Abstract
To evaluate the potential of Branchinella kugenumaensis for cyanobacterial bloom control relative to Daphnia, we conducted several feeding experiments on microcystin-free and microcystin-containing unicellular strains of Microcystis aeruginosa and colonial forms of Microcystis using B. kugenumaensis and Daphnia magna in a laboratory. Branchinella kugenumaensis showed higher filtration rates than those of D. magna in all treatments. In particular, the microcystin-containing unicellular strain supported the highest filtration rates of B. kugenumaensis among treatments. Daphnia magna reduced colonies less than 75 μm in length, whereas B. kugenumaensis could graze colonies less than 100 μm. The middle-sized group of B. kugenumaensis had a higher filtration rate than the small and large sized groups in a continuous feeding experiment for 4 days. In survival experiments, survivorships were not different between the two species, whereas ages at the beginning of the experiments affected their survival time. Our results showed that B. kugenumaensis grazed on toxic and colonial cyanobacteria at relatively high rates, indicating that locally abundant grazers like Branchinella may offer a better potential for bloom control than Daphnia.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Blooms caused by harmful cyanobacteria, such as genera of Microcystis- and Dolichospermum (Anabaena)-producing toxic compounds, have affected domestic animals, humans, and aquatic organisms, such as zooplankton and fish worldwide (Codd et al. 2005; Zurawell et al. 2005). Various methods have been applied to control cyanobacterial blooms in the field and through laboratory experiments, such as reducing nutrient loading (Hamilton et al. 2016), applying inorganic chemicals such as copper sulfate (Le Jeune et al. 2006), ultrasonic irradiation (Lee et al. 2001), allelopathy of aquatic plants (Hu and Hong 2008), and biomanipulation by increasing herbivorous zooplankton by reducing predation pressure of higher trophic levels (Shapiro et al. 1975; Hansson et al. 1998). Although chemical and mechanical approaches can efficiently eliminate target cyanobacteria species, they can result in negative effects on non-target species (Jančula and Maršálek 2011; Matthijs et al. 2016). Moreover, approaches to reduce the influx of nutrients are generally unfeasible due to high costs, risks of being largely ineffective, and producing unexpected effects (Mackay et al. 2014). Therefore, many recent studies have attempted to increase abundances of herbivorous organisms, especially Daphnia, by removing planktivorous fish (Horppila et al. 1998; Olin et al. 2006; Søndergaard et al. 2008) or addition of piscivorous fish (Søndergaard et al. 1997; Drenner et al. 2002). In addition, other studies have used various kinds of organisms, such as protozoa (Pajdak-Stós et al. 2001), zooplankton (Ha et al. 2013), zebra mussels (Dionisio-Pires et al. 2005), and filter-feeding fish (Xie and Liu 2001) to directly consume cyanobacteria in a form of biological control. When consumers of phytoplankton are added to blooms in water, they encounter resistance of harmful cyanobacteria to their grazing by means including toxicity, inedible size, high density, and insufficient nutrient compositions (Visser et al. 2005). Therefore, numerous indoor feeding experiments have focused on growth rate, grazing ability, and survival rates of control organisms on toxic cyanobacterial strains, which contain cyanotoxins such as microcystin and anatoxin at various concentrations and forms, especially Microcystis aeruginosa (DeMott et al. 1991; Dionisio-Pires et al. 2005; Wilson et al. 2006; Ger et al. 2010a, b). Among zooplankton, the large cladoceran Daphnia was primarily tested for feeding experiments due to its high mass-specific grazing rate as compared with other common zooplankton, such as copepods and smaller cladocerans, and ease of use in both laboratory and field experiments (Ger et al. 2016).
The fairy shrimp, a large freshwater branchiopod, can be compared its grazing ability to Daphnia among the consumers of phytoplankton. Most fairy shrimps are known as non-selective filter feeders feeding on algal and detrital diets by particle filtration using thoracic appendages, except some raptorial species, such as Branchinecta gigas (Brendonck 1993a; Munuswamy et al. 1997). Ingestion of Daphnia and fairy shrimps is restricted by morphological conditions, such as filter mesh size and body size along with environmental factors (i.e., temperature) as well as prey toxicity (Lampert 1987a; Brendonck 1993b; Kurmayer and Jüttner 1999). Maximum size of ingested particles are reported to be similar between Daphnia and fairy shrimp up to around 70–75 μm depending on body size in feeding experiments using artificial particles for diets (Burns 1968; Brendonck 1993a).
Daphnia species have been tested to measure filtration rate and survival rate on various algal species, as well as Microcystis in many studies (Lampert 1987a, b; DeMott et al. 1991; Reinikainen et al. 1994). However, only few studies have been conducted in fairy shrimps regarding grazing rates and survival rates on cyanobacteria (Kurmayer and Jüttner 1999), although several feeding experiments were performed on green algae, zooplankton prey, and bacteria (Mertens et al. 1990; Brendonck 1993a, b; Ali et al. 1996; Dierckens et al. 1997). According to previous studies, ingestion rates and clearance rates of fairy shrimps on Cryptomonas (Cryptophyceae), Fragilaria (Diatomophyceae), natural bacteria, and Planktothrix (Cyanobacteria) were lower than those of Daphnia (Kurmayer and Jüttner 1999; Bertilsson et al. 2003). However, the cyanobacteria ingestion of Daphnia and fairy shrimp might be influenced also by consumer’s background such as exposure to cyanobacteria. Zooplankton species coexisting with toxic cyanobacteria tend to have tolerance on toxic cyanobacterial diets (Kurmayer and Jüttner 1999; Chislock et al. 2013).
