Abstract
The emergence of bone tissue engineering as a trend in regenerative medicine is forcing scientists to create highly functional materials and scaffold construction techniques. Bone tissue engineering uses 3D bio-printed scaffolds that allow and stimulate the attachment and proliferation of osteoinductive cells on their surfaces. Bone grafting is necessary to expedite the patient’s condition because the natural healing process of bones is slow. Fused deposition modeling (FDM) is therefore suggested as a technique for the production process due to its simplicity, ability to create intricate components and movable forms, and low running costs. 3D-printed scaffolds can repair bone defects in vivo and in vitro. For 3D printing, various materials including metals, polymers, and ceramics are often employed but polymeric biofilaments are promising candidates for replacing non-biodegradable materials due to their adaptability and environment friendliness. This review paper majorly focuses on the fused deposition modeling approach for the fabrication of 3D scaffolds. In addition, it also provides information on biofilaments used in FDM 3D printing, applications, and commercial aspects of scaffolds in bone tissue engineering.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
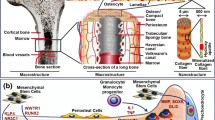
Bone tissue is a unique type of viscous-elastic connective tissue consisting of 45–60% (w/w) inorganic minerals, 20–30% (w/w) organic materials, and 10–20% (w/w) water [54]. Hydroxyapatite (HAp) makes up most of the inorganic portion of bone tissue [15]. The aging process causes the body to go through several changes that might cause bone tissues or their functions to be lost or damaged [33]. A significant bone defect or critical-sized defect cannot be repaired by the patient’s body even though the bone is a robust tissue with the ability to repair itself [95]. Conventional treatment strategies, including autografts, allografts, and xenografts, have been limited because of their associated disadvantages, which include few donor resources and donor locations, extra surgical procedures, immunological response, and the possibility of disease transmission [11, 96]. Bone tissue engineering (BTE) has garnered considerable interest in the development of innovative structures aimed at preserving, enhancing, and/or restoring bone function [20]. Bone tissue engineering is the creation of implantable bone substitutes for the treatment of bone loss due to trauma, infection, and tumor resection [67]. Damaged biological tissue can be restored, maintained, improved, or replaced by combining cells, technology, material techniques, and relevant biochemical and physicochemical parameters [34, 55]. The regulation of morphology and the distribution of pores in scaffolds has proven difficult to achieve using conventional fabrication techniques including solvent-casting, particulate-leaching, and freeze-drying [35]. On the other hand, because of its superior control over scaffold structure, 3D printing, an advanced manufacturing method, is the most promising method for producing artificial bone grafts, and organs [63]. The use of fused deposition modeling (FDM) 3D-printed scaffolds in bone tissue engineering is necessary because they serve as transient templates for cells that direct bone repair and promote the body’s natural processes of bone regeneration [51, 86]. Over the past three decades, fast prototyping has been widely used in the production of 3D scaffolds with specified porosity [73]. In this review article, we focused on the fused deposition modeling (FDM) method for the fabrication of 3D scaffolds. Furthermore, it also provides information on biofilaments used in FDM 3D printing, applications, and commercial aspects of scaffolds in bone tissue engineering.
We addressed the biofilaments, fused deposition modeling 3d printing, and their impact on the fabrication of scaffolds for bone tissue engineering.
Scaffolds
In bone tissue engineering, a scaffold is a three-dimensional biomaterial framework that is used to rebuild bone defects [57]. A scaffold must resemble the extracellular matrix (ECM) and meet particular biological and mechanical criteria to attract and guide osteogenic cells [102]. Scaffold design is the fundamental aspect of bone tissue engineering since it offers the cells a surface on which to adhere, proliferate, migrate, and differentiate after being transplanted into the region of bone defect [6]. To withstand outside stresses and steadily remodel over time as new bone tissue is created, these three-dimensional structures must also offer mechanical support, waste elimination, and nutrition supply [64]. Before creating or designing a scaffold, scientists need to understand the bone biology that supports the natural healing process [102]. Conventional methods (see Fig. 1) can produce scaffolds for bone tissue engineering, but they can’t produce scaffolds with fully continuous interconnectivity and uniform pore morphology [26]. Furthermore, they demonstrated a few additional drawbacks, such as mechanical failure, inadequate stress shielding, and material-associated infections [87].
The most popular manufacturing technique used today to transform biomaterials into three-dimensional scaffolds for tissue engineering is rapid prototyping [2]. Technologies for rapid prototyping (RP) are sometimes referred to as additive manufacturing since the material is placed gradually, layer by layer until the desired shape is attained [48]. Technologies utilized in rapid prototyping include 3D bioprinting, selective laser sintering, stereolithography, and fused deposition molding [45]. With the use of 3D-printing technology, a bone tissue scaffold may be created that closely matches the patient’s original anatomical structure and allows for exact pore size adjustment inside the scaffold [97]. The 3D bioprinter that is controlled by robotics can print the tubular organ using the necessary bioinks [94]. Figure 2 displays the ideal properties of a 3D-printed scaffold for bone tissue engineering [25].
Scaffolds may be entirely tuned for porosity and their shapes can be made to be patient-specific without the requirement for a mold if they are fabricated from a computer-aided design (CAD) file [91]. This method produces artificial bone made of poly(ε-caprolactone)-nanohydroxyapatite that is more closely aligned with the mechanics of real bone, has good cell biocompatibility and biodegradability in vitro, and has the proper ability to build new bone in vivo [18]. While scaffolds with big pore sizes contain less material and are therefore more prone to deformation, scaffolds with tiny pore sizes have a larger capacity to support loads [80]. A non-toxic, biodegradable, and biocompatible 3D-Printing PU/PAAM/Gel Hydrogel Scaffold with osteogenic potential was created by He and colleagues. This unique scaffold holds great potential for non-load-bearing bone repair [36]. Scientists have also created a 3D-printed scaffold made of graphene oxide that may be used to create a cartilage matrix in future studies [21]. Using hydroxyapatite (HAp) and polycaprolactone (PCL), a multi-nozzle three-dimensional (3D) printer was utilized to develop and create a new biphasic scaffold for osteochondral tissue engineering [89]. A poly(l-lactide-co-ε-caprolactone) 3D scaffold coated with collagen-I was fabricated by He and his workers for bone tissue engineering [37]. An excellent example of bioactivity to encourage hydroxyapatite (HAp) nucleation and development was demonstrated by Jiang and his co-workers’ production of a biocompatible and biodegradable polyvinyl alcohol/sodium alginate blend polymers containing Ca2+ doped TiO2 nanocomposite 3D scaffold [41]. Additionally, three-dimensional hydrogel/bioactive glass scaffolds with enhanced mechanical and bioactive qualities were created for vascularized bone [4]. The immobilization of gelatin and CuO nanoparticles on a 3D-printed conductive PCL/GO scaffold demonstrated appropriate cell adhesion and proliferation of H9C2 cardiomyoblast cells at an affordable price [83]. The bioactivity of inner cube 3D-printed PCL scaffolds was explored and enhanced by the application of biomimetic polydopamine (PDA) coating and bionic hydrolysis. The changes greatly enhanced the scaffolds’ biological qualities, hydrophilicity, surface adhesion ability, and surface roughness [59].
