Abstract
Hydrotalcite-like compounds (HTC) are distinguished by their properties for CO2 capture, like high surface area, basic sites, thermal stability and good adsorption/desorption efficiency. Mg–Al e Zn–Al HTCs with Al3+ molar ratios x = 0.20, 0.28 and 0.33 were synthesized by coprecipitation, and subsequently calcined at 400 °C. For both HTCs, X-ray diffraction patterns have attested the formation of mixed oxides through calcination. The amount of basic sites, measured by temperature-programmed desorption of CO2, decreases as x increases. The CO2 adsorption was performed in a thermogravimetric balance using an adsorption temperature of 50 °C. Mg–Al and Zn–Al samples with x = 0.33 molar composition presented the highest CO2 adsorption, 0.91 and 0.21 mmol g−1, respectively. The Langmuir isotherm fitted well to the experimental data. It was also found that increasing the number of adsorption/desorption cycles the CO2 adsorption decreases, which is associated with the irreversible chemisorption.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
Human activities are increasingly consuming the world resources and the concern about environmental degradation is more and more part of our society. The growing emission of greenhouse gases is worrying because of the major environmental hazards associated with these emissions. There are many gases causing the greenhouse effect, and carbon dioxide is the main among them. Since the time of the industrial revolution, the atmospheric CO2 concentration has risen by about 35 % to a value of 400 ppm, and is expected to reach 550 ppm by 2050 even if CO2 emission is stable for the next four decades (IEA Statistics 2014). Consequently, the planet temperature is rising, causing several environmental impacts. Thus, technologies must be developed in order to prevent or reduce CO2 emissions.
There are three main approaches for CO2 separation and capture: membrane purification, liquid absorption and adsorption using solids. Membranes are more promising for concentrated CO2 streams at elevated pressures (Choi et al. 2009). Amines are the most commonly used liquid absorbent for CO2 capture and it is a well-established commercial technology (Choi et al. 2009; D’Alessandro et al. 2010). Unlike liquid absorbents, solid adsorbents can be used over a wider temperature range from ambient temperature to 700 °C, yield less waste during cycling, and the spent solids can be disposed of without undue environmental precautions (Wang et al. 2011). Moreover, solid adsorbents exhibit better energy efficiency in the regeneration stage compared with absorption approaches and better chemical stability in presence of hydrogen sulfide and steam (D’Alessandro et al. 2010). The adsorbent must have high CO2 selectivity and adsorption capacity, fast adsorption/desorption kinetics, stable adsorption capacity after repeated adsorption/desorption cycles, and adequate mechanical strength of adsorbent particles after cyclic exposure to high pressure streams (Yong et al. 2001; Yong and Rodrigues 2002). Zeolites, activated carbons, alkaline oxides and hydrotalcite-like compounds are examples of solid adsorbents for CO2 removal (Choi et al. 2009; D’Alessandro et al. 2010).
Hydrotalcites (HTs) are anionic clays with lamellar structure formed by brucite-like layers [Mg(OH)2], where part of Mg2+ is replaced by Al3+, and an interlayer space containing CO3 2− anions and water molecules. Hydrotalcite-like compounds (HTCs), also known as layered double hydroxides (LDHs), are produced by partial or total substitution of Mg2+ by other divalent cations and Al3+ by other trivalent cations, with similar ionic radius. Equation 1 shows general formula for HTCs (Cavani et al. 1991):
where: M2+ is divalent cation (Mg2+, Ni2+, Zn2+, Cu2+, Mn2+, etc.); M3+ is trivalent cation (Al3+, Fe3+, Cr3+, etc.); An− is the charge compensation anion (CO3 2−, SO4 2−, NO3 −, Cl−, OH−, etc.) and x is the molar ratio of trivalent cation [M3+/(M2++M3+)], normally ranging between 0.17 and 0.33.