Branchinella kugenumaensis is widespread in Asia, including Korea, India, China, and Japan (Yoon 1993; Brendonck and Belk 1997). The fairy shrimp usually inhabits ephemeral pools, such as vernal ponds, and especially rice paddy fields in Korea. The fairy shrimp and other coexisting zooplankton, such as Moina and Daphnia, which hatch from dormant eggs, occurred in irrigated paddy fields in South Korea generally from May to July due to farming methods (Han et al. 2013). Branchinella and other zooplankton appear with cyanobacteria, one of the main microbiota and graze it during the irrigation period in rice paddy fields (Kimura 2005; Wu and Xue 2009).
To compare the grazing rates of the fairy shrimp and Daphnia on single celled and colonial Microcystis, we conducted indoor feeding experiments using B. kugenumaensis and D. magna that were fed unicellular toxic and non-toxic strains of M. aeruginosa in addition to natural colonial forms of Microcystis. Further, we measured filtration rates, survivorships, and feeding ranges of the two consumer species.
Materials and methods
Phytoplankton cultures
A chlorophyte species, Selenastrum capricornutum, and two different strains of cyanobacteria Microcystis aeruginosa were obtained from the Culture Collection of Algae at the University of Texas at Austin, USA (UTEX). While Selenastrum capricornutum (UTEX 1648) was cultured with the synthetic medium L16 (Lindström 1983), ‘toxic’ strain of M. aeruginosa (UTEX 2385) known to produce microcystins (MCs) and ‘non-toxic’ strain (UTEX 2386) known not to produce MCs were cultured using modified L16 medium with enriched nitrogen (×12 NaNO3) for optimizing M. aeruginosa growth. All three algal strains were grown in 2-L glass flask containing 1 L of the medium in semicontinuous batch cultures maintained in exponential growth phase by weekly subculturing at 25 °C under fluorescent light at 40 μmol photons m−2 s−2 in a light/dark cycle of 16 h:8 h. Flasks were capped with sterile cotton balls and gently shaken two times a day. MCs (microcystin-LR and microcystin-RR) in both Microcystis strains were measured by an Agilent Series 1100 high-performance liquid chromatography coupled to a Waters Micromass ZQ 2000 mass spectrometry with electrospray ionization (HPLC–ESI–MS). The UTEX 2385 strain (MC+) contained only MC-LR, whereas both MC-LR and MC-RR were not detected in the UTEX 2386 strain (MC−).
Animal cultures
Daphnia magna was obtained from stock cultures under laboratory conditions at the Han-River Environment Research Center. Daphnia magna was maintained in a state of clonal replication in 2-L experimental glass aquarium containing 1 L of the L16 medium refreshed weekly in triplicate. On the other hand, Branchinella kugenumaensis was obtained from dormant eggs in soil collected from rice paddy fields under organic farming practice in Jangseong, Korea. B. kugenumaensis were separated by hatching time into different 2-L experimental glass aquariums and maintained lower than 10 individuals in each aquarium containing 1 L of the L16 medium refreshed weekly. Selenastrum capricornutum (kept more than 0.5 mg C L−1) was supplied to the animals by daily additions. Algal carbon biomass (mg C L−1) was determined by estimated equation of correlation with total carbon biomass and light absorbance at 800 nm for both green algae and cyanobacteria strains. The animals were maintained in a growth chamber at 25 °C under fluorescent light at 40 μmol photons m−2 s−2 in a light/dark cycle of 16 h:8 h.
Feeding experiments and filtration rate
To investigate grazing ability of B. kugenumaensis on toxic and colonial form of Microcystis, three different laboratorial feeding experiments were conducted. In first feeding experiment, 30 individuals of B. kugenumaensis were selected for covering a wide range of animal sizes and separated into two groups fed with MC+ and MC− strains (body length, MC+ strain group: 5.30–13.65 mm, 9.48 ± 3.07 mm, n = 15; MC− strain group: 5.15–13.05 mm, 9.33 ± 2.94 mm, n = 15, mean ± SD). Length of animals and colonies were measured using a microscope (SMZ 800, Nikon) and a microruler before the experiment. Thirty-two individuals of D. magna selected for covering a wide range of animal sizes and separated into two groups (body length, MC+ strain group: 1.5–3 mm, 2.26 ± 0.50 mm, n = 16; MC− strain group: 1.35–2.75 mm, 2.06 ± 0.50 mm, n = 16). One individual animal regardless of species was used in a 250-mL beaker with 100 mL of mixed L16 medium and diet at 8.08 ± 0.04 × 107 cells L−1 and 8.34 ± 0.03 × 107 cells L−1 for MC+ and MC− strain group, respectively, in a growth chamber at 25 °C under a dark condition for 12 h. To obtain cell density of Microcysits, we converted concentration of chlorophyll a to the number of cells using equations of Sun et al. (2012). Control in triplicate without animals of each group was used for calculating filtration rates. Each beaker was gently shaken once to prevent food settling during the experiment. After feeding, animals were removed and dried in an oven at 60 °C. Dried individual animals were weighed using a microbalance (PerkinElmer, AD 6000 Ultra Microbalance). The medium with cyanobacteria were filtered using GF/C filters (Whatman, USA). The filters were kept in a refrigerator at −20 °C before chlorophyll a measurements.