Biofilaments
In the FDM, the adaptability of filament material is a crucial point. The type of filament materials utilized in the FDM process determines the product’s qualities. To produce desired structures on the printing platform before solidification, melted polymers become flexible [68]. Different materials (such as polymer, ceramic, metal, and composites) are used by different kinds of 3D printers. Figure 3 displays the examples of different polymers used in the 3D-printing machine [58]. Except for directed-energy deposition, a polymer is one of the most often utilized feedstocks in nearly all forms of 3D printing [58]. It is well known that polymers such as proteins and polysaccharides have excellent mechanical properties, high surface-to-volume ratios, biocompatibility, anti-allergic response, favorable enzymatic reactions, porosity, and controlled biodegradation rates [87].
Polylactic acid (PLA) is a biodegradable material and is extensively used in 3D printing due to its low melting temperature, non-toxic fume emissions, and minimal warping [68]. PLA is also suitable for use as filaments in composites containing a broad range of materials, including boron nitride, tricalcium phosphate, calcium carbonate, graphene oxide, and nanohydroxyapatite [93]. Polyhydroxyalkanoates (PHAs) are biodegradable polyesters that can be used to replace traditional polymers and lessen environmental issues. They are produced by prokaryotic organisms from industrial and agricultural waste [70]. Anuar and his collogues fabricated a novel soda lignin/PLA/EPO biocomposite to create a 3D printable filament [9]. By using a single screw extruder for hot-melt extrusion, a 1.7 mm diameter polymer/ceramic composite filament comprising PLA-BCPs was produced by Nevado and his co-worker [71]. To create a filament with improved bioactivity, Shafqat et al. proposed a calcium phosphate-loaded novel polypropylene glycol-based resin [84]. Martins and his colleagues created a sustainable filament of PHB and PHBV filled with 5-weight percent pullulan. These filaments demonstrated a greater degree of heat resistance and sufficient thermal stability for the processing of fused deposition modeling [65]. A new kind of poly(ε-caprolactone) scaffold with customized core/shell-structured filaments has shown improved mechanical characteristics (strain at failure, modulus, and tensile strength) [23].
FDM 3D Printing
FDM is marketed as a powerful instrument for producing intricate structures that are impossible to create with conventional methods, such as scaffolds, and for acquiring precise parts for high-performance specialized applications [104]. 3D printing (additive manufacturing) is a marvel of modern technology that combines several building processes and the field of fabrication science to create a wide range of interesting materials [56]. In 1986, Charles Hull invented the method of stereolithography (SLA), which was followed by other innovations such as powder bed fusion, fused deposition modeling (FDM), inkjet printing, selective laser sintering (SLS), electron beam melting, and contour crafting (CC) [72, 100]. The majority of the initial 3D-printing methods appeared in the late 1990 s and developed quickly, which was linked to advancements in computer technology [77]. In the early 2000 s, 3D-printing technologies were first widely investigated in a variety of industries, including the aerospace and medical sectors [85]. Figure 4 summarizes the history of 3D printing and the advancement of polymer materials [74].
History of polymer development and 3D printing [74]. Reprinted with permission from Elsevier
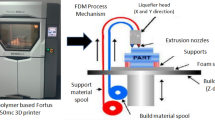
FDM is one of the most popular types of commercial 3D-printing technologies today that primarily employs single or hybrid polymer filaments [53]. The FDM process involves heating a thermoplastic bio-filament at the nozzle to a semi-liquid condition before extruding it onto a platform or over layers that have already been printed [62]. A crucial component of this process is the polymer filament’s thermoplasticity, which enables the filaments to fuse during printing and subsequently harden at ambient temperature after printing [76]. Figure 5 features the characteristics of materials used in FDM technology.
Typically, engineered polymers with excellent mechanical resilience, thermal stability, and flow qualities are selected for the printing of a 3D scaffold [74]. The fundamental idea behind FDM-based manufacturing is to melt raw material and mold it into novel shapes. The material is a roll of filament that is drawn by a drive wheel, heated to a semi-liquid state, and then inserted into a temperature-controlled nozzle head. To create structural parts layer by layer, the nozzle accurately extrudes and directs materials in an incredibly thin layer after layer. This traces the outline of the layer that the program—typically CAD—specifies and inserts into the FDM work system [30, 66]. FDM can produce patient-specific 3D templates in a time-saving and economical way. The complicated demands of bone defect treatment and each patient’s unique course of therapy can be met by the microstructure of 3D-printed scaffolds [46]. This technology has empowered the tailored fabrication of 3D scaffolds with complex geometries and functionalities in surface and mechanical properties [24]. The few processing parameters that influence the mechanical qualities of the printed component are the width, the thickness of the layers, printing speed, and the orientation of the filament [88]. Currently, 51% of the products produced by additive manufacturing technology are made of polymer–plastic filaments. This is because these materials not only meet the requirements to be manufactured and used, but they also contribute to the optimization and management of FDM processes used in product manufacturing [30]. When compared to traditional manufacturing techniques, the 3D-printing process has several benefits, including flexible design, print on demand, quick prototyping, robust and lightweight components, little waste, cost-effectiveness, ease of assessment, and environmental friendliness [38, 75].