In general, the CO2 capture capacities of HTCs are somewhat lower than those of other chemisorbents (typically <1.0 mmol g−1) (Choi et al. 2009); however, the adsorption capacities can be altered substantially by controlling the type and amount of divalent and trivalent cations, and also the thermal treatment. Upon calcination, HTCs gradually lose interlayer water, dehydroxylate and decarbonate, leading to formation of mixed oxides with large surface area and basic sites that enhance CO2 adsorption. Besides the efforts made to increase the CO2 capture capacity, another important issue is to enhance the long-term stability of HTCs during adsorption/desorption cycling operation, which is crucial for the development of practical applications (Wang et al. 2011).
Yong and Rodrigues (2002) studied the effect of different divalent cations (Ni, Cu, Co, Zn and Mg) on CO2 adsorption capacity at 25 °C, showing that Mg–Al sample presented the best value (approximately 0.55 mmol g−1). With regard to this HT, the aluminum content was reported to directly affect the adsorption capacity. The adsorption capacity of Mg–Al sample decreased during about the first 10 cycles of adsorption/desorption under dry or wet conditions. Oliveira et al. (2008) studied three commercial Mg–Al HTs with different Mg/Al ratio and the CO2 adsorption capacities were lower than 0.1 mmol g−1 at 400 °C.
Ram Reddy et al. (2006) reported the effect of calcination temperature on the CO2 adsorption capacity of Mg–Al HT with x = 0.25. The sample calcined at 400 °C showed the highest adsorption capacity, which was associated with a compromise between the surface area and availability of basic sites. Another parameter varied by Ram Reddy et al. (2006) was the adsorption temperature (100–400 °C), where 200 °C showed the highest adsorption (0.49 mmol g−1). The increase in adsorption temperature (200–400 °C) decreased CO2 adsorption capacity, because the higher levels of kinetic energy cause CO2 desorption. On the other hand, at 100 °C there was lack of energy to promote activation of basic sites.
The aim of this paper is to evaluate the CO2 adsorption capacity of Mg–Al and Zn–Al hydrotalcite-like compounds of different compositions (x = 0.20, 0.28 and 0.33), after calcination, correlating with surface area and amount of basic sites. The choice of two divalent cations aims to study the effect of basicity on CO2 adsorption; moreover, Zn-based HTCs are less reported in the literature. The CO2 adsorption equilibrium using Langmuir model and the effect of adsorption/desorption cycles are also presented.
2 Experimental
2.1 Preparation of adsorbents
Mg–Al and Zn–Al HTCs were prepared by coprecipitation method at room temperature based on Corma et al. (1994). 200 mL of solution A, containing nitrate precursors (Al/(M2++Al) = 0.20, 0.28 or 0.33 and [M2++Al] = 1.5 mol L−1, where M2+ represents Mg2+ or Zn2+), was slowly dropped (1 mL min−1) in a Teflon reactor under vigorous stirring to 200 mL of solution B, containing appropriated amounts of Na2CO3 and NaOH (CO3 2−/Al3+ = 0.375 and OH−/Al3+ = 6.3). Following the end of dripping of solution A, the gel formed in the reactor was maintained under agitation for 1 h to complete the precipitation. The suspension was aged in an oven at 60 °C for 18 h. Then, the precipitate was vacuum filtered and washed with deionized water at 90 °C until the filtrate was pH neutral in order to ensure complete removal of the base. The washed precipitate was dried at 110 °C overnight. The mixed oxides were obtained by calcination of the HTCs at 400 °C for 2 h using a heating rate of 10 °C min−1 under air flow (60 mL min−1). The samples will be referred to as MgAlXXX or ZnAlXXX, where XXX = 020, 028 or 033, depending on x = Al/(M2++Al) molar ratio of the synthesis gel.
2.2 Characterization of adsorbents
The chemical composition was determined by X-ray fluorescence (XRF), performed on a Rigaku RIX 3100 spectrometer equipped with rhodium tube.
