Abstract
Oxygenator thrombosis during extracorporeal membrane oxygenation (ECMO), is a complication that necessitates component replacement. ECMO centers monitor clot burden by intermittent measurement of pressure drop across the oxygenator. An increase in pressure drop at a preset flow rate suggests an increase in resistance/clot formation within the oxygenator. This monitoring method comes with inherent disadvantages such as monitoring gaps, and increased risk of air embolism and infection. We explored utilizing flow measurement, which avoids such risks, as an indicator of ECMO circuit obstructions. The hypothesis that flow rate through a shunt tube in the circuit will increase as distal resistances in the circuit increases was tested. We experimentally simulated controlled levels of oxygenator obstructions using glass microspheres in an ex vivo veno-venous ECMO circuit and measured the change in shunt flow rate using over the tube ultra-sound flow probes. A mathematical model was also used to study the effect of distal resistances in the ECMO circuit on shunt flow. Results of both the mathematical model and the experiments showed a clear and measurable increase in shunt flow with increasing levels of oxygenator obstruction. Therefore, flow monitoring appears to be an effective non-contact and continuous method to monitor for obstruction during ECMO.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
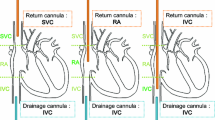
Extracorporeal membrane oxygenation (ECMO) is a form of cardiopulmonary bypass circuit that acts as a heart-lung machine, usually administered to critically ill patients with cardiac and/or respiratory failure.11 With improvements in technology, ECMO has been brought out of the operating room and to the patient bedside, thereby allowing clinicians to aid critically ill patients requiring continuous pulmonary or cardiac support.6 The circuit is usually administered to the patient in two distinct configurations—venovenous (VV) ECMO, wherein the blood is drawn into the circuit from a patient’s vein (such as the vena cava and/or a large vein such as the femoral vein) and sent back into a vein and/or into the right atrium after oxygenation2, 14; and venoarterial (VA) ECMO, wherein the blood drawn from a vein is then sent back into the patient’s artery after oxygenation. VV ECMO generally provides respiratory support to the patient, whereas VA ECMO can provide both respiratory and hemodynamic support.6, 15 Complications for patients on ECMO are very common and are associated with high mortality and morbidity.9 Between 12 and 18% of all ECMO patients face oxygenator related events or complications with high associated mortality (40–80%).1, 11, 12 Thrombogenic clot formation within the membrane oxygenator is a common complication associated with prolonged ECMO duration with especially high prevalence among pediatric patients.4, 7 Oxygenator thrombosis is considered a serious complication, because the blockage of gas exchange fibers increases blood flow resistance, reduces gas transfer capability, activates coagulation processes, increases risk of hemolysis and eventually can lead to oxygenator failure.7, 10 Imminent oxygenator failure and switch out can be expensive as well as dangerous for critically ill patients. Therefore, developing a monitoring system that can identify potential complications early and provide more time to perform interventions is very valuable.