The biomedical area has benefited from the 3D printer’s contributions, which include applications utilizing biocompatible materials for dental implants, blood vessel synthesis, tissue formation without causing damage to living cells, and customized medical prostheses [16]. Natural (alginate, polylactic acid, hyaluronan, collagen, etc.) and synthetic polymers (PLA, PGA, PLGA, etc.) are used in 3D printing for healthcare applications because of their chemical and structural compatibilities to the environment found in bodily tissues [60, 90]. One of the main advantages of synthetic origins is their ability to modify various biomolecules peacefully while still meeting their physicochemical properties [73]. Because of its low melting point, non-toxicity, non-irritability, and biocompatibility, PLA (polylactic acid) material is widely used in FDM technology, which is its most important feature for 3D printing [61]. 3D-printing technological innovation satisfies the current demands of the healthcare system, they combine medical and technical applications [75]. This technology is successfully used in surgical applications, organ printing, medical imaging, vet medicine applications, the production of patient-specific medical prostheses and implants, skin engineering, and the pharmaceutical industry (Fig. 6) [16].
Applications of FDM 3D Printing in BTE
The creation of scaffolds for tissue regeneration has given rise to a need for polymeric materials. When it comes to bone regeneration, polymeric materials have an advantage over bioinert metals due to their wide range of natural and biocompatible synthetic polymers with specific functions that are readily accessible on the market [52]. Because natural polymers may offer customized scaffolding systems for the structural and functional organization of cells, they have emerged as key players in the 3D bioprinting of tissues and organs [99]. 3D printing is majorly used to (1) fabricate bone grafts that match the shape of the missing bone, (2) control the microarchitecture of scaffolds, (3) create different binders to create bone scaffolds with the required mechanical strength, and (4) print cells, growth factors, and proteins [17, 22, 40]. FDM allows for the creation of intricate and customized 3D structures with high precision. This is particularly beneficial in bone tissue engineering, where the ability to replicate complex tissue architectures is essential for successful integration and functionality [5, 7]. Consequently, the goal of 3D-printing technology is to create an artificial bone structure with the necessary dimensions, mechanical qualities, biocompatibility, degradability, and porosity for the regeneration of bone [1]. Scientists must also investigate the characteristics of various biomaterials to fully comprehend how 3D model design is crucial to the customization of bone implants [79]. A great deal of work has gone into creating polymer–polymer composites that improve the functionality of bone scaffolds. Polyethylene glycol (PEG)–PLA [13], PLA–polyaniline (PANI) [19], chitosan–collagen–hyaluronic acid [44], and many more combinations are examples of these composite scaffolds. A bone scaffold material should also be biodegradable, which means that it should be able to decompose over time into non-toxic byproducts that the body can metabolize and eliminate [31]. Increased permeability promotes better bone ingrowth, and vascularization, and prevents cartilaginous tissue from forming in the regenerated area [92]. The porosity, orientation, size, distribution, and interconnectedness of the pores all affect permeability [47]. Alternatively, scaffolds with variable porosity and pore size distribution have been created using 3D printing to patient specifications [10]. Any type of scaffold must have pores that are at least 100 μm in diameter for bone to mineralize. Additionally, studies have shown that scaffolds with hole diameters between 250 and 600 μm may promote the growth of new bone, capillary development, extracellular matrix synthesis, and cell proliferation [49]. A non-toxic and biocompatible composite-based 3D scaffold has been prepared using polylactic acid (PLA) and cellulose nanocrystals (CNC) which revealed an average pore size of approximately 400 µm. This combination increases bone tissue growth by allowing optimal vascularization, hydroxyapatite nucleation, and mineral maturation [69]. However, polycaprolactone (PCL) has relatively low mechanical strength and hydrophobic nature compared to some other polymers [32, 39, 98]. Romero-Araya et al. prepared a PCL 3D-printed scaffold which revealed low wettability, a smooth, uniform surface, moderate porosity (60%) with pores of around 500 μm, and compressive strength of 91 MPa for the applications in hard tissue engineering [79]. Jirofti et al. prepared a 3D-printed biocompatible and less toxic crocin-loaded chitosan/collagen/hydroxyapatite-based scaffolds for the regeneration of damaged bone tissue [42]. Hydroxyapatite (HAp) can improve the biocompatibility, mechanical strength, and hydrophilicity of the scaffold, which increases the rate of formation of neo-tissue-bone [82]. Using antimicrobial peptide-modified silk fibroin (SF) and silica, a micro extrusion-based 3D-printing technique was used to create a 3D aerogel-based hybrid scaffold [50]. In another research work, BMP2 mimetic peptide and antimicrobial peptide PSI10 (RRWPWWPWRR) were added to the hydroxyapatite (HAp) to increase the antibacterial and osteoinduction efficacy of the scaffold [101]. The human cell viability is increased and bacteria adhering to the scaffold are eliminated by a 3D-printed electroactive scaffold composed of PCL with reduced graphene oxide (TrGO) [8]. According to antibacterial studies, the hydrophilic characteristic of TrGO composites allowed the PCL/TrGO scaffolds to decrease germs by 26% when compared to pure PCL [78]. Deng et al. created a bioactive ceramic scaffold called Sr5(PO4)2SiO4 (SPS) via extrusion 3D printing to create bioactive scaffolds for cartilage and subchondral interface. Strontium (Sr) and silicon (Si) ions were added to these scaffolds since the release of these ions from the scaffold is crucial for the regeneration of osteochondral defects [29]. For the treatment of osteosarcoma, Dang et al. employed doxorubicin (DOX), a chemotherapy medication used at varying dosages, and tricalcium phosphate (TCP) coated with TiN (TN) microparticles as a 3D-printed bioceramics scaffold [27]. In another study, Zhuang et al. created Pluronic F-127 and akermanite-iron (Fe-AKT) bone scaffolds that were 3D-printed and had high osteogenic activity [103]. Das et al. fabricated a hybrid porous scaffold by using alginate–bioglass composite hydrogels with poly(lactic acid) (PLA). The good in vitro cytocompatibility, biocompatibility, and mechanical strength of this hybrid scaffold make it attractive for large bone regeneration [28]. Overall, it can be concluded that traditional manufacturing methods may struggle to produce complex geometric structures required for bone scaffolds. 3D printing allows the fabrication of intricate and porous structures, mimicking the natural architecture of bone tissue. Fused Deposition Modeling (FDM) 3D printing has shown promise in bone tissue engineering applications, but there are several limitations associated with this technology. Understanding these limitations is crucial for researchers and practitioners to make informed decisions and improvements in the field. Some of the key limitations are displayed in Fig. 7.