X-ray powder diffraction (XRD) was performed on a Rigaku Miniflex II diffractometer with graphite monochromator and Cu Kα radiation (30 kV and 15 mA). Analysis was conducted between 2θ = 5° and 90° with steps of 0.05° using a counting time of 2 s by step.
The textural characteristics, such as specific surface area, average pore size and pore size distribution, were investigated from nitrogen physisorption analysis using the Brunauer, Emmett and Teller (BET) and Barrett, Joyner and Hallenda (BJH) methods. The N2 adsorption and desorption isotherms were measured at −196 °C in a Micromeritics TriStar II 3020 device.
The amount and nature of the basic sites were determined by temperature- programmed desorption of CO2 (TPD-CO2), carried out in conventional equipment coupled to a PrismaPlus (Pfeiffer) mass spectrometer. The sample was pretreated at 400 °C for 1 h under He flow (30 mL min−1). CO2 adsorption was performed under 40 mL min−1 of 10 %CO2/He at room temperature for 30 min; then the sample was treated with He flow for 1 h in order to remove CO2 physically adsorbed. Finally, TPD profile was recorded when the sample was heated at 20 °C min−1 to 700 °C using He as the carrier gas at a flow rate of 40 mL min−1. Relations m/e = 2, 12, 15, 16, 18, 28, 32 and 44 were monitored and m/e = 44 was used for CO2 quantification.
2.3 Measurements of CO2 adsorption capacity
CO2 adsorption capacity was measured by thermogravimetric (TG) analysis using TA SDT-Q600 device. Before the adsorption, in situ heat treatment at 400 °C for 1 h was performed. Then, the sample was cooled to 50 °C, where the CO2 adsorption was processed. After adsorption, the desorption step was performed heating the sample to 300 °C and then the sample was cooled again to 50 °C. A second adsorption was performed in order to find CO2 physically adsorbed. Nitrogen flowed at 50 mL min−1 in the heat treatment, cooling and desorption. In the adsorption steps a mixture with 10 % CO2/He flowed at 50 mL min−1. The cooling and heating rates were 2 and 10 °C min−1, respectively.
2.4 CO2 adsorption isotherms
The experimental CO2 sorption isotherm experiments were performed on the same TG equipment. Different CO2 concentrations were obtained diluting a mixture of 10 %CO2/He with appropriate amounts of He. Firstly, an in situ heat treatment at 400 °C for 1 h was performed. Then, the sample was cooled at 2 °C min−1 up to 50 °C, the adsorption temperature.
The CO2 adsorption isotherms were fitted with a Langmuir model (Eq. 2) and the model was linearized (Eq. 3) to calculate qm and b parameters:
where q is the CO2 combined adsorbed amount (mmol CO2 g−1), qm is the CO2 adsorbed amount corresponding to a monolayer (mmol CO2 g−1), b is the equilibrium constant (L mmol CO −12 ) and C is the CO2 concentration in CO2/He mixture (mmol CO2 L−1).
2.5 CO2 adsorption/desorption cycles
Three CO2 adsorption/desorption cycles were evaluated using the same TG apparatus. Firstly, an in situ heat treatment at 400 °C for 1 h was performed. Then, the sample was cooled at 2 °C min−1 up to 50 °C, when the first adsorption took place. After adsorption, the desorption was performed at 300 °C under flowing N2. This procedure was repeated three times. The same flow and heating/cooling rates as described in Sect. 2.3 were used.
3 Results and discussion
3.1 Characterization of adsorbents
The chemical composition of the HTCs is presented in Table 1. The composition of the as-synthesized samples is similar to that of the gel of synthesis, indicating an approximately complete incorporation of the cations in HTC structure.