Current ECMO monitoring techniques involve the periodic measurement of pressure drop across the oxygenator. It is common practice to check the circuit pressures pre- and post-oxygenator periodically, where circuit access sites are usually present.13 An increase in pressure drop between the pre- and post-oxygenator measurements suggests an increase in the resistance within the membrane oxygenator due to acute thrombosis.1, 3, 10, 13, 14 Though an effective estimator of oxygenator blockage, this method of monitoring has several limitations. The introduction of added connectors, stopcocks and accessing indwelling pressure lines to the path of blood every hour can increase the potential sites of turbulence and elevates risk of hemolysis, infection, embolization and air entrainment.5 Because this type of monitoring is not continuous but periodic (once every hour at our institution), it requires the laborious and intermittent involvement of an examiner with the accompanying possibility of delayed identification of oxygenator or circuit related complications. Pressure drop is also a marker specific to the oxygenator and does not indicate elevated resistances at distal parts of the circuit that would be pertinent to clinical practice such as a recovering heart (VA config), elevated systemic vascular resistance (SVR) or a cannula obstruction.13
We proposed shunt flow monitoring during ECMO as a potential continuous, automated and non-blood contacting alternative.1 The proposed method leverages basic fluid mechanics and a specific aspect of the ECMO circuit. A typical ECMO circuit is set up as shown in Fig. 1a; venous blood from the patient is drained into the circuit using an inflow cannula, the blood passes through a mechanical pump (centrifugal pump) that then maintains a steady flow of blood through the membrane oxygenator, where gas exchange occurs. Subsequently, the blood returns to the patient via an outflow cannula. Distal to the pump but proximal to the oxygenator, a small tube or shunt of about 1.8 mm diameter (compared to 6.35 mm diameter of the main line) bifurcates from the main line returning a small amount of blood back, to provide an access point for infusion of drugs, retrieving blood samples and utilization of blood parameter sensors (CDI manifold).1 Since the shunt is significantly smaller in diameter in comparison to the main line, it recirculates an inconsequential amount of blood back to the pump. In theory, when distal resistance to flow within the circuit is increased due to partial oxygenator obstruction (clotting) or any other downstream events, the flow through the shunt should start to increase due to an increased diversion of blood from the main line to the shunt. Therefore, we hypothesized that blood flow monitoring in the shunt tubing of the circuit—which can be done continuously and with ‘no contact’ over-the-tube flow sensors (safer)—can serve as an effective early indicator of oxygenator (distal) obstruction during ECMO treatment. This principle was tested using constrictor clamps to reduce the effective diameter of the tube downstream to the oxygenator to mimic an increase in resistance in the main line.1 Badheka et al. demonstrated that shunt flow increased with increasing constriction of the main line tube. However, these experiments1 had some limitations such as limited realism in simulation of oxygenator obstruction and use of saline whose viscosity was much lower than blood. More realistic experimental conditions—where the membrane oxygenator is obstructed at the fiber level to elevate circuit resistance8 and the use of blood analog fluid—are necessary to assess not just proof of concept that the shunt flow will increase due to oxygenator obstruction but also that such an increase is measurable in practice and therefore, a clinically viable alternative.
In this study, we have used a mathematical model to create a preliminary theoretical framework for shunt flow, conducted experiments that more realistically simulate ECMO circuit conditions, and tested a novel usable metric that is a more consistent indicator of circuit obstructions. We assessed shunt flow fraction, defined as the fraction of the flow out of the pump being diverted into the shunt tubing from the main line, as a measurable novel metric of circuit obstruction. The hypothesis to be tested in this study is that shunt flow fraction will increase as obstruction/resistance within the oxygenator increases. This hypothesis was first tested using a mathematical (0-dimensional) model. The mathematical model gives us the opportunity to test a desired range of clinical situations with ease and allows us to make theoretical predictions albeit under idealized simplified conditions. Subsequently, the hypothesis was tested experimentally using an actual ECMO circuit, albeit ex vivo, under more realistic conditions by inducing various levels of oxygenator obstruction using clot-simulating microsphere injections and observing the change in flow rate through the shunt tubing. We have focused on studying flow monitoring for VV ECMO.