Commercial Aspects
3D bioprinting has become more and more popular in recent years, both in the academic and in the industrial domains. In the years 2014 to 2015, the market saw the entry of a large number of 3D bioprinting firms, and new start-ups, spin-offs, and subsidiaries kept popping up [43]. The usage of 3D printing has grown due to supply chain robustness and enabled local, on-demand production. The expansion of 3D printing has slowed in recent years due to COVID-19 which caused business disruption [3]. Businesses have been forced to expand and improve their current production and distribution capacities due to the 3D bioprinting industry’s expansion, which is mostly driven by technical advancements in biomaterials and 3D bioprinters [81]. More than 2.2 million bone transplants are carried out globally each year because pathological and unintentional stress can occasionally cause damage to bone tissue [2]. The market for bone grafts and replacements was projected to be valued at $3.07 billion in 2022 and would grow to $4.5 billion by 2030. By 2025, the ceramic 3D-printing market is projected to grow to a value of USD 384 million worldwide. In the medical field, 3D ceramic implants have the second-highest compound annual growth rate (26.5%). It is anticipated to increase to USD 82 million by 2025 from USD 25 million in 2020 [17]. Modern commercial 3D-printing technology is primarily based on thermoplastic material extrusion, with prices as low as $250 being easily found on the market [12]. To encourage bone regeneration, FDM 3D-printed porous bioresorbable polycaprolactone-based bone implants called OsteoplugTM and OsteomeshTM are utilized. Orbital fractures, dental sockets, buccal abnormalities, craniosynostosis, septal extension, mandible reconstruction, etc., can all be treated with these implants [14]. Furthermore, developments in hybrid materials and 4D/5D printing are gaining ground and signal the market’s potential growth in the years to come.
Conclusion and future perspectives
The review has consolidated and produced the existing knowledge on Fused Deposition Modeling (FDM) 3D-printed scaffolds for bone tissue engineering. By bringing together information from various studies, it provides a comprehensive overview of the current state of the field. A lot of studies have been done on using 3D printing for polymer composites to add reinforcement materials like nanoparticles and short or continuous fibers to overcome these mechanical performance restrictions. Furthermore, in some functional applications, 3D-printed polymer composites can outperform traditional composites if functional elements like metal or metal oxide and live cells are added to the polymers. The complicated geometry of bone defects, the characteristics of the material, and the incorporation of cells and biomolecules provide difficulties in the 3D printing of bone tissue scaffolds. Medical imaging, processing, and the resulting CAD model make it simpler to get over this impediment. However, there are still limitations with printable materials, especially when bioprinting and functionally graded materials are involved.
Future research could focus on the development of new biomaterials and composites suitable for FDM 3D printing to enhance the mechanical and biological properties of scaffolds. Exploring multi-material 3D-printing techniques with FDM could enable the fabrication of scaffolds with complex structures and gradients. Combining different materials in a single scaffold could mimic the heterogeneous composition of natural bone tissue, enhancing functionality and promoting tissue integration. Future research could explore innovative strategies, such as incorporating microchannels or biomimetic vasculature networks, to enhance the scaffolds’ ability to support blood vessel formation and tissue integration. This approach, coupled with telemedicine initiatives, could improve accessibility to advanced bone tissue engineering solutions in diverse geographical locations. Since this technology makes it possible to print organs and tissues, a large amount of research is now being done on the potential applications of this technology in many biological contexts. As a result, many people will benefit from it in future. Furthermore, it is possible to build organs using this technology that will carry out the same biological tasks as the original organs.
Data availability
All data are given in the manuscript
References
Abbasi, N., S. Hamlet, R. M. Love, and N.-T. Nguyen. Porous scaffolds for bone regeneration. J. Sci. Adv. Mater. Devices. 5:1–9, 2020.
Abdelaziz, A. G., H. Nageh, S. M. Abdo, M. S. Abdalla, A. A. Amer, A. Abdal-hay, and A. Barhoum. A review of 3D polymeric scaffolds for bone tissue engineering: principles, fabrication techniques. Immunomodulatory Roles Chall. 10:204, 2023.
Ahmed, A., A. Azam, M. M. Aslam Bhutta, F. A. Khan, R. Aslam, and Z. Tahir. Discovering the technology evolution pathways for 3D printing (3DP) using bibliometric investigation and emerging applications of 3DP during COVID-19. Clean. Environ. Syst. 3:100042, 2021.
Aldhaher, A., F. Shahabipour, A. Shaito, S. Al-Assaf, A. A. M. Elnour, E. B. Sallam, S. Teimourtash, and A. A. Elfadil. 3D hydrogel/ bioactive glass scaffolds in bone tissue engineering: status and future opportunities. Heliyon.9:e17050, 2023.
Alzoubi, L., A. A. A. Aljabali, and M. M. Tambuwala. Empowering precision medicine: the impact of 3D printing on personalized therapeutic. AAPS PharmSciTech. 24:228, 2023.
Amirazad, H., M. Dadashpour, and N. Zarghami. Application of decellularized bone matrix as a bioscaffold in bone tissue engineering. J. Biol. Eng. 16:1, 2022.
Amiri, E., P. Sanjarnia, B. Sadri, S. Jafarkhani, and M. Khakbiz. Recent advances and future directions of 3D to 6D printing in brain cancer treatment and neural tissue engineering. Biomed. Mater. 18:52005, 2023.
Angulo-Pineda, C., K. Srirussamee, P. Palma, V. M. Fuenzalida, S. H. Cartmell, and H. Palza. Electroactive 3D printed scaffolds based on percolated composites of polycaprolactone with thermally reduced graphene oxide for antibacterial and tissue engineering applications. Nanomaterials. 10:428, 2020.
Anuar, H., N. A. A. Rahman, M. R. Manshor, Y. A. Alli, O. A. Alimi, F. Alif, and J. Suhr. Novel soda lignin/PLA/EPO biocomposite: a promising and sustainable material for 3D printing filament. Mater. Today Commun.35:106093, 2023.
Bahraminasab, M. Challenges on optimization of 3D-printed bone scaffolds. Biomed. Eng. Online. 19:69, 2020.
