiments by Ponder et al. (1989). This aim tested three alternative hypotheses: that species with the same pattern of distribution would have similar responses (H1), that species from the same family would have similar responses (H2), or that species from the same region would have similar responses (H3).
Only a subset of the treatments used by Ponder et al. (1989) were used here (namely, all conductivity levels, all temperatures and desiccation treatments). Conductivity levels in Ponder et al. (1989) were converted to mS/cm for the presentation of results and analyses (6‰ = 10 mS/cm, 9‰ = 15 mS/cm and 12‰ = 20 mS/cm). Experiments at Edgbaston were replicated in July and November, and populations as previous tests suggest responses differ across season and population (see “Results” section), and the months chosen for these experiments flank the seasonal timing of the experiments of Ponder et al. (1989) (August to September). Unlike the experiments for Aims 1 and 2, table salt was used to manipulate conductivity instead of NaCl, as this was the method used by Ponder et al. (1989). For two species (J. acuminata and J. edgbastonensis), we were unable to collect enough individuals in each season and spring combination and, thus, the full comparison was not possible.
As replication was low in both studies (n < 3), and some season-spring combinations were not included, we chose to assess each hypothesis using ordination. All raw data presented by Ponder et al. (1989) were input into a data frame representing the Kati Thanda species, alongside the results for species endemic to Pelican Creek, and were analysed using the methods described in the previous section (Aim 1.2-3). This analysis differed only in that 26 input values were used for the comparison [one for each time (1, 2, 6 and 24 h) for the dry, 6-ppm, 9-ppm and 12-ppm treatments and one for each of the ten temperature increments].
Results
Aim 1: Short-term tests of lethal responses
No individual of any species (J. acuminata, J. jesswiseae, J. edgbastonensis, Glyptophysa sp., Gy. edgbastonensis, Ga. fontana) suffered mortalities at any time in the pool treatment. No deaths occurred within 24 h for any species in the tail treatments moist, tail pH (9.5) and average tail conductivity (3500 μS/cm). Mortalities did occur in most species in response to all extreme tail treatments: dry, 10 mS/cm conductivity and at the temperature extremities.
Species differed from each other in their response to these extreme treatments. All species apart from Ga. fontana suffered mortalities in the dry treatment (Fig. 3, left). J. acuminata suffered greatest mortality: 100% of individuals were dead at all times (Fig. 3c). All Gy. edgbastonensis and J. jesswiseae survived in the 1-h dry treatment, but mortalities occurred at 2 h and none survived in the 6- and 24-h treatments (Fig. 3a, d). Eighty per cent of individuals of Glyptophysa sp. were alive in the 2- and 6-h treatments, but only 20% survived 24 h under dry conditions (Fig. 3b). G. fontana and J. edgbastonensis were the only species in which 100% of individuals were alive in the 6-h dry treatment (Fig. 3e, f), and Ga. fontana was the only species in which all individuals were alive after 24-h dry.
When exposed to extreme conductivity (10 mS/cm) (Fig. 3, centre), all species apart from Ga. fontana suffered mortalities (Fig. 3f). All individuals of all species apart from J. edgbastonensis were alive in the 1- and 2-h treatments (Fig. 3e). G. edgbastonensis and J. acuminata had lower proportions of individuals alive in the 6-h treatment, and 100% of individuals were dead in the 24-h treatment (Fig. 3a, c). Similar patterns occurred in Glyptophysa sp. and J. jesswiseae, except that in both species a small number of individuals remained alive in the 24-h treatment (Fig. 3b, d).
When exposed to increasing or decreasing temperatures, all species had all or some individuals that lost traction and ceased movement at temperatures below 10 °C (Fig. 3, right), though individuals of the two species of Planorbidae (Glyptophysa sp. and Gy. edgbastonensis) were the only ones to remain active at 5 °C (Fig. 3a, b). Under increasing temperatures, all species of Jardinella and Ga. fontana began losing traction and ceasing activity at 35 °C. All individuals of J. acuminata ceased movement at 40 °C (Fig. 3c) and all other species had < 50% of individuals active at 40 °C. Of the two planorbid species this began at higher temperatures, 40 °C for Gy. edgbastonensis and 45 °C for Glyptophysa sp. (Fig. 3a, b). All individuals of all species had entered heat coma at 50 °C, and no individuals of any species revived once the water returned to 25 °C after reaching 50 °C.
Aim 1.2-3: Differences across populations and seasons
In all species, there was no mortality in response to the pool treatment or the tail conductivity (3500 μS/cm), tail pH (9.5) and tail moist treatments across any populations or season (the same result as previous tests). Differences arose in response only to the extreme treatments (dry, 10 mS/cm conductivity and extreme thermal limits).