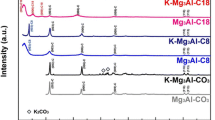
The XRD patterns of hydrotalcite-like precursors (Fig. 1) exhibit sharp and symmetrical reflections at 11.7°, 23.2°, 60.6° and 61.8° (ascribed to the diffraction by the (003), (006), (110) and (113) planes) and broad and asymmetric reflections at 34.7°, 38.8° and 46.0° (ascribed to the diffraction by the (102), (105) and (108) planes), characteristic of a well-crystallized Mg–Al HT (JCPDS 41-1428) or Zn–Al HTC (JCPDS 38-0486), with rhombohedral symmetry (Silva et al. 2010; León et al. 2010). In this later case, the absence of other phases suggests that Zn2+ has isomorphically replaced Mg2+ cations in the brucite-like layers.
After calcination at 400 °C, the characteristic lamellar structure disappears by loss of interlayer water, dehydroxylation and decarbonation, which occurs in sequence as temperature increases (Yang et al. 2002). Consequently, the materials become nearly amorphous and XRD patterns (Fig. 2) show the presence of only MgO periclase (JCPDS 45-0946) or ZnO phase (JCPDS 36-1451). These results indicate that aluminum oxide is well dispersed in MgO or ZnO matrix with formation of a solid solution, in accordance with the literature (Yang et al. 2002; Hutson et al. 2004; Sampieri and Lima 2009; León et al. 2010).
The specific surface area, pore volume and average pore diameter of HTC samples, before and after calcination, are presented in Table 1. Adsorption–desorption isotherms of nitrogen for all samples exhibited the type IV pattern, which are typical of mesoporous solids (pore size between 20 and 500 Å), according to the IUPAC classification (Sing et al. 1985). They all presented H3 hysteresis loop, which is observed with aggregates of plate-like particles giving rise to slit-shaped pores, and is affected by phenomena such as delayed pore condensation, percolation, and pore blocking (Sing et al. 1985; Hutson et al. 2004). After calcination, it was observed an increase in surface area and pore volume, as shown in Table 1, which can be attributed to the elimination of carbonate anions as CO2, leading to the destruction of the layered structure (as observed by XRD). The strains associated with the resulting 3-D structure and with the compression-expansion of the octahedral layer are related to the significant increase in surface area (Hutson et al. 2004; León et al. 2010).
The non-calcined samples presented similar surface areas, irrespective to the M2+ cation. However, after calcination, Mg(Al)O mixed oxides showed much larger surface area than Zn(Al)O samples, as observed by Béres et al. (1999). The obtained values of surface areas are in accordance with others in the literature. Silva et al. (2010) reported BET surface areas of 83 m2 g−1 for Mg–Al HT (x = 0.33) and 202 m2 g−1 after calcination at 400 °C, while the values measured by Abelló et al. (2005) were 57 m2 g−1 for Mg–Al HT (x = 0.22) and 210 m2 g−1 after calcination at 450 °C. For Mg–Al calcined samples, the surface area decreased with increasing Al content (higher values of x), similarly to that observed by Corma et al. (2005): 214 m2 g−1 for x = 0.25, 173 m2 g−1 for x = 0.30 and 162 m2 g−1 for x = 0.33 (samples were calcined at 450 °C).
The basicity of the mixed oxides obtained from HTC calcination was investigated by TPD-CO2 (Fig. 3). The complex desorption profiles are clearly related to the presence of basic sites with different strengths. For all samples the profiles consist of: (i) a low temperature peak, with maximum desorption at around 120–130 °C, attributed to CO2 interaction with weak basic sites, (ii) a small desorption peak in the range 170–250 °C, related to medium basic strength sites, and (iii) a large desorption peak at high temperatures (>500 °C), associated with CO2 desorption from sites of strong basicity. The weak basic sites correspond to OH− groups on the surface, the medium strength sites are related to the oxygen in both M2+-O2− and Al3+-O2− pairs and the strong basic sites correspond to isolated O2− anions (Di Cosimo et al. 1998; Bolognini et al. 2002; Liu et al. 2014).