Beheshtizadeh, N., M. Gharibshahian, Z. Pazhouhnia, M. Rostami, A. R. Zangi, R. Maleki, H. K. Azar, V. Zalouli, H. Rajavand, A. Farzin, N. Lotfibakhshaiesh, F. Sefat, M. Azami, T. J. Webster, and N. Rezaei. Commercialization and regulation of regenerative medicine products: Promises, advances and challenges. Biomed. Pharmacother.153:113431, 2022.
Bhagia, S., K. Bornani, R. Agrawal, A. Satlewal, J. Ďurkovič, R. Lagaňa, M. Bhagia, C. G. Yoo, X. Zhao, V. Kunc, Y. Pu, S. Ozcan, and A. J. Ragauskas. Critical review of FDM 3D printing of PLA biocomposites filled with biomass resources, characterization, biodegradability, upcycling and opportunities for biorefineries. Appl. Mater. Today.24:101078, 2021.
Bhaskar, B., R. Owen, H. Bahmaee, Z. Wally, P. Sreenivasa Rao, and G. C. Reilly. Composite porous scaffold of PEG/PLA support improved bone matrix deposition in vitro compared to PLA-only scaffolds. J. Biomed. Mater. Res. Part A. 106:1334–1340, 2018.
Bloom, O., N. Goddard, B. Yannoulias, and S. Eccles. The successful use of a bespoke OssDsign cranial plate to reconstruct an occipital defect following excision of a recurrent epithelioid sarcoma. JPRAS Open. 24:71–76, 2020.
Bogala, M. R. Three-dimensional (3D) printing of hydroxyapatite-based scaffolds: a review. Bioprinting.28:e00244, 2022.
Bozkurt, Y., and E. Karayel. 3D printing technology; methods, biomedical applications, future opportunities and trends. J. Mater. Res. Technol. 14:1430–1450, 2021.
Budharaju, H., S. Suresh, M. P. Sekar, B. De Vega, S. Sethuraman, D. Sundaramurthi, and D. M. Kalaskar. Ceramic materials for 3D printing of biomimetic bone scaffolds—current state-of-the-art & future perspectives. Mater. Des.231:112064, 2023.
Cestari, F., M. Petretta, Y. Yang, A. Motta, B. Grigolo, and V. M. Sglavo. 3D printing of PCL/nano-hydroxyapatite scaffolds derived from biogenic sources for bone tissue engineering. Sustain. Mater. Technol.29:e00318, 2021.
Chen, J., M. Yu, B. Guo, P. X. Ma, and Z. Yin. Conductive nanofibrous composite scaffolds based on in-situ formed polyaniline nanoparticle and polylactide for bone regeneration. J. Colloid Interface Sci. 514:517–527, 2018.
Chen, Y., and X. Li. The utilization of carbon-based nanomaterials in bone tissue regeneration and engineering: respective featured applications and future prospects. Med. Nov. Technol. Devices.16:100168, 2022.
Cheng, Z., L. Xigong, D. Weiyi, H. Jingen, W. Shuo, L. Xiangjin, and W. Junsong. Potential use of 3D-printed graphene oxide scaffold for construction of the cartilage layer. J. Nanobiotechnol. 18:97, 2020.
Chinnasami, H., M. K. Dey, and R. Devireddy. Three-dimensional scaffolds for bone tissue engineering. Bioengineering. 10:759, 2023.
Choi, J.-W., K. Lee, Y.-H. Koh, and H.-E. Kim. Novel poly(ε-caprolactone) scaffolds comprised of tailored core/shell-structured filaments using 3D plotting technique. Mater. Lett.269:127659, 2020.
Chowdhury, S. R., N. Keshavan, and B. Basu. Urinary bladder and urethral tissue engineering, and 3D bioprinting approaches for urological reconstruction. J. Mater. Res. 36:3781–3820, 2021.
Chung, J., H. Im, S.-H. Kim, J. Park, and Y. Jung. Toward biomimetic scaffolds for tissue engineering: 3D printing techniques in regenerative medicine. Front. Bioeng. Biotechnol. 2020. https://doi.org/10.3389/fbioe.2020.586406.
Collins, M. N., G. Ren, K. Young, S. Pina, R. L. Reis, and J. M. Oliveira. Scaffold fabrication technologies and structure/function properties in bone tissue engineering. Adv. Funct. Mater. 31:2010609, 2021.
Dang, W., K. Yi, E. Ju, Y. Jin, Y. Xu, H. Wang, W.-C. Chen, K. Wang, Y. Wang, Y. Tao, and M. Li. 3D printed bioceramic scaffolds as a universal therapeutic platform for synergistic therapy of osteosarcoma. ACS Appl. Mater. Interfaces. 13:18488–18499, 2021.
Das, M., O. Sharabani-Yosef, N. Eliaz, and D. Mandler. Hydrogel-integrated 3D-printed poly(lactic acid) scaffolds for bone tissue engineering. J. Mater. Res. 36:3833–3842, 2021.
Deng, C., H. Zhu, J. Li, C. Feng, Q. Yao, L. Wang, J. Chang, and C. Wu. Bioactive scaffolds for regeneration of cartilage and subchondral bone interface. Theranostics. 8:1940–1955, 2018.
Dizon, J. R. C., A. H. Espera, Q. Chen, and R. C. Advincula. Mechanical characterization of 3D-printed polymers. Addit. Manuf. 20:44–67, 2018.
Dubey, A., H. Vahabi, and V. Kumaravel. Antimicrobial and biodegradable 3D printed scaffolds for orthopedic infections. ACS Biomater. Sci. Eng. 9:4020–4044, 2023.
Dwivedi, R., S. Kumar, R. Pandey, A. Mahajan, D. Nandana, D. S. Katti, and D. Mehrotra. Polycaprolactone as biomaterial for bone SCAFFOLDS: REVIEW OF literature. J. Oral Biol. Craniofacial Res. 10:381–388, 2020.
Fang, H., Z. Deng, J. Liu, S. Chen, Z. Deng, and W. Li. The mechanism of bone remodeling after bone aging. Clin. Interv. Aging. 17:405–415, 2022.
Gao, J., X. Yu, X. Wang, Y. He, and J. Ding. Biomaterial-related cell microenvironment in tissue engineering and regenerative medicine. Engineering. 13:31–45, 2022.
Haider, A., S. Haider, M. Rao Kummara, T. Kamal, A.-A.A. Alghyamah, F. Jan Iftikhar, B. Bano, N. Khan, M. Amjid Afridi, S. Soo Han, A. Alrahlah, and R. Khan. Advances in the scaffolds fabrication techniques using biocompatible polymers and their biomedical application: a technical and statistical review. J. Saudi Chem. Soc. 24:186–215, 2020.