Whether snails from different springs or snails examined in different seasons differed from one another depended on the variable tested (Fig. 4). When exposed to the extreme dry treatment, all species responded in a species-specific fashion (Fig. 4, far left). Individuals from different springs elicited similar responses, but across all species suffered larger mortalities (> 50% dead) in earlier time treatments in November compared to July: in the 2-h treatment instead of the 6 or 24 h for Gy. edgbastonensis (Fig. 4a), in the 6-h treatment versus the 24-h treatment for Glyptophysa (Fig. 4b), in the 1-h treatment instead of the 2-h treatment for J. jesswiseae (Fig. 4c) and mortality after 6 h compared to none within 24 h in Ga. fontana (Fig. 4d).
The proportion of individuals of a G. edgbastonensis, b Glyptophysa sp., c J. jesswiseae and d G. fontana from two populations (circles = E509, squares = SW50) tested in two seasons (dark line, filled = July, dashed line, open = November) alive at each time or temperature when exposed to extreme dry treatment (far left), extreme conductivity treatment (10 mS/cm) (centre left) and the full temperature range experienced in nature (split according to population, centre right = E509, right—SE50)
Exposure to extreme conductivity treatments (Fig. 4, centre left) revealed different patterns across species. In the two planorbid species (Gy. edgbastonensis and Glyptophysa sp.) higher mortality rates occurred sooner in July compared to November, but snails from E509 (the spring with higher conductivity and pH on average, Table 4) experienced greater mortality than those from SW50 (Fig. 4a, b). In contrast, J. jesswiseae showed the same pattern as for the extreme dry treatment—mortalities occurred sooner in November than in July for snails from both springs (Fig. 4c). No mortalities of Ga. fontana occurred in response to extreme conductivity levels in any season for snails from both springs (Fig. 4d).
For increasing temperature (Fig. 4 centre right and far right), a threshold of > 50% mortality occurred at lower temperatures in the July observations compared to the November observations in snails of all species from both springs. Specifically, Gy. edgbastonensis and Glyptophysa sp. suffered 100% mortality at 45 °C (Fig. 4a, b), for J. jesswiseae at 35 versus 40 °C (Fig. 4c) and for Ga. fontana at 35 versus 45 °C (Fig. 4d). Patterns in the lower thermal limit were contingent on the spring from which snails came, the species and the season: Gy. edgbastonensis from both springs had a lower thermal limit in November (Fig. 4a), Glyptophysa from E509 had a lower thermal limit in July, but those from SW50 were similar across seasons (Fig. 4b), J. jesswiseae from E509 had a lower thermal limit in July, but those from SW50 were the opposite (Fig. 4c) and Ga. fontana thermal limit was lower in snails from E509 but similar to those from SW50 (Fig. 4d centre right versus right).
When all responses are considered simultaneously (Fig. 5), populations of each species were generally more similar to each other within a season. For example, the responses of Ga. fontana from E509 tested in July were more similar to Ga. fontana from SW50 tested in July than those from E509 tested in November. Glyptophysa sp. is an exception, and both populations in each season are approximately equidistant. Despite this variability, species remain dissimilar to each other—no 95% confidence interval for a species overlaps with another, particularly within seasons.
An nMDS showing the response to dry, 10 mS/cm conductivity and temperature treatments of two populations of four species of snail endemic to Edgbaston tested in two seasons (filled = July, open = November) bound within 95% confidence interval ellipses for each species. Symbols denote families (circle = Tateidae, triangle = Planorbidae, diamond = Bithyniidae). Colours and initials within the ellipse denote species with J. jesswiseae (JJ) as navy, G. edgbastonensis (GE) and Glyptophysa sp. (GL) as light green and dark green and G. fontana (GF) as yellow. Final stress = 0.12. (Color figure online)
Aim 2: Sub-lethal and interactive effects of variables representative of tail conditions
Individual Glyptophysa sp. exposed to tail moist, tail pH (9.5) and tail conductivity (3500 μS/cm) generally had a similar response time to those exposed to pool conditions (pH 8.5, conductivity 800 μS/cm) (Fig. 6a–c). However, in the group exposed for 2 h to tail conductivity, average response time was longer than all other treatments, but there was high between-individual variance (i.e. some had response times similar to those in the pool treatment, and others had response times considerably longer than that) rendering differences in the averages statistically insignificant (Fig. 6b). In extreme treatments, although all Glyptophysa sp. were able to survive both the 1- and 2-h treatments under dry conditions, the response time of individuals in these treatments was significantly longer than all other treatments (p < 0.001) (Fig. 6a). Likewise in 10 mS/cm conductivity treatment, although 50% of individuals survived in the 1-h treatment, the response time of the remaining individuals was significantly longer (Fig. 6b).
The average response time (in seconds, plus SE of n = 6) (left) and proportion of individuals alive (only for treatments where mortalities occurred, right) of Glyptophysa sp. individuals exposed to pool conditions (grey), tail conditions (white, dashed line) or extreme conditions (black, black line) a desiccation potential, b conductivity and c pH
Glyptophysa sp. experienced no mortality after 24 h exposed to treatments that combined tail conductivity and pH treatments. Individuals exposed to this treatment for 2 h had a longer response time at the 2-h time period, again with high variance, identical to the pattern that occurred when exposed to tail conductivity in isolation.