Materials and Methods
Mathematical Model
A lumped parameter 0D model of VV ECMO circuit was developed (Fig. 1b), with both the oxygenator and the patient modeled as variable resistances and the shunt with constant resistance. The circuit is designed to be an idealized linear system without a temporal component. This is reasonable for VV ECMO since highly temporal phenomenon such as pulsatility of blood flow would only be expected in a VA ECMO configuration. Accordingly, the patient resistance (Rpt) is considered negligible in a VV ECMO configuration because both the inflow and outflow cannula are from low-resistance venous vessels. The cannulas used for inflow of blood from the patient into the circuit and outflow of blood back to the patient taper in size and are smaller than the circuit tubes. These cannulas present an increased resistance (Rcan) to flow and therefore included in the model. The pump is modeled as a constant current/flow source, wherein the pump flow or Qp remains constant. The relationship between flow, pressure and resistance is governed by the equation:
Clinical situations such as an oxygenator blockage would manifest as an increase in oxygenator resistance and likewise a cannula obstruction would manifest itself as a sudden increase in cannula resistance.13 Hence, to understand how flow through the shunt changes with respect to changes in oxygenator, patient, and cannula resistance, we express shunt flow as a function of the independent variables:
where Qp is pump flow, ∆Ppump is pressure gradient across the pump, Rox is oxygenator resistance, Rpt is patient resistance, Rcan is cannula resistance (since the inflow and outflow cannulas are resistances in series, we can assume a single term for cannulae resistance), Rs is shunt resistance, Rcircuit is total circuit resistance, Pv is venous-side pressure which can also be defined as pre-pump pressure.
In this circuit,
Considering the whole ECMO circuit, Rox, Rcan and Rpt are in series and Rs is parallel to them (see Fig. 1). Combining these resistances, the total pressure drop across the pump, ∆Ppump will be,
In a VV ECMO configuration, the patient resistance is negligible as both the inlet and outlet cannulae are located at the venous side (Rpt = 0). From (4),
Finally, shunt fraction, Qf is defined as follows:
Equation (6) describes how the shunt fraction is impacted by changes in the resistances of the oxygenator and cannula. A set of initial values that approximate those in clinical practice and observed in our experiments were used to interrogate the model:
Qp = 1000 mL/min (typical pediatric ECMO flowrate), Pv = 2 mmHg (realistic pre-pump pressure), Rs = 4.1 mmHg min/mL (experimentally estimated by measuring pressure drop across the shunt tube used for a range of realistic flow rates of blood-analog fluid); PpreOx = 120 mmHg, PpostOx = 105 mmHg (typical baseline pressures in an ECMO circuit). Baseline (no obstruction) oxygenator resistance (ROx) and cannula resistance (Rcan) were determined using these initial values in the following relationships13:
The model was then interrogated to understand the effect that a gradual increase in oxygenator obstruction (simulated by increasing Rox) and cannula obstruction (simulated by increasing Rcan) can have on shunt fraction.
Experimental Investigation
An experimental ex vivo ECMO circuit was set up in an identical manner to treatment in clinic, with the use of the tubes, connectors, membrane oxygenator, pump (Maquet Intellipack1—Pediatric oxygenator and Maquet cardiopulmonary centrifugal pump, Germany), shunt tube with CDI manifold (Terumo Cardiovascular Systems Corporation, Ann Arbor, MI), and a liquid reservoir hung at a height to which the inflow and outflow cannula were connected as previously demonstrated by Badheka et al.1 The shunt tube used was approximately 1.8 mm in diameter and 34 in. in length including the CDI manifold. Over the tube ultrasound flow probes (Transonic Systems, Inc., NY) were used to measure flow rates through the circuit. The shunt flow rate was determined using two such flow probes—flow probe 1, which is placed distal to the pump but before the shunt bifurcation and flow probe 2 which is placed distant to the shunt bifurcation but before the oxygenator. Flow probe 1 measures the total flow rate coming out of the pump, the pump flow (Qp); flow probe 2 measures the flow rate going through the oxygenator post shunt, the oxygenator flow (Qox). Therefore, flow rate through the shunt is the difference between the pump flow and the oxygenator flow (Qs = Qp − Qox). An additional access port was added proximal to the oxygenator, to facilitate the injection of microspheres into the oxygenator (Fig. 2).