A deconvolution procedure using multi-peak Gaussian fitting was applied to TPD-CO2 profiles and the amount of CO2 desorbed from each site is shown in Table 2. It can be clearly seen that strong basic sites are predominant over weak and medium basic sites, in accordance with other works in the literature (Di Cosimo et al. 1998; Silva et al. 2010). The amount of basic sites for MgAl HTC found here is much lower than the value reported by Hutson and Attwood (2008) (692 µmol g−1 for MgAl with x = 0.25), but their TPD profile did not keep a constant baseline as those presented in Fig. 3. On the other hand, Kustrowski et al. (2004) found by TPD-CO2 an amount of basic sites of only 46.7 µmol g−1 for MgAl HT with x = 0.33, calcined at 600 °C. These same authors reported values of 39.6 µmol g−1 for MgAl with x = 0.33, 126.9 µmol g−1 for x = 0.25 and 166.7 µmol g−1 for x = 0.20, all of them calcined at 450 °C (Kustrowski et al. 2005). These values are close to those calculated in this work.
Table 2 also shows that the total basicity decreases with increasing x and MgAl samples are more basic than ZnAl ones. Using the Sanderson electronegativity model, Rodrigues (2005) showed that the increase on x = M3+/(M2++M3+) ratio in HTCs increases the electronegativity and decreases basicity, as observed experimentally in the present work. Moreover, as electronegativity of Zn2+ is higher than Mg2+, ZnAl HTC is expected to have lower basicity than MgAl, as observed by Sampieri and Lima (2009).
3.2 CO2 adsorption
Table 3 shows the combined, physical and chemical CO2 adsorption capacities and also reversible fraction for MgAl and ZnAl HTCs calcined at 400 °C, in which the term combined refers to the sum of chemical and physical adsorption. The adsorption capacities were lower than 1 mmol g−1, in accordance with Choi et al. (2009).
The much higher CO2 adsorption capacities of MgAl samples when compared to ZnAl can be related to their larger surface area (Table 1) and higher number of basic sites (Table 2). When analyzing MgAl or ZnAl series separately, the combined adsorption capacities do not follow the total number of basic sites. For MgAl series, the highest adsorption capacity was presented by the sample with lower amount of strong basic sites (x = 0.33), which shows that the presence of weak and medium basic sites are more important for CO2 adsorption in this case. The correlation between CO2 adsorption capacity and the amount of strong basic sites is shown in Fig. 4. Yong et al. (2001, 2002) observed that increasing the Al3+ content on Mg–Al HTs is favorable for CO2 adsorption due to the increase of the layer charge density in HTCs and the reduction of high strength basic sites. Sharma et al. (2008) also showed that CO2 chemisorption capacity of Mg–Al HTs increases with Al content; the maximum adsorption capacity (0.91 mmol g−1 at 30 °C) was obtained with x = 0.37.
For ZnAl series, the sample with the highest combined CO2 adsorption capacity was also that with x = 0.33, which can be associated not only to its lower number of strong basic sites but also to its much higher surface area. Comparing both series, the reversible fractions were higher for ZnAl samples, especially for x = 0.33, showing that substitution of Mg2+ by Zn2+ favors physisorption over chemisorption. For MgAl samples it was observed by Torres-Rodríguez et al. (2011) formation of MgCO3 after CO2 adsorption, which explains the enhanced contribution of chemisorption.
Tsuji et al. (1993) measured a CO2 adsorption capacity of 0.6 mmol g−1 at 25 °C for Mg–Al HT (x = 0.45) calcined at 400 °C, and 0.14 mmolg−1 for Zn–Al HTC (x = 0.45) calcined at 200 °C. The CO2 adsorption capacity at 50 °C reported by Léon et al. (2010) for Mg–Al HT (x = 0.25) calcined at 450 °C was 0.96 mmol g−1. Hutson et al. (2004) measured the adsorption capacity at 200 °C: 0.9 mmol g−1 for Mg–Al HT (x = 0.25) calcined at 400 °C; the reversible fraction was 81.6 %. At the same adsorption temperature and the same HT composition and calcination, Wang et al. (2012) obtained an adsorption capacity of 0.58 mmol g−1. Thus, the adsorption capacities obtained here are coherent with other values in the literature.