When Glyptophysa sp. snails were exposed to treatments for longer than 24 h, there was a significant difference in the time until death across treatments (n = 6, p < 0.05) (Fig. 7). No mortalities occurred in the pool treatment within the 38-day test period (Fig. 7). Short-term exposure to either tail pH or conductivity in isolation caused no deaths, but a single mortality occurred in each after 35 days (with no significant difference between the two or between these treatments and the pool treatment) (Fig. 7). Glyptophysa sp. in the combined tail pH and conductivity treatment had a significantly shorter time until death than in treatments of either variable alone. Over half of the individuals exposed to a combination of tail pH and conductivity died within 7 days, and only 20% remained alive beyond 28 days (Fig. 7).
Aim 3: Similarities between species with different patterns of distribution from opposite sides of the GAB
In ordinations of species endemic to Kati Thanda (Lake Eyre), two broadly dissimilar clusters formed corresponding to the conclusions of Ponder et al. (1989) (Fig. 8). Individuals of the “amphibious” F. zeidleri form A from all three locations were more similar to each other than to the “large aquatic” species (F. accepta and F. aquatica), both of which were dissimilar to the “small aquatic” species (F. variabilis and T. punicea).
nMDS ordination of all species endemic to Pelican Creek springs and those from the Kati Thanda (Lake Eyre) complex studied by Ponder et al. (1989). For species endemic to Pelican Creek, species from different families are represented as different symbols (circle = Tateidae, triangle = Planorbidae, diamond = Bithyniidae) and multiple populations tested within two seasons are shown (filled = July, open = November) and are bound within a 95% confidence interval ellipse coloured and labelled with an abbreviation of the species name [navy (JJ) = J.jesswiseae, light blue (JE) = J. edgbastonensis, blue (JA) = J. acuminata, light green (GE) = G. edgbastonensis, dark green (GL) = Glyptophysa sp., yellow (GF) = G. fontana]. Data presented in Ponder et al. (1989) are presented as squares, coloured according to the category applied to the species by the authors (black = amphibious, grey = large aquatic and white = aquatic). Final stress = 0.10. (Color figure online)
The Pelican Creek species responded in a similar fashion to the treatments of Ponder et al. (1989) (Table 6). Confidence intervals (95%) for Pelican Creek species generally corresponded to the groups formed by the Kati Thanda (Lake Eyre) species (Fig. 8) (i.e. Pelican Creek species were not more similar to each other than to Kati Thanda species). The tail occupying Ga. fontana was dissimilar to all other Pelican Creek species and had a confidence interval that overlaps with the “amphibious” F. zeidleri form A (Fig. 8). The large planorbid Glyptophysa sp. has a confidence interval that overlaps with the “large aquatic” species F. aquatic and F. accepta (Fig. 8). All Tateidae (J. acuminata, J. jesswiseae and J. edgbastonensis) and the smaller planorbid (Gy. edgbastonensis) had confidence intervals that overlapped with each other and were most similar to the “small aquatic” species of Ponder et al. (1989) (F. aquatica and T. punicea). G. edgbastonensis and J. acuminata are therefore all more similar to Kati Thanda species with similar patterns of distribution. In contrast, the response of J. edgbastonensis is highly dissimilar to the Kati Thanda species that has the same pattern of distribution in the field, the “amphibious” F. zeidleri (Fig. 8).
Discussion
Most species tested here are found only where water is deep and permanent, and pH and conductivity are lowest. Therefore, it could have been assumed that, when left without standing water or left exposed to levels of conductivity or pH beyond the limits of their distribution in the field (Table 1), individuals of these species of snail would perish. Instead, pool-restricted species were able to persist for 24 h in water with conductivity two times their field limits, or with pH of 9.5, and where there was little more than moist substrate. However, all species suffered mortalities within 24 h of exposure to treatments imitating the desiccation, conductivity and temperature extremes that prevail when springs dry and retract (Rossini et al. 2017). Further to this, in one species with the most restricted distribution in the field, short-term tolerance is not indicative of effects in the long term. as treatments tolerable for 24 h caused sub-lethal responses and mortality within a week. This was particularly true in scenarios that more closely resemble nature (i.e. factors in combination over extended periods). This alludes to the subtlety with which environmental variance may be shaping patterns of distribution in this system.
Most species tested here were able to endure conductivity levels far beyond their field limits, a seemingly anomalous result considering the fact that only two of them are found in tail areas where conductivity is consistently high. The particular ions responsible for shifts in conductivity strongly influence their toxicity (Zalizniak et al. 2006). Though tests used here shifted conductivity by adjusting major ions, shallow tail areas are likely to see all ions concentrate due to evaporation [e.g. potassium concentration is also highly variable in springs (Table 5)]. These tests also overlooked life stages other than the adult, but high abundance is contingent on the ability of the organism to fit all elements of its life cycle into the prevailing structure of its environment (Walter and Hengeveld 2014). For example, early life stages are often more sensitive than adults (Kefford et al. 2007; Pineda et al. 2012). Thus, although adults of pool-restricted species survived treatments representing tail conditions used here, tests of single variables over short time periods are not likely to be indicative of their true limitations in the field.