He, M., Y. Hou, C. Zhu, M. He, Y. Jiang, G. Feng, L. Liu, Y. Li, C. Chen, and L. Zhang. 3D-printing biodegradable PU/PAAM/Gel hydrogel scaffold with high flexibility and self-adaptibility to irregular defects for nonload-bearing bone regeneration. Bioconjug. Chem. 32:1915–1925, 2021.
He, Y., W. Liu, L. Guan, J. Chen, L. Duan, Z. Jia, J. Huang, W. Li, J. Liu, J. Xiong, L. Liu, and D. Wang. A 3D-printed PLCL scaffold coated with collagen type I and its biocompatibility. Biomed Res. Int. 2018:5147156, 2018.
Iftekar, S. F., A. Aabid, A. Amir, and M. Baig. Advancements and limitations in 3D printing materials and technologies: a critical review. Polymers. 15:2519, 2023.
Ilyas, R. A., M. Y. M. Zuhri, M. N. Norrrahim, M. S. Misenan, M. A. Jenol, S. A. Samsudin, N. M. Nurazzi, M. R. M. Asyraf, A. B. M. Supian, S. P. Bangar, R. Nadlene, S. Sharma, and A. A. B. Omran. Natural fiber-reinforced polycaprolactone green and hybrid biocomposites for various advanced applications. Polymer. 14:182, 2022.
Jariwala, S. H., G. S. Lewis, Z. J. Bushman, J. H. Adair, and H. J. Donahue. 3D printing of personalized artificial bone scaffolds. 3D Print. Addit. Manuf. 2:56–64, 2015.
Jiang, N., B. Qi, X. Fan, L. Yao, Y. Wang, Z. Zhao, Y. Xu, and M. Hasmizam Razali. Fabrication of biocompatible and biodegradable polyvinyl alcohol/sodium alginate blend polymers incorporating Ca2+ doped TiO2 nanocomposite 3D scaffold for biomedical applications. J. Saudi Chem. Soc. 27:101758, 2023. https://doi.org/10.1016/j.jscs.2023.101758.
Jirofti, N., M. Hashemi, A. Moradi, and F. Kalalinia. Fabrication and characterization of 3D printing biocompatible crocin-loaded chitosan/collagen/hydroxyapatite-based scaffolds for bone tissue engineering applications. Int. J. Biol. Macromol.252:126279, 2023.
Jovic, T. H., E. J. Combellack, Z. M. Jessop, and I. S. Whitaker. 3D bioprinting and the future of surgery. Front. Surg.7:609836, 2020.
Kaczmarek, B., A. Sionkowska, J. Kozlowska, and A. M. Osyczka. New composite materials prepared by calcium phosphate precipitation in chitosan/collagen/hyaluronic acid sponge cross-linked by EDC/NHS. Int. J. Biol. Macromol. 107:247–253, 2018.
Kafle, A., E. Luis, R. Silwal, H. M. Pan, P. L. Shrestha, and A. K. Bastola. 3D/4D printing of polymers: fused deposition modelling (FDM), selective laser sintering (SLS), and stereolithography (SLA). Polymer. 13:3101, 2021.
Kang, X., X. B. Zhang, X. D. Gao, D. J. Hao, T. Li, and Z. W. Xu. Bioprinting for bone tissue engineering. Front. Bioeng. Biotechnol. 10:1–7, 2022.
Kang, Y., and J. Chang. Channels in a porous scaffold: a new player for vascularization. Regen. Med. 13:705–715, 2018.
Kanishka, K., and B. Acherjee. Revolutionizing manufacturing: a comprehensive overview of additive manufacturing processes, materials, developments, and challenges. J. Manuf. Process. 107:574–619, 2023.
Kanwar, S., and S. Vijayavenkataraman. Design of 3D printed scaffolds for bone tissue engineering: a review. Bioprinting.24:e00167, 2021.
Karamat-Ullah, N., Y. Demidov, M. Schramm, D. Grumme, J. Auer, C. Bohr, B. Brachvogel, and H. Maleki. 3D printing of antibacterial, biocompatible, and biomimetic hybrid aerogel-based scaffolds with hierarchical porosities via integrating antibacterial peptide-modified silk fibroin with silica nanostructure. ACS Biomater. Sci. Eng. 7:4545–4556, 2021.
Kennedy, S. W., N. Roy Choudhury, and R. Parthasarathy. 3D printing soft tissue scaffolds using Poly(caprolactone). Bioprinting. 30:e00259, 2023.
Krishani, M., W. Y. Shin, H. Suhaimi, and N. S. Sambudi. Development of scaffolds from bio-based natural materials for tissue regeneration applications: a review. Gels. 9:100, 2023.
Kristiawan, R. B., F. Imaduddin, and D. Ariawan. A review on the fused deposition modeling (FDM) 3D printing: filament processing, materials, and printing parameters. Open Eng. 11:639–649, 2021.
Kumar, P., B. S. Dehiya, and A. Sindhu. Synthesis and characterization of nHA-PEG and nBG-PEG scaffolds for hard tissue engineering applications. Ceram. Int. 45:8370–8379, 2019.
Kumar, P., M. Saini, B. S. Dehiya, A. Sindhu, V. Kumar, R. Kumar, L. Lamberti, C. I. Pruncu, and R. Thakur. Comprehensive survey on nanobiomaterials for bone tissue engineering applications. Nanomaterials. 10:2019, 2020.
Lai, J., and M. Wang. Developments of additive manufacturing and 5D printing in tissue engineering. J. Mater. Res. 38:4692–4725, 2023.
Lee, S. S., X. Du, I. Kim, and S. J. Ferguson. Scaffolds for bone-tissue engineering. Matter. 5:2722–2759, 2022.
Lin, C., Y. Wang, Z. Huang, T. Wu, W. Xu, W. Wu, and Z. Xu. Advances in filament structure of 3D bioprinted biodegradable bone repair scaffolds. Int. J. Bioprint. 7:426, 2021.
Lin, Y., Y. Ou, M. Xu, and J. Chen. Enhancing bone regeneration with bionic hydrolysis and biomimetic polydopamine coating on 3D-printed PCL scaffolds: a comparative study. Mater. Today Commun.37:107262, 2023.