We repeated a similar feeding experiment adding the colonial treatment in the second experiment. Seven animals for each species were used in the MC+ strain, MC− strain, and colonial treatment. In this time, large animals were selected compared to the first experiment (B. kugenumaensis: 10.86 ± 2.10 mm, n = 21, D. magna: 2.39 ± 0.32 mm, n = 20). The colonial forms of cyanobacteria were collected from surface of the Seoho Reservoir in Suwon, Korea, in September 2008 during a cyanobacterial bloom. The Seoho Reservoir is a eutrophic reservoir dominated with Microcystis when the cyanobacterial bloom occurred (Yang et al. 2016). A relative abundance of Microcystis spp. is regarded as more than 99% of total phytoplankton biomass during the cyanobacterial bloom on the surface of the Seoho Reservoir based on unpublished data. Collected colonies were filtered with a 368-μm mesh screen before the second feeding experiment. Initial cyanobacterial densities were 8.82 ± 0.18 × 107 cells L−1, 9.07 ± 0.02 × 107 cells L−1, and 7.05 ± 0.70 × 107 cells L−1 for MC+, MC− strain and colonial form, respectively. Culture conditions and procedure to calculate filtration rates of the second experiment were same as the first experiment. A 20 mL aliquot of each sample was fixed with Lugol’s iodine solution for counting segmentalized colonies in the colonial treatment. Three samples each among initial, control, and treatments were used for counting. The medium left were filtered using GF/C filters (Whatman, USA). The filters were refrigerated at −20 °C before chlorophyll a measurements.
The third feeding experiment was conducted to test persistence of the fairy shrimp with the MC+ strain at high densities. The animals were assigned into three size groups (group I: 5.64 ± 0.62 mm, group II: 8.34 ± 0.34 mm, and group III: 12.02 ± 0.96 mm; each group had five animals for replications). One individual B. kugenumaensis was fed on the MC+ strain in 250-mL beaker with 100 mL of mixed L16 medium and diet at about 12× higher density (1.09 ± 0.14 × 109 cells L−1) than first and second experiments at 25 °C under a light/dark cycle of 12 h:12 h for 4 days. Medium with food were refreshed and collected every 12 h during the experiment. Collected mediums were filtered using GF/C filters (Whatman, USA) and kept at −20 °C in a refrigerator before chlorophyll a measurements.
Chlorophyll a concentrations were measured with a fluorometer (Trilogy, Turner designs, USA) following the EPA Method 445.0, except for the acidification step.
Filtration rate (F, in mL ind−1 h−1) was calculated as following equation:
where V is test volume in a beaker in mL, t is the length of time for feeding, N is the number of animals in the beaker, a growth rate constant (b) is calculated with the initial (CC 0) and final (CC t ) concentrations in the control, changes in the experimental container yields (a) is calculated with the initial (C 0) and the final (C t ) concentration in the treatment. Chlorophyll a concentration (μg L−1) was used for cell concentration in the experiments. The initial and control had three replicates each, and we used averages of those concentrations for calculation except first day on the third experiment. Same initial concentrations were used for calculation of filtering rates for both controls (CC 0) and treatments (C 0).
Survivorship on the MC+ strain
To investigate survivorship on the MC+ strain, two consecutive experiments were conducted using cohorts with different ages. Neonates of B. kugenumaensis and D. magna hatched and born in distilled water within 24 h were used for the first experiment. In the second experiment, animals cultured by feeding the S. capricornutum (0.5 mg C L−1 day−1) with L16 medium for a week after hatching were used. Ten individuals of each species were used for the experiments. One individual animal was incubated in 100 mL of the L16 medium with MC+ strain at 0.5 mg C L−1 in 250 mL beaker in the growth chamber at 25 °C under 16 h light/8 h dark photoperiod during experiments. On each day, the survivors were recorded and transferred to fresh medium.
Statistical analysis
Variations in filtration rates, survival times on toxic strain, and ability of grazing colonies between species were analyzed by standard t test and analysis of variance (ANOVA) using S-Plus 6 for Windows (Insightful Corp., USA).
Results
In the first feeding experiment, filtration rates of B. kugenumaensis were significantly higher than those of D. magna on both of MC+ and MC− strains (B. kugenumaensis: 4.66 ± 2.88 mL ind−1 h−1 and 1.97 ± 1.44 mL ind−1 h−1; D. magna: 0.16 ± 0.25 mL ind−1 h−1 and 0.49 ± 0.64 mL ind−1 h−1 on MC+ stain and MC− strain, respectively, p < 0.001) (Fig. 1a). In particular, B. kugenumaensis appeared to filter the MC+ strain much more than the MC− strain (p < 0.01), whereas D. magna appeared to filter the MC− strain more than the MC+ strain (p < 0.05). In the experiment, we used various sizes of animals. So, we compared relationships with lengths of animals and mass-specific filtration rates on different strains in B. kugenumaensis (Fig. 1b) and D. magna (Fig. 1c). The mass-specific filtration rates were higher in small B. kugenumaensis than those in large individuals on both strains, especially in animals in the range of 6 and 8 mm in the MC+ strain (Fig. 1b). The filtration rates of D. magna were not distinguished among sizes, although variance in their filtration rates was higher on the MC− strain than on MC+ strain (Fig. 1c). The mass-specific filtration rates were not different between species on MC− strain; however, B. kugenumaensis filtered much more than D. magna on the MC+ strain (p < 0.001).