Studies of the effects of environmental variance as it occurs for organisms in nature are vital for understanding the subtleties of how species respond to environmental variability, and how this affects their distributions in the field (Côté et al. 2016; Jackson et al. 2016). Extremes of environmental variance coincide in springs: conductivity, pH, temperature and the potential to dry must all be endured simultaneously in space (i.e. in shallow tail areas) and in time (i.e. diurnally at midday, seasonally when springs evaporate and shrink) (Rossini et al. 2017). Temperature and salinity (Layman et al. 2000), pH, bicarbonate concentration or alkalinity and salinity (Havas and Rosseland 1995; Zalizniak et al. 2009; Vera et al. 2014) or salinity and desiccation (Pallarés et al. 2015) have all been shown to interact with one another. In this study, levels of conductivity and pH that were tolerable in isolation resulted in death within a week if experienced in combination. This emphasises the importance of studying interactions (Piggott et al. 2015) and suggests that when experienced simultaneously, stressors in springs are far more lethal.
Many species that live in regions characterised by climatic extremes, like the arid zone, have adaptations that make them resilient to environmental variance (Cloudsley-Thompson 1975; Williams 1985). Spring snails lack many of the adaptations common to other arid zone freshwater species [e.g. ability to diapause at some point in the lifecycle through dry periods (Ponder and Slatyer 2010)]; they retain traits of their mesic ancestors but, because of this, are now trapped in permanent springs. Despite this, these results demonstrate that they have some resistance to environmental variance. All species had higher upper thermal limits in summer when the ambient temperatures were ~ 10 °C higher (Rossini et al. 2017). Many also appeared to employ behavioural mechanisms to avoid lethal temperatures in the field (> 35 °C), such as perching at the waterline in the shade or burrowed during the day and active at night (Rossini pers. obs.). Such behavioural responses may be key for their persistence; for example, burrowing was shown to be essential for the Desert Goby (Chlamydogobius eremius), one of the few fish that occurs in spring shallows (Kodric-Brown and Brown 1993), to remain within its thermal limits (Glover 1982).
In some cases, however, acclimation or adaptation does not appear to be occurring. It could have been expected that populations from springs with higher average pool conductivity (E509), or species that occupy areas of highest conductivity on average (J. edgbastonensis), would be more tolerant to conductivity extremes. However, Glyptophysa sp. and Gy. edgbastonensis from the spring with higher average conductivity (E509) suffered mortality faster than others, and J. edgbastonensis (the species that occupies the broadest conductivity gradient in the field) was the least tolerant of salinity extremes. Likewise, in all species tested, mortalities in response to desiccation and extreme conductivity occurred faster in the hottest, driest season when conductivity and pH are higher on average (November) (Rossini et al. 2017). Perpetually persisting in areas of sub-lethal environmental conditions can have fitness effects (Kefford and Nugegoda 2005) and make individuals less resistant of environmental extremes (Sultan 2015). Therefore, whilst these species appear able to acclimate and respond to some forms of environmental variance (i.e. temperature), some appear to be already living at their limit, which effects their ability to endure extreme events.
When seeking generalisations across the Kati Thanda and Pelican Creek systems, Pelican Creek species share stronger similarities with those from Kati Thanda that have comparable patterns of distribution than those that are more closely related (e.g. Tateidae are as similar to Planorbidae) or are from the same region. However, the divide between “aquatic” and “amphibious”, and the strong connection to patterns of distribution in the field shown by Ponder et al. (1989), is not readily applicable to the Pelican Creek system. In some cases, the generalisation works. Species with high abundance in pool areas at the Pelican Creek springs (J. acuminata, Gy. edgbastonensis, Glyptophysa sp.) were similar to the pool-restricted and sensitive “aquatic” species from Kati Thanda (F. variabilis, F. aquatic, F. accepta and T. punicea), with a similar pattern also occurring in that larger species (Glyptophysa sp., F. aquatic, F. accepta) were more tolerant than small (see Fig. 1 for sizes). Likewise, the Bithyniidae Ga. fontana, which can be found in relatively high abundance in shallow areas of springs at Pelican Creek (Table 2), was most similar to the “amphibious” F. zeidleri from Kati Thanda. However, J. edgbastonensis does not fit this mould—it was expected to be the most tolerant of environmental extremes because it is the only species at Pelican Creek that occupies shallow areas in high abundance, and can be found in springs that completely lack pools. This was not the case, and whilst it was tolerant of desiccation for up to 6 h, it was the most sensitive to conductivity extremes.