Liu, F., and X. Wang. Synthetic polymers for organ 3D printing. Polymers. 12:1765, 2020.
Liu, Z., Y. Wang, B. Wu, C. Cui, Y. Guo, and C. Yan. A critical review of fused deposition modeling 3D printing technology in manufacturing polylactic acid parts. Int. J. Adv. Manuf. Technol. 102:2877–2889, 2019.
Mallikarjuna, B., P. Bhargav, S. Hiremath, K. G. Jayachristiyan, and N. Jayanth. A review on the melt extrusion-based fused deposition modeling (FDM): background, materials, process parameters and military applications. Int. J. Interact. Des. Manuf. 2023. https://doi.org/10.1007/s12008-023-01354-0.
Mani, M. P., M. Sadia, S. K. Jaganathan, A. Z. Khudzari, E. Supriyanto, S. Saidin, S. Ramakrishna, A. F. Ismail, and A. A. M. Faudzi. A review on 3D printing in tissue engineering applications. J. Polym. Eng. 42:243–265, 2022.
Marin, E. Forged to heal: the role of metallic cellular solids in bone tissue engineering. Mater. Today Bio.23:100777, 2023.
Martins, L. S., L. I. C. C. O. Cortat, N. C. Zanini, R. F. S. Barbosa, A. G. Souza, S. F. Medeiros, D. S. Rosa, and D. R. Mulinari. A versatile filler in polyhydroxyalcanoates filaments for FDM: a diverse panorama for pullulan application. Mater. Today Commun.28:102690, 2021.
Mitchell, A., U. Lafont, M. Hołyńska, and C. Semprimoschnig. Additive manufacturing—a review of 4D printing and future applications. Addit. Manuf. 24:606–626, 2018.
Mohammadi Zerankeshi, M., R. Bakhshi, and R. Alizadeh. Polymer/metal composite 3D porous bone tissue engineering scaffolds fabricated by additive manufacturing techniques: a review. Bioprinting.25:e00191, 2022.
Musa, L., N. Krishna Kumar, S. Z. Abd Rahim, M. S. Mohamad Rasidi, A. E. Watson Rennie, R. Rahman, A. Yousefi Kanani, and A. A. Azmi. A review on the potential of polylactic acid based thermoplastic elastomer as filament material for fused deposition modelling. J. Mater. Res. Technol. 20:2841–2858, 2022.
N’Gatta, K. M., H. Belaid, J. El Hayek, E. F. Assanvo, M. Kajdan, N. Masquelez, D. Boa, V. Cavaillès, M. Bechelany, and C. Salameh. 3D printing of cellulose nanocrystals based composites to build robust biomimetic scaffolds for bone tissue engineering. Sci. Rep. 12:21244, 2022.
Naser, A. Z., I. Deiab, and B. M. Darras. Poly(lactic acid) (PLA) and polyhydroxyalkanoates (PHAs), green alternatives to petroleum-based plastics: a review. RSC Adv. 11:17151–17196, 2021.
Nevado, P., A. Lopera, V. Bezzon, M. R. Fulla, J. Palacio, M. A. Zaghete, G. Biasotto, A. Montoya, J. Rivera, S. M. Robledo, H. Estupiñan, C. Paucar, and C. Garcia. Preparation and in vitro evaluation of PLA/biphasic calcium phosphate filaments used for fused deposition modelling of scaffolds. Mater. Sci. Eng. C.114:111013, 2020.
Ngo, T. D., A. Kashani, G. Imbalzano, K. T. Q. Nguyen, and D. Hui. Additive manufacturing (3D printing): a review of materials, methods, applications and challenges. Compos. Part B Eng. 143:172–196, 2018.
Nikolova, M. P., and M. S. Chavali. Recent advances in biomaterials for 3D scaffolds: a review. Bioact. Mater. 4:271–292, 2019.
Park, S., W. Shou, L. Makatura, W. Matusik, and K. Kelvin Fu. 3D printing of polymer composites: materials, processes, and applications. Matter. 5:43–76, 2022.
Pavan Kalyan, B. G., and L. Kumar. 3D printing: applications in tissue engineering, medical devices, and drug delivery. AAPS PharmSciTech. 23:92, 2022.
Rahmatabadi, D., K. Soltanmohammadi, M. Pahlavani, M. Aberoumand, E. Soleyman, I. Ghasemi, M. Baniassadi, K. Abrinia, M. Bodaghi, and M. Baghani. Shape memory performance assessment of FDM 3D printed PLA-TPU composites by Box–Behnken response surface methodology. Int. J. Adv. Manuf. Technol. 127:935–950, 2023.
Rayna, T., and L. Striukova. From rapid prototyping to home fabrication: how 3D printing is changing business model innovation. Technol. Forecast. Soc. Change. 102:214–224, 2016.
Rivera-Briso, A. L., F. L. Aachmann, V. Moreno-Manzano, and Á. Serrano-Aroca. Graphene oxide nanosheets versus carbon nanofibers: enhancement of physical and biological properties of poly(3-hydroxybutyrate-co-3-hydroxyvalerate) films for biomedical applications. Int. J. Biol. Macromol. 143:1000–1008, 2020.
Romero-Araya, P., V. Cárdenas, A. Nenen, G. Martínez, F. Pavicic, P. Ehrenfeld, G. Serandour, C. Covarrubias, M. Neira, I. Moreno-Villoslada, and M. E. Flores. Polycaprolactone scaffolds prepared by 3D printing electrosprayed with polyethylene glycol-polycaprolactone block copolymers for applications in bone tissue engineering. Polymer (Guildf).288:126448, 2023.
Rotbaum, Y., C. Puiu, D. Rittel, and M. Domingos. Quasi-static and dynamic in vitro mechanical response of 3D printed scaffolds with tailored pore size and architectures. Mater. Sci. Eng. C. 96:176–182, 2019.
Santoni, S., S. G. Gugliandolo, M. Sponchioni, D. Moscatelli, and B. M. Colosimo. 3D bioprinting: current status and trends—a guide to the literature and industrial practice. Bio-Design Manuf. 5:14–42, 2022.
Seethalakshmi, K., M. Kaviya, B. Venkatachalapathy, S. Mubeena, A. M. Punnoose, and T. M. Sridhar. Nanohydroxyapatite-doped polycaprolactone-based nanoscaffolds as a viable drug delivery agent in bone tissue engineering. J. Mater. Res. 36:420–430, 2021.