The measured adsorption isotherms were fitted into Langmuir model, as shown in Fig. 5, while Table 4 presents the Langmuir parameters. According to the correlation coefficients (R2), it was observed that the Langmuir model fitted relatively well to the experimental points. Experimental error was calculated performing triplicates for MgAl sample with x equal to 0.28 and ZnAl with x equal to 0.20 in the CO2 concentration of 2.25 and 0.28 mmol L−1, respectively. Two other models were also tested, Freundlich and Temkin, but they failed to fit with the experimental data.
The qm parameter for MgAl samples did not show the expected behavior when compared to CO2 adsorption capacity (Table 3). The expected behavior was that the sample with highest adsorption presented the highest value for qm, and this divergence is probably due to the high experimental error (8.7 %). ZnAl samples showed the expected behavior for the qm parameter.
Ding and Alpay (2000, 2001) showed that CO2 adsorption equilibrium on Mg–Al HTC, in both dry and wet conditions at 400–450 °C, could be adequately described by the Langmuir model. On the other hand, Sharma et al. (2008) observed that the Freundlich isotherm gave the best fit with the experimental data of CO2 adsorption on Mg–Al HT at 30 °C.
Cyclic adsorption/desorption experiments showed that the adsorption capacity decreases with increasing the number of cycles (Table 5). The adsorption fraction in the third cycle follows the order x = 0.28 < 0.33 < 0.20, which shows that samples with x = 0.20 have greater resistance to the deactivation of adsorption sites as a function of cycle number.
A possible explanation for the decrease of the CO2 adsorption capacity in cyclic experiments is the increase of irreversible chemisorption, which decreases the amount of the sites able to CO2 adsorption (Yong and Rodrigues 2002; Du et al. 2010; León et al. 2010). Kim et al. (2004) showed that Mg–Al HT reached a steady-state behavior for CO2 adsorption at 150 °C after the 9th cycle. As MgAl adsorbents have higher chemical adsorption fraction (Table 3) it was expected a higher decrease in adsorption capacity of these samples with cycle number, but this was only true for x = 0.20 (although the difference in this case is very small). Thus, MgAl samples are more resistant to deactivation with adsorption/desorption cycles and more adequate for long-term use.
4 Conclusions
Mg–Al and Zn–Al hydrotalcite-like compounds were synthesized by coprecipitation and obtained as pure phases, as demonstrated by the XRD analyses. The calcination of adsorbents at 400 °C formed mixed oxides with the desirable basic sites responsible for the CO2 adsorption, and increase in the specific surface area. The increase in Al content decreased the number of basic sites. Mg–Al and Zn–Al samples with Al molar composition of 0.33 showed greater CO2 adsorption capacity at 50 °C: 0.91 and 0.21 mmol g−1, respectively. Langmuir isotherm fitted well to the experimental data, since the correlation coefficients showed satisfactory values. CO2 adsorption/desorption cycles showed that the adsorption decreases with the increasing number of cycles, demonstrating that there is deactivation due to irreversible chemisorption. When comparing the samples, it becomes clear that the adsorbent that presented the best characteristics to adsorb CO2 was Mg–Al.