This alludes again to the potential subtleties by which environmental variance influences patterns of distribution in these species. In the field, J. edgbastonensis is common and abundant in areas less than 10 mm deep that dry for up to 6 h daily, but has highest abundance where conductivity is low (< 1500 μS/cm) (Rossini et al. 2017). Therefore, the desiccation tolerance of this species may let it persist in springs and areas shallower than most, but it is environmentally limited to areas where conductivity is low. This may explain why abundance of this species was highest in springs that were shallow but had low conductivity (spring SW66 in Rossini et al. (2017)). Such surprising results are not uncommon in tests of environmental limits (e.g. Klockmann et al. 2016) and emphasise the importance of empirical tests of all environmental correlates of abundance, rather than assumptions of limits based purely on descriptive study. The match between an organism requirement and the structure and spatiotemporal dynamics of its environment is important for explaining patterns of distribution at a range of scales (Hengeveld and Haeck 1981, 1982; Hengeveld 1993). This study demonstrates that combinations of naturally occurring environmental variables can act as a mechanism that shapes the distribution of snails endemic to the GAB springs system. However, broadly applicable generalisations about how this occurs that encompass all species are not available.
One generalisation that can be made, however, is that all species considered here are dead within a day or two when left on dry substrates, when exposed to high conductivity (> 10 mS/cm) or high temperatures (> 40 °C), and mortality is likely to be more severe when all extremes are experienced simultaneously (as they are in nature). Dry and hot conditions occur often in this arid context, emphasising the vital role permanent spring-fed aquatic habitat plays in arid zone aquatic ecosystems (Davis et al. 2017). Organisms other than snails are also restricted to deep spring pools, and many have been shown to retreat to the pool margin or spring vent when the springs shrink in size (Fairfax et al. 2007; Rossini et al. 2017). Therefore, what unites springs across opposite sides of the GAB within Australia (e.g. Kati Thanda and Pelican Creek), and in different contexts across the globe (e.g. arid vs. alpine), is the strong role of groundwater-mediated environmental stability. In alpine (von Fumetti and Blattner 2017), temperate (Smith et al. 2003) and arid (Rossini et al. 2017; Blinn 2008) systems alike, areas close to the vent are the most stable, and this stability helps species stay within their physiological limits (e.g. never dry (Klockmann et al. 2016), never freeze (Küry et al. 2017), never get above 50 °C (Rossini et al. 2017)).
Without permanent and strong groundwater flow, such environmental stability will be lost, alongside all species that rely on it. Considering the fact that springs across the globe are hot spots of diversity (Fensham et al. 2011; Cantonati et al. 2012; Hershler et al. 2014; Cantonati et al. 2016; Davis et al. 2017), such losses represent a huge concern for aquatic conservation. Aquatic ecosystems are already among the most anthropogenically altered and threatened systems (Dudgeon et al. 2006), and the endemic invertebrates within them the most at risk (Strayer 2006; Cardoso et al. 2011). Groundwater systems face their own challenges (Danielopol et al. 2003; Boulton 2005), and unfortunately, poor basin management (Richey et al. 2015) has already led to severe losses (Unmack and Minckley 2008; Powell et al. 2015; Powell and Fensham 2016) and threats to groundwater have been (Fairfax and Fensham 2002, 2003), and remain (Nevill et al. 2010) concerning in Australia.
References
Benke M, Brandle M, Albrecht C, Wilke T (2011) Patterns of freshwater biodiversity in Europe: lessons from the spring snail genus Bythinella. J Biogeogr 38:2021–2032
Blinn DW (2008) The extreme environment, trophic structure, and ecosystem dynamics of a large fishless desert spring (Montezuma Well, Arizona). In: Stevens LE, Meretsky VJ (eds) Aridland springs in North America; ecology and conservation. The University of Arizona Press, Tucson, pp 98–126
Borcard D, Gillet F, Legendre P (2011) Numerical ecology with R. Springer, Berlin
Boulton AJ (2005) Chances and challenges in the conservation of groundwaters and their dependent ecosystems. Aquat Conserv 15:319–323
Brown DS (2001) Freshwater snails of the genus Gyraulus (Planorbidae) in Australia: taxa of the mainland. Molluscan Res 21:17–107
Cañedo-Argüelles M, Hawkins CP, Kefford BJ, Schäfer RB, Dyack BJ, Brucet S, Buchwalter D, Dunlop J, Frör O, Lazorchak J, Coring E (2016) Saving freshwater from salts. Science 351:914–916