Mean (±SD) filtration rates (mL ind−1 h−1) for B. kugenumaensis and D. magna on MC+ and MC− strains of M. aeruginosa in the first feeding experiment (a). Relationship between filtration rates (mL mg−1 h−1) which is standardized to 1 mg dry mass on the MC+ and MC− strains of M. aeruginosa and length for B. kugenumaensis (b) and D. magna (c). SD standard deviation. Asterisk indicates statistical differences (t test, *** p < 0.001)
In the second feeding experiment, filtration rates of B. kugenumaensis were significantly higher than those of D. magna for unicellular and colonial form of Microcystis (B. kugenumaensis: 16.97 ± 3.27, 4.84 ± 1.45 and 2.46 ± 1.39 mL ind−1 h−1; D. magna: 0.25 ± 0.44, −0.08 ± 0.19 and −1.09 ± 10.63 mL ind−1 h−1 on MC+ strain, MC− strain and colonial form, respectively, p < 0.001, Fig. 2a). The filtration rate of B. kugenumaensis on MC+ strain was significantly higher than that on other treatment (p < 0.001), whereas D. magna on the colonial form was much lower than others (p < 0.001). We used larger animals in the second feeding experiment than in the first feeding experiment, so filtration rates on MC+ and MC− strains in the second experiment were higher than those in the first experiment. Relationships between the mass-specific filtration rates and animal sizes on the unicellular strain in the second experiment were similar to those of the first experiment. Feeding on the colonial form was not dependent on sizes of B. kugenumaensis (Fig. 2b). It is notable that small-sized D. magna rather augmented cyanobacterial concentration (Fig. 2c). In the feeding experiment on the colonial form, only B. kugenumaensis decreased the number of colonies (Fig. 3a). In detail, B. kugenumaensis decreased the number of colonies smaller than 100 μm. Daphnia magna decreased number of colonies, smaller than 75 μm and increased lager than 75 μm (Fig. 3b). Consequently, both B. kugenumaensis and D. magna increased the proportion of colonies larger than 75 μm (Fig. 3c). In the final feeding experiment, the higher cell density (about 12 times higher) of MC+ strain than those of the previous experiments, the filtration rates of B. kugenumaensis were lower than that of the previous experiment. In addition, the filtration rates decreased with sustained exposure to MC+ strain in all of groups (Fig. 4). Light conditions did not affect filtration rates. The middle-sized group (8.34 ± 0.34 mm) showed higher filtration rates than the other groups during the experiment except at the light condition on day 2. Both survival experiments showed that B. kugenumaensis and D. magna had similar survivorship and survival times (Fig. 5). However, survival times of animals depended on the age at the beginning of the experiments (B. kugenumaensis: 3.9 ± 1.10 and 7.5 ± 2.07 days, p < 0.001, D. magna: 3.8 ± 1.08 and 8 ± 2.5 days, p < 0.001, at newborn and 7 days old, respectively, not significant between species). In particular, survival times of B. kugenumaensis at 7 days had relationships with its initial size (correlation coefficient, r = 0.55).
Mean (±SD) filtration rates (mL ind−1 h−1) for B. kugenumaensis and D. magna on MC+ and MC− strains of M. aeruginosa and colonies of Microcystis in the second feeding experiment (a). Relationship between filtration rates (mL mg−1 h−1) which is standardized to an individual of 1 mg dry mass on the MC+ and MC− strains and colonies of Microcystis and lengths for B. kugenumaensis (b) and D. magna (c). SD standard deviation. Asterisk indicates statistical differences (t test, *** p < 0.001)
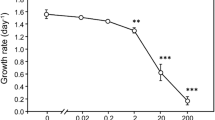
Mean (±SD) number of total dividing cells. Initial group, control, and treatments with B. kugenumaensis and D. magna on colonies of Microcystis in the second feeding experiment (a). Mean (±SD) number of dividing cells on range of major axis length of colonies (b). Mean (±SD) frequency of dividing cells on range of major axis length of colonies (c). SD standard deviation. Asterisk indicates statistical differences (ANOVA, * p < 0.05, ** p < 0.01 and *** p < 0.001). Bars labeled with the same letter are not significantly different between treatments after Tukey’s test
Mean (±SD) filtration rates (mL ind−1 h−1) for different size groups of B. kugenumaensis on MC+ strain of M. aeruginosa in dark and light switched conditions for four days. SD standard deviation. Asterisk indicates statistical differences (ANOVA, * p < 0.05, ** p < 0.01 and *** p < 0.001). Bars labeled with the same letter are not significantly different between treatments after Tukey’s test
Discussion
Our results show that compared to a laboratory strain of D. magna, a wild strain of the fairy shrimp B. kugenumaensis had a higher mass-specific ingestion rate of Microcystis, ingested more of the MC+ strain, and ingested larger colonies. These results suggest that local zooplankton, as opposed to laboratory maintained Daphina strains, may offer more potential in controlling toxic cyanobacteria. In two feeding experiments, compared to D. magna, B. kugenumaensis showed higher cyanobacteria filtration rates per capita regardless of toxicity and cyanobacterial forms (Figs. 1, 2a). However, filtration rates of zooplankton have positive relations with body length and dry weight (Peters and Downing 1984). Mass-specific filtration rates also showed higher values in B. kugenumaensis than in D. magna, especially for the MC+ strain (Figs. 1b, c, 2b, c). In terms of mass-specific filtration rates, D. magna had consistent feeding ability on unicellular diets over the whole range of body length, whereas B. kugenumaensis showed particularly higher filtering ability in smaller sizes of animals. In other word, Daphnia species had a linear relation between filtration rates per individual and body length (Burns 1969), and B. kugenumaensis exhibited a logarithmic relation similar to that by other anostracan species (Brendonck 1993b) and a mussel (Thompson and Bayne 1974). The fairy shrimp grows rapidly to mature within 10 days, though there is a difference between individuals. Therefore, immature fairy shrimps need more energy or nutrient to develop stage and ingest diet more than adults as compared with their body weight (Dumont et al. 1994). Similarly, mass-specific ingestion rates of juvenile Daphnia are higher than those of adults (McCauley et al. 1990). However, body size of adult fairy shrimp is much larger than Daphnia. For that reason, we think that the small-sized fairy shrimps ingested M. aeruginosa strains more than the large one.