References
Abelló, S., Medina, F., Tichit, D., Pérez-Ramírez, J., Groen, J.C., Sueiras, J.E., Salagre, P., Cesteros, Y.: Aldol condensations over reconstructed Mg–Al hydrotalcites: structure–activity relationships related to the rehydration method. Chem. A. Eur. J. 11, 728–739 (2005)
Béres, A., Pálinkó, I., Kiricsi, I., Nagy, J.B., Kiyozumi, Y., Mizukami, F.: Layered double hydroxides and their pillared derivatives – materials for solid base catalysis; synthesis and characterization. Appl. Catal. A 182, 237–247 (1999)
Bolognini, M., Cavani, F., Scagliarini, D., Flego, C., Perego, C., Saba, M.: Heterogeneous basic catalysts as alternatives to homogeneous catalysts: reactivity of Mg/Al mixed oxides in the alkylation of m-cresol with methanol. Catal. Today 75, 103–111 (2002)
Cavani, F., Trifiro, F., Vaccari, A.: Hydrotalcite-type anionic clays: preparation, properties and applications. Catal. Today 11, 173–301 (1991)
Choi, S., Drese, J.H., Jones, C.W.: Adsorbent materials for carbon dioxide capture from large anthropogenic point sources. ChemSusChem 2, 796–854 (2009)
Corma, A., Iborra, S., Primo, J., Rey, F.: One-step synthesis of citronitril on hydrotalcite derived base catalysts. Appl. Catal. A 114, 215–225 (1994)
Corma, A., Hamid, S.B.A., Iborra, S., Velty, A.: Lewis and Brönsted basic active sites on solid catalysts and their role in the synthesis of monoglycerides. J. Catal. 234, 340–347 (2005)
D’Alessandro, D.M., Smit, B., Long, J.R.: Carbon dioxide capture: prospects for new materials. Angew. Chem. Int. Ed. 49, 6058–6082 (2010)
Di Cosimo, J.I., Díez, V.K., Xu, M., Iglesia, E., Apesteguia, C.R.: Structure and surface and catalytic properties of Mg-Al basic oxides. J. Catal. 178, 499–510 (1998)
Ding, Y., Alpay, E.: Equilibria and kinetics of CO2 adsorption on hydrotalcite adsorbent. Chem. Eng. Sci. 55, 3461–3474 (2000)
Ding, Y., Alpay, E.: High temperature recovery of CO2 from flue gases using hydrotalcite adsorbent. Trans. IChemE 79, 45–51 (2001)
Du, H., Ebner, A.D., Ritter, J.A.: Temperature dependence of the nonequilibrium kinetic model that describes the adsorption and desorption behavior of CO2 in K-promoted HTlc. Ind. Eng. Chem. Res. 49, 3328–3336 (2010)
Hutson, N.D., Speakman, S.A., Payzant, E.A.: Structural effects on the high temperature adsorption of CO2 on a synthetic hydrotalcite. Chem. Mater. 16, 4135–4143 (2004)
Hutson, N.D., Attwood, B.C.: High temperature adsorption of CO2 on various hydrotalcite-like compounds. Adsorption 14, 781–789 (2008)
International Energy Agency: CO2 emissions from fuel combustion highlights, www.iea.org. Accessed Sept 2014
Kim, Y., Yang, W., Liu, P.K.T., Sahimi, M., Tsotsis, T.T.: Thermal evolution of the structure of a Mg-Al-CO3 layered double hydroxide: sorption reversibility aspects. Ind. Eng. Chem. Res. 43, 4559–4570 (2004)
Kustrowski, P., Chmielarz, L., Bozek, E., Sawalha, M., Roessner, F.: Acidity and basicity of hydrotalcite derived mixed Mg–Al oxides studied by test reaction of MBOH conversion and temperature programmed desorption of NH3 and CO2. Mater. Res. Bull. 39, 263–281 (2004)
Kustrowski, P., Sułkowska, D., Chmielarz, L., Rafalska-Łasocha, A., Dudek, B., Dziembaj, R.: Influence of thermal treatment conditions on the activity of hydrotalcite-derived Mg–Al oxides in the aldol condensation of acetone. Microp. Mesop. Mater. 78, 11–22 (2005)