Cantonati M, Fureder L, Gerecke R, Juttner I, Cox EJ (2012) Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshw Sci 31:463–480
Cantonati M, Segadelli S, Ogata K, Tran H, Sanders D, Gerecke R, Celico F (2016) A global review on ambient limestone-precipitating springs (LPS): hydrogeological setting, ecology, and conservation. Sci Total Environ 568:624–637
Cardoso P, Erwin TL, Borges PAV, New TR (2011) The seven impediments in invertebrate conservation and how to overcome them. Biol Cons 144:2647–2655
Cloudsley-Thompson JL (1975) Adaptations of Arthropoda to arid environments. Annu Rev Entomol 20:261–283
Côté IM, Darling ES, Brown CJ (2016) Interactions among ecosystem stressors and their importance in conservation. Proc R Soc B Biol Sci 283:1824
Danielopol DL, Griebler C, Gunatilaka A, Notenboom J (2003) Present state and future prospects for groundwater ecosystems. Environ Conserv 30:104–130
Davis JA, Kerezsy A, Nicol S (2017) Springs: conserving perennial water is critical in arid landscapes. Biol Cons 211:30–35
Dowd WW, King FA, Denny MW (2015) Thermal variation, thermal extremes and the physiological performance of individuals. J Exp Biol 218:1956–1967
DSITIA (2015) Lake eyre basin springs assessment project: groundwater dependent ecosystem mapping report. Department of Science, I.T.a.I.A. (ed), Department of Science, Information Technology and Innovation, Brisbane
Dudgeon D, Arthington AH, Gessner MO, Kawabata ZI, Knowler DJ, Leveque C, Naiman RJ, Prieur-Richard AH, Soto D, Stiassny MLJ, Sullivan CA (2006) Freshwater biodiversity: importance, threats, status and conservation challenges. Biol Rev 81:163–182
Dussart GBJ (1979) Life cycles and distribution of the aquatic gastropod molluscs Bithynia tentaculata (L.), Gyraulus albus (Muller), Planorbis planorbis (L.) and Lymnaea peregra (Muller) in relation to water chemistry. Hydrobiologia 67:223–239
Fairfax RJ, Fensham RJ (2002) In the footsteps of J. Alfred Griffiths: a cataclysmic history of Great Artesian Basin springs in Queensland. Aust Geogr Stud 40:210–230
Fairfax RJ, Fensham RJ (2003) Great Artesian Basin springs in Southern Queensland 1911–2000. Mem Queensl Mus 49:285–293
Fairfax RJ, Fensham RJ, Wager R, Brooks S, Webb A, Unmack P (2007) Recovery of the red-finned blue-eye: an endangered fish from springs of the Great Artesian Basin. Wildl Res 34:156–166
Fensham RJ, Fairfax RJ (2003) Spring wetlands of the Great Artesian Basin, Queensland, Australia. Wetlands Ecol Manage 11:343–362
Fensham RJ, Silcock JL, Kerezsy A, Ponder WF (2011) Four desert waters: setting arid zone wetland conservation priorities through understanding patterns of endemism. Biol Conserv 144:2459–2467
Fleishman E, Murphy DD, Sada DW (2006) Effects of environmental heterogeneity and disturbance on the native and non-native flora of desert springs. Biol Invasions 8:1091–1101
Gaston KJ, Blackburn TM, Lawton JH (1997) Interspecific abundance-range size relationships: an appraisal of mechanisms. J Anim Ecol 66:579–601
Glover CJM (1982) Adaptations of fishes in arid Australia. In: Barker WR, Greenslade PJM (eds) Evolution of the Flora and Fauna of Arid Australia. Peacock Publications, Frewville, South Australia, pp 241–246
Guisan A, Thuiller W (2005) Predicting species distribution: offering more than simple habitat models. Ecol Lett 8:993–1009
Habermehl M (1982) Springs in the Great Artesian Basin, Australia: their origin and nature. Canberra, Australia, Australian Government Publishing Service for the Bureau of Mineral Resources, Geology and Geophysics
Hart BT, Bailey P, Edwards R, Hortle K, James K, McMahon A, Meredith C, Swadling K (1991) A review of the salt sensitivity of the Australian freshwater biota. Hydrobiologia 210:105–144
Havas M, Rosseland BO (1995) Response of zooplankton, benthos, and fish to acidification: an overview. Water Air Soil Pollut 85:51–62
Hengeveld R (1993) Ecological biogeography. Prog Phys Geogr 17:448–460
Hengeveld R, Haeck J (1981) The distribution of abundance 2. Models and implications. Proc Koninklijke Nederlandse Akademie Van Wetenschappen Ser C Biol Med Sci 84:257–284
Hengeveld R, Haeck J (1982) The distribution of abundance 1. Measurements. J Biogeogr 9:303–316
Hershler R, Liu HP, Howard J (2014) Springsnails: a new conservation focus in Western North America. Bioscience 64:693–700
Jackson MC, Loewen CJG, Vinebrooke RD, Chimimba CT (2016) Net effects of multiple stressors in freshwater ecosystems: a meta-analysis. Glob Change Biol 22:180–189
Kearney M (2006) Habitat, environment and niche: what are we modelling? Oikos 115:186–191
Kefford BJ, Nugegoda D (2005) No evidence for a critical salinity threshold for growth and reproduction in the freshwater snail Physa acuta. Environ Pollut 134:377–383
Kefford BJ, Nugegoda D, Zalizniak L, Fields EJ, Hassell KL (2007) The salinity tolerance of freshwater macroinvertebrate eggs and hatchlings in comparison to their older life-stages: a diversity of responses. Aquat Ecol 41:335–348