In our results, B. kugenumaensis showed significantly higher filtering ability on MC+ strain than those on MC− strain in the first and second feeding experiments (MC+: 34.77 ± 26.18 mL mg−1 h−1, n = 22; MC−: 13.45 ± 9.38 mL mg−1 h−1, n = 22, p < 0.01) in addition to a higher filtering ability than D. magna. In contrast to our results, a fairy shrimp, Thamnocephalus platyurus, showed lower ingestion rates on a toxic fragmented cyanobacterium than did Daphnia hyalina in a previous study (Kurmayer and Jüttner 1999). Kurmayer and Jüttner (1999) used a filamentous cyanobacterium Planktothrix rubescens containing microcystins to be consumed by coexisting species such as D. hyaline in a lake. The fairy shrimps they used were obtained from a company and smaller in size than the Daphnia in their experiments. Zooplankton genotypes which co-occur with toxic cyanobacteria in nature or previous exposure to the toxic cyanobacteria showed physiological resistance and tolerance to the toxic cyanobacteria (Kurmayer and Jüttner 1999; Barros et al. 2001; Gustafsson and Hansson 2004; Chislock et al. 2013; Jiang et al. 2013). We used B. kugenumaensis which has been exposed to cyanobacteria in the rice paddy fields where we obtained their dormant eggs. The results showing higher ingestion on both MC+ and MC− strains in B. kugenumaensis compared to D. magna could be attributed to their exposure to cyanobacteria and their relatively large body size. Based on our results from the first and second feeding experiments, we assumed that B. kugenumaensis used in our experiments had specific grazing ability on MC+ strain. Although toxic concentration and toxicity on M. aeruginosa can be influenced by culture conditions (Watanabe and Oishi 1985; Kardinaal and Visser 2005), the MC+ strain used in this study, known to produce microcystin (Ouahid et al. 2005; Ouellette et al. 2006), also produced microcystin-LR in our laboratory conditions. Of course, microcystins might not influence grazers (Wilson et al. 2006) and other secondary metabolites in Microcystis could affect the consumer’s grazing on the MC− strain (Codd et al. 2005). However, mass-specific filtration rates on the MC− strain were not significantly different between species in the first feeding experiment by using wide size distribution of animals (p = 0.381). Therefore, we suppose that B. kugenumaensis has better grazing ability on MC producing M. aeruginosa based on our results.
The mass-specific filtration rates of D. magna were higher on the MC− strain than on the MC+ strain (MC+: 2.42 ± 6.44 mL mg−1 h−1, n = 23; MC−: 5.81 ± 10.68 mL mg−1 h−1, n = 22), although it was not significantly different (p = 0.208). Because the filtration rate was influenced by algal concentration, as well as toxicity and morphological type (Peters and Downing 1984), it is not appropriate to compare our present results to previous studies with different experimental conditions, such as body size of animals and exposure time. Nonetheless, filtration rates of D. magna in our study support the results of other studies showing higher filtration rates of Daphnia sp. on non-toxic strain than on toxic strain (DeMott et al. 1991; Blanchette and Haney 2002). Some studies showed higher filtration rates of D. magna than those in the present study. We think that such results are related to the diet concentration, because our study was conducted at a high cell density (MC+ strain 8.45 ± 0.42 × 107 cells L−1, n = 6; MC− strain: 8.70 ± 0.40 × 107 cells L−1, n = 6). Some studies showed that Daphnia exhibited a type II functional response (DeMott 1982; McCauley et al. 1990).
The fairy shrimp showed the ability to graze small-sized colonial forms of cyanobacteria, whereas D. magna showed limited ability to forage colonies (Figs. 2a, 3a, respectively). In particular, small Daphnia presented highly negative filtration rate (Fig. 2c). Earlier studies showed similar results that daphnids appeared to have higher filtration rates on single cells than on colonial or filamentous forms of green alga and cyanobacteria, such as M. aeruginosa and Planktothrix agardhii, due to selectively feed on small phytoplankton (Dionisio-Pires et al. 2007). Edible diet sizes of filter-feeding herbivores would be determined by the size of their filtration appendages and mouth. Daphnia magna can feed on diet up to a size of around 75 μm in diameter (Burns 1968), while a fairy shrimp Streptocephalus proboscideus can take particles less than around 70 μm of artificial particles (Brendonck 1993a), although this species could consume small cladocerans such as Bosmina longirostris and rotifers at low abundance in a feeding experiment (Ali et al. 1996). The present results show that B. kugenumaensis could consume up to 100 μm of colony size, whereas D. magna fed on colonies less than 75 μm, mainly 50 μm (Fig. 3b). When Burns (1968) equation was applied to D. magna on the colonial form feeding, to estimate maximum particle ingested, the result obtained was similar to our result of 57.89 ± 4.78 μm. Additionally, D. magna increased number of colonies larger than 75 μm (Fig. 3b). Coexistence of animals such as a mixotrophic chrysophyte and Daphnia appeared to alter size distributions and morphology of the colonial form of cyanobacteria and a green alga due to their feeding activities and infochemicals (Fig. 3b, c) (Lürling et al. 1997; Van Donk et al. 2009).