León, M., Díaz, E., Bennici, S., Vega, A., Ordóñez, S., Auroux, A.: Adsorption of CO2 on hydrotalcite-derived mixed oxides: sorption mechanisms and consequences for adsorption irreversibility. Ind. Eng. Chem. Res. 49, 3663–3671 (2010)
Liu, Q., Wang, B., Wang, C., Tian, Z., Qu, W., Ma, H., Xu, R.: Basicities and transesterification activities of Zn–Al hydrotalcites-derived solid bases. Green Chem. 16, 2604–2613 (2014)
Oliveira, E.L.G., Grande, C.A., Rodrigues, A.E.: CO2 sorption on hydrotalcite and alkali-modified (K and Cs) hydrotalcites at high temperatures. Sep. Purif. Technol. 62, 137–147 (2008)
Ram Reddy, M.K., Xu, Z.P., Lu, G.Q., Costa, J.C.D.: Layered double hydroxides for CO2 capture: structure evolution and regeneration. Ind. Eng. Chem. Res. 45, 7504–7509 (2006)
Rodrigues, A.C.C.: Influence of the composition on the electronegativity and on the oxygen charge distribution in a binary hydrotalcite-like by modified Sanderson method. J. Math. Chem. 37, 347–351 (2005)
Sampieri, A., Lima, E.: On the acid-base properties of microwave irradiated hydrotalcite-like compounds containing Zn2+ and Mn2+. Langmuir 25, 3634–3639 (2009)
Sharma, U., Tyagi, B., Jasra, R.V.: Synthesis and characterization of Mg-Al-CO3 layered double hydroxide for CO2 adsorption. Ind. Eng. Chem. Res. 47, 9588–9595 (2008)
Silva, C.C.C.M., Ribeiro, N.F.P., Souza, M.M.V.M., Aranda, D.A.G.: Biodiesel production from soybean oil and methanol using hydrotalcites as catalyst. Fuel Process. Technol. 91, 205–210 (2010)
Sing, K.S.W., Everett, D.H., Haul, R.A.W., Moscou, L., Pierotti, R.A., Rouquerol, J., Siemieniewska, T.: Reporting physisorption data for gas/solid system with special reference to the determination of surface area and porosity. Pure Appl. Chem. 57, 603–619 (1985)
Torres-Rodríguez, D.A., Lima, E., Valente, J.S., Pfeiffer, H.: CO2 capture at low temperatures (30–80°C) and in the presence of water vapor over a thermally activated Mg-Al layered. J. Phys. Chem. A 115, 12243–12250 (2011)
Tsuji, M., Mao, G., Yoshida, T., Tamaura, Y.: Hydrotalcites with an extended Al3+-substitution: synthesis, simultaneous TG-DTA-MS study, and their CO2 adsorption behaviors. J. Mater. Res. 8, 1137–1142 (1993)
Wang, Q., Luo, J., Zhong, Z., Borgna, A.: CO2 capture by solid adsorbents and their applications: current status and new trends. Energy Environ. Sci. 4, 42–55 (2011)
Wang, Q., Tay, H.H., Guo, Z., Chen, L., Liu, Y., Chang, J., Zhong, Z., Luo, J., Borgna, A.: Morphology and composition controllable synthesis of Mg–Al–CO3 hydrotalcites by tuning the synthesis pH and the CO2 capture capacity. Appl. Clay Sci. 55, 18–26 (2012)
Yang, W., Kim, Y., Liu, P.K.T., Sahimi, M., Tsotsis, T.T.: A study by in situ techniques of the thermal evolution of the structure of a Mg–Al–CO3 layered double hydroxide. Chem. Eng. Sci. 57, 2945–2953 (2002)
Yong, Z., Mata, V., Rodrigues, A.E.: Adsorption of carbon dioxide onto hydrotalcite-like compounds (HTLCs) at high temperatures. Ind. Eng. Chem. Res. 40, 204–209 (2001)
Yong, Z., Rodrigues, A.E.: Hydrotalcite-like compounds as adsorbents for carbon dioxide. Energy Convers. Manag. 43, 1865–1876 (2002)
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rossi, T.M., Campos, J.C. & Souza, M.M.V.M. CO2 capture by Mg–Al and Zn–Al hydrotalcite-like compounds. Adsorption 22, 151–158 (2016). https://doi.org/10.1007/s10450-015-9732-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10450-015-9732-2