Klockmann M, Scharre M, Haase M, Fischer K (2016) Does narrow niche space in a ‘cold-stenothermic’ spring snail indicate high vulnerability to environmental change? Hydrobiologia 765:71–83
Kodric-Brown A, Brown IH (1993) Highly structured fish communities in Australian desert springs. Ecology 74:1847–1855
Küry D, Lubini V, Stucki P (2017) Temperature patterns and factors governing thermal response in high elevation springs of the Swiss Central Alps. Hydrobiologia 793:185–197
Layman CA, Smith DE, Herod JD (2000) Seasonally varying importance of abiotic and biotic factors in marsh-pond fish communities. Mar Ecol Prog Ser 207:155–169
Leblanc M, Tweed S, Lyon B, Bailey J, Franklin C, Harrington G, Suckow A (2015) On the hydrology of the bauxite oases, Cape York Peninsula, Australia. J Hydrol 528:668–682
Losos JB (2008) Phylogenetic niche conservatism, phylogenetic signal and the relationship between phylogenetic relatedness and ecological similarity among species. Ecol Lett 11:995–1003
McMahon T, Murphy R, Little P, Costelloe J, Peel M, Chiew F, Hayes S, Nathan R, Kandel D (2005) Hydrology of Lake Eyre Basin. The University of Melbourne, Melbourne
Mitchell R (1974) The evolution of thermophily in hot springs. Q Rev Biol 49:229–242
Mitchell SC (2005) How useful is the concept of habitat? A critique. Oikos 110:634–638
Murphy NP, Guzik MT, Cooper SJB, Austin AD (2015) Desert spring refugia: museums of diversity or evolutionary cradles? Zoolog Scr 6:693–701
Nevill JC, Hancock PJ, Murray BR, Ponder WF, Humphreys WF, Phillips ML (2010) Groundwater-dependent ecosystems and the dangers of groundwater overdraft: a review and an Australian perspective. Pac Conserv Biol 16:187–208
Oksanen J, Blanchet FG, Kindt R, Legendre P, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Stevens MHH, Wagner H (2015) Vegan: community ecology package. R package version 2.2-1
Pallarés S, Arribas P, Bilton DT, Millán A, Velasco J (2015) The comparative osmoregulatory ability of two water beetle genera whose species span the fresh-hypersaline gradient in inland waters (Coleoptera: Dytiscidae, Hydrophilidae). PLoS ONE 10:e0124299
Perez KE, Ponder WF, Colgan DJ, Clark SA, Lydeard C (2005) Molecular phylogeny and biogeography of spring-associated hydrobild snails of the Great Artesian Basin, Australia. Mol Phylogenet Evol 34:545–556
Piggott JJ, Lange K, Townsend CR, Matthaei CD (2012) Multiple stressors in agricultural streams: a mesocosm study of interactions among raised water temperature, sediment addition and nutrient enrichment. PLoS ONE 7:49873
Piggott JJ, Townsend CR, Matthaei CD (2015) Reconceptualizing synergism and antagonism among multiple stressors. Ecol Evol 5:1538–1547
Pineda MC, McQuaid CD, Turon X, López-Legentil S, Ordóñez V, Rius M (2012) Tough adults, frail babies: an analysis of stress sensitivity across early life-history stages of widely introduced marine invertebrates. PLoS ONE 7:e46672
Ponder WF (1995) Mound spring snails of the Australian Great Artesian Basin. The IUCN Species Survival Commission on the Conservation Biology of Molluscs, Edinburgh, Scotland, IUCN/SSC Mollusc Specialist Group
Ponder WF (2003) Monograph of the Australian Bithyniidae (Caenogastropoda: Rissooidea). Zootaxa 230:1–126
Ponder WF, Clark GA (1990) A radiation of hydrobiid Snails in threatened artesian springs in western Queensland. Rec Aust Mus 42:301–363
Ponder WF, Slatyer C (2010) Freshwater molluscs in the Australian arid zone. In: Dickman C, Lunney D, Burgin S (eds) Animals of Arid Australia: out on their own. Royal Zoological Society of New South Wales, Mosman, pp 1–13
Ponder WF, Hershler R, Jenkins B (1989) An endemic radiation of hydrobiid snails from artesian springs in Northern South Australia—their taxonomy, physiology, distribution and anatomy. Malacologia 31:1–140
Ponder WF, Vial M, Jefferys E (2010) The aquatic macroinvertebrates in the springs on Edgbaston Station, Queensland. Queensland Museum, Brisbane
Powell O, Fensham RJ (2016) The history and fate of the Nubian Sandstone Aquifer springs in the oasis depressions of the Western Desert, Egypt. Hydrogeol J 24:395–406
Powell O, Silcock J, Fensham RJ (2015) Oases to oblivion: the rapid demise of springs in the South-Eastern Great Artesian Basin, Australia. Groundwater 53:171–178
Pritchard G (1991) Insects in thermal springs. Mem Entomol Soc Can 123:89–106
R Studio Team (2015) RStudio: integrated development for R. RStudio, Inc., Boston. http://www.rstudio.com/
Richey AS, Thomas BF, Lo MH, Reager JT, Famiglietti JS, Voss K, Swenson S, Rodell M (2015) Quantifying renewable groundwater stress with GRACE. Water Resour Res 51:5217–5238
Rosati M, Cantonati M, Primicerio R, Rossetti G (2014) Biogeography and relevant ecological drivers in spring habitats: a review on ostracods of the Western Palearctic. Int Rev Hydrobiol 99:409–424