In continuous feeding experiment at high concentrations, filtration rates of B. kugenumaensis decreased along with exposure time (Fig. 4). Increased exposure time under toxic conditions would influence the feeding ability of grazers negatively (DeMott et al. 1991). Nevertheless, the filtration rates in all size groups were maintained at positive values until day 3 in this study. Particularly, the middle size group (Group II: 8.34 ± 0.34 mm) showed higher filtration rates than did the other groups and maintained positive rates during the experiment. Previous studies showed comparable results as logarithmic curves in relationship between clearance rates of fairy shrimps on green algae and body length (Mertens et al. 1990; Brendonck 1993b).
To control cyanobacterial bloom in nature, it is important to survive in concentrated toxic conditions for long times. Survival rates on toxic cyanobacteria in laboratorial experiments vary according to cell density, consumer species, and age at beginning experiments (DeMott et al. 1991; Reinikainen et al. 1994; Ger et al. 2010b). Young individuals (1-day-old) of a fairy shrimp showed lower survival rates on extracts of toxic cyanobacterium and fragmented cyanobacteria such as Planktothrix and Anabaena than did Daphnia previously exposed to cyanobacteria in a referenced study (Kurmayer and Jüttner 1999). Our results showed that survival times on MC+ strain were not significantly different between B. kugenumaensis and D. magna regardless of the age at the beginning of experiments (Fig. 5). However, the age of animals and size at the beginning experiments were important in their survival time in all species (p < 0.001). Survivorship as well as population growth rates of animals fed on cyanobacteria would be affected by toxicity and low food quality (Wilson et al. 2006). In addition, the survivorships could be affected by nutritive conditions of animals. Animals fed green alga for seven days prior to the experiment could survive longer than newborn zooplankton fed without prior diet. Our results supported the finding of previous studies that used different age of consumers for grazing toxic cyanobacteria (Lampert 1987b; Reinikainen et al. 1994).
In conclusion, locally adapted B. kugenumaensis had higher feeding ability on MC producing M. aeruginosa and ability to small-sized Microcystis colonies compared to Daphnia. Prior to determine the specific feeding ability, further study would be needed to know whether results of specific grazing on MC+ strain by the fairy shrimp were attributed to local adaptation of cyanobacteria in this study. In the future, we will do further study to evaluate ingestion rates of naïve fairy shrimp and other coexisting zooplankton in paddy fields. Despite insufficient evidence of adaptation to toxic cyanobacteria, locally abundant grazers like Branchinella may offer a better potential for bloom control than Daphnia based on our results.
References
Ali AJ, Sarma SSS, Murugan G, Dumont HJ (1996) Effect of zooplankton type and abundance on prey consumption by the fairy shrimp, Streptocephalus proboscideus (Anostraca: Crustacea). Hydrobiologia 319:191–202
Barros P, Fidalgo ML, Soares AMVM (2001) Resistance of cladoceran species to toxic Microcystis. Limnetica 20:173–177
Bertilsson S, Hansson LA, Graneli W, Philibert A (2003) Size-selective predation on pelagic microorganisms in Arctic freshwaters. J Plankton Res 25:621–632
Blanchette ML, Haney JF (2002) The effect of toxic Microcystis aeruginosa on four different populations of Daphnia. UNH Center Freshw Biol Res 4:1–10
Brendonck L (1993a) Feeding in the fairy shrimp Streptocephalus proboscideus (Frauenfeld) (Branchiopoda: Anostraca). I. Aspects of the feeding biology. J Crustacean Biol 13:235–244
Brendonck L (1993b) Feeding in the fairy shrimp Streptocephalus proboscideus (Frauenfeld) (Branchiopoda: Anostraca). II. Influence of environmental conditions on feeding rate. J Crustacean Biol 13:245–255
Brendonck L, Belk D (1997) Branchinella maduraiensis Raj (Crustacea, Branchiopoda, Anostraca) shown by new evidence to be a valid species. Hydrobiologia 359:93–99
Burns CW (1968) The relationship between body size of filter-feeding Cladocera and the maximum size of particle ingested. Limnol Oceanogr 13:675–678
Burns CW (1969) Relation between filtering rate, temperature, and body size in four species of Daphnia. Limnol Oceanogr 14:693–700
Chislock MF, Sarnelle O, Olsen BK, Doster E, Wilson AE (2013) Large effects of consumer offense on ecosystem structure and function. Ecology 94:2375–2380
Codd GA, Lindsay J, Young FM, Morrison LF, Metcalf JS (2005) Harmful cyanobacteria. In: Huisman J, Matthijs HCP, Visser PM (eds) Harmful cyanobacteria. Springer, Dordrecht, pp 1–23
DeMott WR (1982) Feeding selectivities and relative ingestion rates of Daphnia and Bosmina. Limnol Oceanogr 27:518–527
DeMott WR, Zhang QX, Carmichael WW (1991) Effects of toxic cyanobacteria and purified toxins on the survival and feeding of a copepod and three species of Daphnia. Limnol Oceanogr 36:1346–1357
Dierckens KR, Beladjal L, Vandenberghe J, Swings J, Mertens J (1997) Filter-feeding shrimps (Anostraca) grazing on bacteria. J Crustacean Biol 17:264–268