Shabankhah, M., A. Moghaddaszadeh, and N. Najmoddin. 3D printed conductive PCL/GO scaffold immobilized with gelatin/CuO accelerates H9C2 cells attachment and proliferation. Prog. Org. Coat.186:108013, 2024.
Shafqat, Z., N. Munir, N. Inayat, M. A. Khan, M. A. Fareed, and M. S. Zafar. Calcium phosphate-loaded novel polypropylene glycol-based dental resin composites: evaluation of in vitro bioactivity. J. Compos. Sci. 7:140, 2023.
Shahrubudin, N., P. Koshy, J. Alipal, M. H. A. Kadir, and T. C. Lee. Challenges of 3D printing technology for manufacturing biomedical products: a case study of Malaysian manufacturing firms. Heliyon.6:e03734, 2020.
Su, X., T. Wang, and S. Guo. Applications of 3D printed bone tissue engineering scaffolds in the stem cell field. Regen. Ther. 16:63–72, 2021.
Suamte, L., A. Tirkey, J. Barman, and P. Jayasekhar Babu. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf.1:100011, 2023.
Sultana, J., M. M. Rahman, Y. Wang, A. Ahmed, and C. Xiaohu. Influences of 3D printing parameters on the mechanical properties of wood PLA filament: an experimental analysis by Taguchi method. Prog. Addit. Manuf. 2023. https://doi.org/10.1007/s40964-023-00516-6.
Suo, H., Y. Chen, J. Liu, L. Wang, and M. Xu. 3D printing of biphasic osteochondral scaffold with sintered hydroxyapatite and polycaprolactone. J. Mater. Sci. 56:16623–16633, 2021.
Tappa, K., and U. Jammalamadaka. Novel biomaterials used in medical 3D printing techniques. J. Funct. Biomater. 9:17, 2018.
Top, N., İ Şahin, H. Gökçe, and H. Gökçe. Computer-aided design and additive manufacturing of bone scaffolds for tissue engineering: state of the art. J. Mater. Res. 36:3725–3745, 2021.
Turnbull, G., J. Clarke, F. Picard, P. Riches, L. Jia, F. Han, B. Li, and W. Shu. 3D bioactive composite scaffolds for bone tissue engineering. Bioact. Mater. 3:278–314, 2018.
Vallejos Baier, R., J. I. Contreras Raggio, C. Toro Arancibia, M. Bustamante, L. Pérez, I. Burda, A. Aiyangar, and J. F. Vivanco. Structure-function assessment of 3D-printed porous scaffolds by a low-cost/open source fused filament fabrication printer. Mater. Sci. Eng. C.123:111945, 2021.
Vasanthan, K. S., V. Srinivasan, V. Mathur, P. Agarwal, N. Negi, and S. Kumari. 3D Bioprinting for esophageal tissue regeneration: a review. J. Mater. Res. 37:88–113, 2022.
Wei, S., J.-X. Ma, L. Xu, X.-S. Gu, and X.-L. Ma. Biodegradable materials for bone defect repair. Mil. Med. Res. 7:54, 2020.
Xu, C., Y. Sun, J. Jansen, M. Li, L. Wei, Y. Wu, and Y. Liu. Calcium phosphate ceramics and synergistic bioactive agents for osteogenesis in implant dentistry. Tissue Eng. Part C Methods. 29:197–215, 2023.
Xu, Y., F. Zhang, W. Zhai, S. Cheng, J. Li, and Y. Wang. Unraveling of advances in 3D-printed polymer-based bone scaffolds. Polymers. 14:566, 2022.
Yaseri, R., M. Fadaie, E. Mirzaei, H. Samadian, and A. Ebrahiminezhad. Surface modification of polycaprolactone nanofibers through hydrolysis and aminolysis: a comparative study on structural characteristics, mechanical properties, and cellular performance. Sci. Rep. 13:9434, 2023.
Zhang, B., L. Gao, L. Ma, Y. Luo, H. Yang, and Z. Cui. 3D bioprinting: a novel avenue for manufacturing tissues and organs. Engineering. 5:777–794, 2019.
Zhang, J., K. He, D. Zhang, J. Dong, B. Li, Y. Liu, G. Gao, and Z. Jiang. Three-dimensional printing of energetic materials: a review. Energ. Mater. Front. 3:97–108, 2022.
Zhongxing, L., W. Shaohong, L. Jinlong, Z. Limin, W. Yuanzheng, G. Haipeng, and C. Jian. Three-dimensional printed hydroxyapatite bone tissue engineering scaffold with antibacterial and osteogenic ability. J. Biol. Eng. 15:21, 2021.
Zhu, G., T. Zhang, M. Chen, K. Yao, X. Huang, B. Zhang, Y. Li, J. Liu, Y. Wang, and Z. Zhao. Bone physiological microenvironment and healing mechanism: basis for future bone-tissue engineering scaffolds. Bioact. Mater. 6:4110–4140, 2021.
Zhuang, H., R. Lin, Y. Liu, M. Zhang, D. Zhai, Z. Huan, and C. Wu. Three-dimensional-printed bioceramic scaffolds with osteogenic activity for simultaneous photo/magnetothermal therapy of bone tumors. ACS Biomater. Sci. Eng. 5:6725–6734, 2019.
Zieliński, P. S., P. K. R. Gudeti, T. Rikmanspoel, and M. K. Włodarczyk-Biegun. 3D printing of bio-instructive materials: toward directing the cell. Bioact. Mater. 19:292–327, 2023.
Funding
No funding is available for this work.
Author information
Authors and Affiliations
Contributions
PK: Conception and writing of the manuscript. S, MM, and TA: Writing and validation. JB and HSS: Validation and writing. AB: Writing and drafting.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no known competing financial interests or personal interests that could have appeared to influence the work reported in this paper.
Consent to Participate
Informed consent was obtained from all the authors involved in the study.
Consent to Publication
Informed consent was obtained from all the authors involved in this paper.
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kumar, P., Shamim, Muztaba, M. et al. Fused Deposition Modeling 3D-Printed Scaffolds for Bone Tissue Engineering Applications: A Review. Ann Biomed Eng 52, 1184–1194 (2024). https://doi.org/10.1007/s10439-024-03479-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-024-03479-z