Rossini RA, Fensham RJ, Walter GH (2017) Spatiotemporal variance in environmental conditions of Australian artesian springs affects the abundance and distribution of six endemic snail species. Aquat Ecol. doi:10.1007/s10452-017-9633-4
Shah AA, Gill BA, Encalada AC, Flecker AS, Funk CW, Guayasamin JM, Ghalambor CK (2017) Climate variability predicts thermal limits of aquatic insects across elevation and latitude. Funct Ecol. doi:10.1111/1365-2435.12906
Shepard WD (1993) Desert springs—both rare and endangered. Aquat Conserv 3:351–359
Slatyer RA, Hirst M, Sexton JP (2013) Niche breadth predicts geographical range size: a general ecological pattern. Ecol Lett 16:1104–1114
Smith H, Wood PJ, Gunn J (2003) The influence of habitat structure and flow permanence on invertebrate communities in karst spring systems. Hydrobiologia 510:53–66
Spitale D, Leira M, Angeli N, Cantonati M (2012) Environmental classification of springs of the Italian Alps and its consistency across multiple taxonomic groups. Freshw Sci 31:563–574
Stendera S, Adrian R, Bonada N, Cañedo-Argüelles M, Hugueny B, Januschke K, Pletterbauer F, Hering D (2012) Drivers and stressors of freshwater biodiversity patterns across different ecosystems and scales: a review. Hydrobiologia 696:1–28
Stomp M, Huisman J, Mittelbach GG, Litchman E, Klausmeier CA (2011) Large-scale biodiversity patterns in freshwater phytoplankton. Ecology 92:2096–2107
Strayer DL (2006) Challenges for freshwater invertebrate conservation. J N Am Benthol Soc 25:271–287
Sultan SE (2015) Organism and environment: ecological development, niche construction, and adaptation. Oxford University Press, Oxford
Underwood A, Chapman M, Connell S (2000) Observations in ecology: you can’t make progress on processes without understanding the patterns. J Exp Mar Biol Ecol 250:97–115
Unmack P, Minckley WL (2008) The demise of desert springs. Aridland Springs in North America; Ecology and Conservation. LE Stevens and VJ Meretsky. The University of Arizona Press, Tucson, pp 11–34
Vanhorne B (1983) Density as a midleading indicator of habitat quality. J Wildl Manage 47:893–901
Vera CL, Hyne RV, Patra R, Ramasamy S, Pablo F, Julli M, Kefford BJ (2014) Bicarbonate toxicity to Ceriodaphnia dubia and the freshwater shrimp Paratya australiensis and its influence on zinc toxicity. Environ Toxicol Chem 33:1179–1186
von Fumetti S, Blattner L (2017) Faunistic assemblages of natural springs in different areas in the Swiss National Park: a small-scale comparison. Hydrobiologia 793:175–184
Walter GH, Hengeveld R (2014) Autecology: organisms, interactions and environmental dynamics. CRC Press, Boca Raton
Wiegert RG, Mitchell R (1973) Ecology of yellowstone thermal effluent systems: intersects of blue-green algae, grazing flies (Paracoenia, Ephydridae) and water mites (Partnuniella, Hydrachnellae). Hydrobiologia 41:251–271
Williams WD (1985) Biotic adaptations in temporary lentic waters, with special reference to those in semi-arid and arid regions. Hydrobiologia 125:85–110
Zalizniak L, Kefford BJ, Nugegoda D (2006) Is all salinity the same? I. The effect of ionic compositions on the salinity tolerance of five species of freshwater invertebrates. Mar Freshw Res 57:75–82
Zalizniak L, Kefford BJ, Nugegoda D (2009) Effects of pH on salinity tolerance of selected freshwater invertebrates. Aquat Ecol 43:135–144
Zullini A, Gatti F, Ambrosini R (2011) Microhabitat preferences in springs, as shown by a survey of nematode communities of Trentino (south-eastern Alps, Italy). J Limnol 70:93–105
Acknowledgements
We would like to acknowledge the traditional owners of the land on which we work: the Jagera and Turrbal (Brisbane) and the Iningai and Bidjara people (Edgbaston and surrounds). We thank Bush Heritage Australia and their supporters for guaranteeing the conservation of Edgbaston, allowing us access and providing on-ground support. We are grateful for the advice of Prof Craig Franklin and Dr Rebecca Cramp and for the voluntary assistance of Sasha Jooste, Karlee Taylor and Ian Rossini. RAR was funded by an Australian Postgraduate Award Scholarship and a top-up scholarship from the Great Artesian Basin Coordinating Committee. A Student Research Grant awarded to RAR and HLT by the Ecological Society of Australia financially supported the construction of the experimental aquaria system.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Piet Spaak.
Rights and permissions
About this article
Cite this article
Rossini, R.A., Tibbetts, H.L., Fensham, R.J. et al. Can environmental tolerances explain convergent patterns of distribution in endemic spring snails from opposite sides of the Australian arid zone?. Aquat Ecol 51, 605–624 (2017). https://doi.org/10.1007/s10452-017-9639-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9639-y