Dionisio-Pires LM, Bontes BM, Van Donk E, Ibelings BW (2005) Grazing on colonial and filamentous, toxic and non-toxic cyanobacteria by the zebra mussel Dreissena polymorpha. J Plankton Res 27:331–339
Dionisio-Pires LM, Bontes BM, Samchyshyna L, Jong J, Van Donk E, Ibelings BW (2007) Grazing on microcystin-producing and microcystin-free phytoplankters by different filter-feeders: implications for lake restoration. Aquat Sci 69:534–543
Drenner RW, Baca RM, Gilroy JS, Ernst MR, Jensen DJ, Marshall DH (2002) Community responses to piscivorous largemouth bass: a biomanipulation experiment. Lake Reserv Manag 18:44–51
Dumont HJ, Ali AJ, Sarma SSS, Mertens J (1994) Predatory filter-feeding in fairy shrimps: functional response of Streptocephalus proboscideus (Crustacea: Anostraca) fed Anuraeopsis fissa (Rotifera). Int Rev Hydrobiol 79:511–519
Ger KA, Arneson P, Goldman CR, The SJ (2010a) Species specific differences in the ingestion of Microcystis cells by the calanoid copepods Eurytemora affinis and Pseudodiaptomus forbesi. J Plankton Res 32:1479–1484
Ger KA, The SJ, Baxa DV, Lesmeister S, Goldman CR (2010b) The effects of dietary Microcystis aeruginosa and microcystin on the copepods of the upper San Francisco Estuary. Freshw Biol 55:1548–1559
Ger KA, Faassen EJ, Pennino MG, Lürling M (2016) Effect of the toxin (microcystin) content of Microcystis on copepod grazing. Harmful Algae 52:34–45
Gustafsson S, Hansson LA (2004) Development of tolerance against toxic cyanobacteria in Daphnia. Aquat Ecol 38:37–44
Ha JY, Saneyoshi M, Park HD, Toda H, Kitano S, Homma T, Shiina T, Moriyama Y, Chang K-H, Hanazato T (2013) Lake restoration by biomanipulation using piscivore and Daphnia stocking; results of the biomanipulation in Japan. Limnology 14:19–30
Hamilton DP, Salmaso N, Paerl HW (2016) Mitigating harmful cyanobacterial blooms: strategies for control of nitrogen and phosphorus loads. Aquat Ecol 50:351–366
Han MS, Cho KJ, Nam HK, Kang KK, Na YE, Kim M, Kim MH (2013) Variation in population size of mudfish by agricultural practices in paddy fields. Korean J Environ Agric 32:24–34
Hansson LA, Annadotter H, Bergman E, Hamrin SF, Jeppesen E, Kairesalo T, Luokkanen E, Nilsson P-Å, Søndergaard M, Strand J (1998) Biomanipulation as an application of food-chain theory: constraints, synthesis, and recommendations for temperate lakes. Ecosystems 1:558–574
Horppila J, Peltonen H, Malinen T, Luokkanen E, Kairesalo T (1998) Top-down or bottom-up effects by fish: issues of concern in biomanipulation of lakes. Restor Ecol 6:20–28
Hu H, Hong Y (2008) Algal-bloom control by allelopathy of aquatic macrophytes—a review. Front Environ Sci Eng 2:421–438
Jančula D, Maršálek B (2011) Critical review of actually available chemical compounds for prevention and management of cyanobacterial blooms. Chemosphere 85:1415–1422
Jiang X, Yang W, Zhao S, Liang H, Zhao Y, Chen L, Li R (2013) Maternal effects of inducible tolerance against the toxic cyanobacterium Microcystis aeruginosa in the grazer Daphnia carinata. Environ Pollut 178:142–146
Kardinaal WEA, Visser PM (2005) Dynamics of cyanobacterial toxins. In: Huisman J, Matthijs HCP, Visser PM (eds) Harmful cyanobacteria. Springer, Dordrecht, pp 41–64
Kimura M (2005) Populations, community composition and biomass of aquatic organisms in the floodwater of rice fields and effects of field management. Soil Sci Plant Nutr 51:159–181
Kurmayer R, Jüttner F (1999) Strategies for the co-existence of zooplankton with the toxic cyanobacterium Planktothrix rubescens in Lake Zurich. J Plankton Res 21:659–683
Lampert W (1987a) Feeding and nutrition in Daphnia. In: Peters RH, De Bernardi R (eds) Daphnia. Istituto Italiano di Idrobiologia, Verbania Pallanza, pp 143–192
Lampert W (1987b) Laboratory studies on zooplankton–cyanobacteria interactions. N Z J Mar Freshw Res 21:483–490
Le Jeune AH, Charpin M, Deluchat V, Briand JF, Lenain JF, Baudu M, Amblard C (2006) Effect of copper sulphate treatment on natural phytoplanktonic communities. Aquat Toxicol 80:267–280
Lee TJ, Nakano K, Matsumara M (2001) Ultrasonic irradiation for blue-green algae bloom control. Environ Technol 22:383–390
Lindström K (1983) Selenium as a growth factor for plankton algae in laboratory experiments and in some Swedish lakes. Hydrobiologia 101:35–47
Lürling M, De Lange HJ, Van Donk E (1997) Changes in food quality of the green alga Scenedesmus induced by Daphnia infochemicals: biochemical composition and morphology. Freshw Biol 38:619–628
Mackay EB, Maberly CS, Gang P, Reitzel K, Bruere A, Corker N, Douglas G, Egemose S, Hamilton D, Hatton-Ellis T, Huser B, Li W, Meis S, Moss B, Lürling M, Phillips G, Yasseri S, Spears BM (2014) Geoengineering in lakes: welcome attraction or fatal distraction? Inland Waters 4:349–356
Matthijs HCP, Jančula D, Visser PM, Maršálek B (2016) Existing and emerging cyanocidal compounds: new perspectives for cyanobacterial bloom mitigation. Aquat Ecol 50:443–460
McCauley E, Murdoch WW, Nisbet RM, Gurney WS (1990) The physiological ecology of Daphnia: development of a model of growth and reproduction. Ecology 71:703–715
Mertens J, Munuswamy N, De Walsche C, Dumont HJ (1990) On predatory tendencies in the feeding ecology of the fairy shrimp Streptocephalus proboscideus (Frauenfeld, 1873) (Crustacea: Anostraca). Hydrobiologia 198:119–123
Munuswamy N, Nazar AKA, Velu CS, Dumont HJ (1997) Culturing the fairy shrimp Streptocephalus dichotomus Baird using livestock waste—a reclamation study. Hydrobiologia 358:199–203
Olin M, Rask M, Ruuhijärvi J, Keskitalo J, Horppila J, Tallberg P, Taponen T, Lehtovaara A, Sammalkorpi I (2006) Effects of biomanipulation on fish and plankton communities in ten eutrophic lakes of southern Finland. Hydrobiologia 553:67–88
Ouahid Y, Pérez-Silva G, Campo FFD (2005) Identification of potentially toxic environmental Microcystis by individual and multiple PCR amplification of specific microcystin synthetase gene regions. Environ Toxicol 20:235–242
Ouellette AJ, Handy SM, Wilhelm SW (2006) Toxic Microcystis is widespread in Lake Erie: PCR detection of toxin genes and molecular characterization of associated cyanobacterial communities. Microb Ecol 51:154–165
Pajdak-Stós A, Fialkowska E, Fyda J (2001) Phormidium autumnale (Cyanobacteria) defense against three ciliate grazer species. Aquat Microb Ecol 23:237–244
Peters RH (1984) Methods for the study of feeding, grazing and assimilation by zooplankton. In: Downing JA, Rigler FH (eds) A manual on methods for the assessment of secondary productivity in fresh waters, 2nd edn. Blackwell Scientific Publications, London, pp 336–412
Peters RH, Downing JA (1984) Empirical analysis of zooplankton filtering and feeding rates. Limnol Oceanogr 29:763–784
Reinikainen M, Ketola M, Walls M (1994) Effects of the concentrations of toxic Microcystis aeruginosa and an alternative food on the survival of Daphnia pulex. Limnol Oceanogr 39:424–432
Shapiro J, Lamarra VA, Lynch M (1975) Biomanipulation: an ecosystem approach to lake restoration. In: Brezonik PL, Fox JL (eds) Water quality management through biological control. University of Florida, Gainesville, pp 85–96
Søndergaard M, Jeppesen E, Berg S (1997) Pike (Esox lucius L.) stocking as a biomanipulation tool 2. Effects on lower trophic levels in Lake Lyng, Denmark. Hydrobiologia 342:319–325
Søndergaard M, Liboriussen L, Pedersen AR, Jeppesen E (2008) Lake restoration by fish removal: short-and long-term effects in 36 Danish lakes. Ecosystems 11:1291–1305
Sun F, Pei HY, Hu WR, Song MM (2012) A multi-technique approach for the quantification of Microcystis aeruginosa FACHB-905 biomass during high algae-laden periods. Environ Technol 33:1773–1779
Thompson RJ, Bayne BL (1974) Some relationships between growth, metabolism and food in the mussel Mytilus edulis. Mar Biol 27:317–326
Van Donk E, Cerbin S, Wilken S, Helmsing NR, Ptacnik R, Verschoor AM (2009) The effect of a mixotrophic chrysophyte on toxic and colony-forming cyanobacteria. Freshw Biol 54:1843–1855
Visser PM, Ibelings BW, Mur LR, Walsby AE (2005) The ecophysiology of the harmful cyanobacterium Microcystis. In: Huisman J, Matthijs HCP, Visser PM (eds) Harmful Cyanobacteria. Springer, Dordrecht, pp 109–142
Watanabe MF, Oishi S (1985) Effects of environmental factors on toxicity of a cyanobacterium (Microcystis aeruginosa) under culture conditions. Appl Environ Microb 49:1342–1344
Wilson AE, Sarnelle O, Tillmanns AR (2006) Effects of cyanobacterial toxicity and morphology on the population growth of freshwater zooplankton: meta-analyses of laboratory experiments. Limnol Oceanogr 51:1915–1924
Wu H, Xue J (2009) Analysis of the diet of the tadpole shrimp, Triops sinensis, in paddy fields of Shouchang River watershed. Front Biol China 4:569–573
Xie P, Liu J (2001) Practical success of biomanipulation using filter-feeding fish to control cyanobacteria blooms: a synthesis of decades of research and application in a subtropical hypereutrophic lake. Sci World J 1:337–356
Yang D, Nam S, Hwang SJ, An KG, Park YS, Shin KH, Park S (2016) Fatty acid biomarkers to verify cyanobacteria feeding abilities of herbivorous consumers. J Freshw Ecol 31:77–91
Yoon SM (1993) The systematics and molecular evolution of the Korean branchiopods (Crustacea, Branchiopoda). Doctoral dissertation, Seoul National University
Zurawell RW, Chen H, Burke JM, Prepas EE (2005) Hepatotoxic cyanobacteria: a review of the biological importance of microcystins in freshwater environments. J Toxicol Environ Health B 8:1–37
Acknowledgements
This work was supported by Basic Science Research Program through the National Research Foundation of Korea (NRF), [2013R1A1A2011780]. The authors would like to thank the anonymous reviewer for very helpful suggestions and comments on this paper.
Author information
Authors and Affiliations
Corresponding author
Additional information
Handling Editor: Bas W. Ibelings.
Rights and permissions
About this article
Cite this article
Yang, D., Park, S. Freshwater anostracan, Branchinella kugenumaensis, as a potential controlling consumer species on toxic cyanobacteria Microcystis aeruginosa . Aquat Ecol 51, 449–461 (2017). https://doi.org/10.1007/s10452-017-9628-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10452-017-9628-1