In each experiment, the circuit was primed with either saline (0.9% NaCl) as the flowing medium or blood analog fluid and the pump set to flow steadily at a predetermined target flow rate. Blood analog fluid was prepared as a mixture of 35% glycerine v/v and 65% water v/v to achieve a viscosity of 3.5 cP as verified using a Brookfield LVDV-II+CP Pro Digital Cone/Plate viscometer (Brookfield Engineering Labs, Middleboro, MA). Baseline readings of pressure and flow were documented to represent an oxygenator with no obstruction. Hollow-glass microspheres of 125–150 μm diameter and 0.07 g/cm3 density (Cospheric LLC, Santa Barbara, CA) were used to simulate oxygenator obstruction by clogging the exchange fibers in the oxygenator. This size of microspheres was optimal for occluding the oxygenator membrane surface without passing through its pores. Initial dose–response experiments were performed to ascertain the relationship between the weight of these microspheres injected and the obstruction caused in the oxygenator as quantified by pressure drop across it for three pediatric pump flow rates (1000, 1500, 2000 mL/min). Separate dose–response curves were generated each for saline and blood analog fluid. It was noted that depending on the fluid medium used (saline/blood analog) a clinically realistic range of oxygenator obstruction levels (as quantified by oxygenator pressure drop) were possible by injecting aliquots of 50 to 200 mg of hollow glass microspheres into the circuit proximal to the oxygenator. During experiments, the pump was maintained at the target flow rate by adjusting its RPM as is done in the ECMO clinic. After the flow in the circuit reached steady state, four sets of pressure and flow measurements were documented at 1-min intervals and averaged for use in data analysis. The injections of microspheres were then repeated while recording the flow rates, until the pressure drop across the oxygenator reached 80–120 mmHg. At the end of every experiment, the oxygenator was washed thoroughly with deionized water flow in the opposite direction to flush out the microspheres. The no-obstruction pressure drop in the oxygenator was checked after flushing to ensure its return to baseline for subsequent experiments.
Results
Mathematical Model
The mathematical model showed that the shunt fraction and shunt flow increase linearly as oxygenator resistance is increased from baseline (pressure drop < 10 mmHg) to severe obstruction (pressure drop > 100 mmHg)—see Fig. 3a. A similar linear increase was noted when the cannula resistance was increased from its baseline to four times that (simulating a moderate kink in the inflow or outflow cannula)—see Fig. 3b.
Mathematical model prediction on change in shunt flow and shunt fraction due to increase in oxygenator resistance (a) and cannula resistance (b). The cannula and oxygenator resistances were each maintained at their baselines when the other was perturbed (0.1 mmHg min/mL for Rcan and 10 mmHg pressure drop across the oxygenator).
Experimental Investigation
An initial series of experiments were performed with saline as the flowing medium followed by a second series of experiments with blood analog fluid as the flowing medium. For both media, microsphere injection was noted to simulate oxygenator obstruction well—specifically, they do not pass through the membrane oxygenator and instead clog up the inlet part of the oxygenator. This is consistent with how thrombus obstruction occurs in the oxygenator, wherein thrombotic clots are generally located in the inlet part (venous) of the device and do not pass inside or seen to occur in the outlet/exit layers of the oxygenator.5, 7
For saline as the medium at two flowrates—1000 and 1500 mL/min—and microsphere injections to simulate gradually increasing thrombus formation in the oxygenator, the shunt flow was found to increase with increasing levels of obstruction. Figure 4 illustrates these experimental results. They also demonstrate that, consistent with our hypothesis and mathematical model, the shunt flow (Fig. 4b) and shunt fraction (Fig. 4c) increase as the level of oxygenator obstruction increases. Interestingly, while the effect of oxygenator obstruction on shunt flow depends on the pump flow rate, its effect on shunt fraction, while equally strong, is less confounded by pump flow rate. Also notable is some variation between experiments even within a given pump flow rate especially, for 1000 mL/min (Fig. 4b). Each experimental run in our study was after cleaning, dismantling and reassembling of the apparatus. This is especially so for the oxygenator which needed to be thoroughly cleaned as it may have residual microspheres injected from a previous run. Across all the experiments done in this study we used three new oxygenators (same brand, type, and model). The differences reflect the fact that every time we set up the circuit for a new run, there were subtle differences from the previous run. This could be due to subtle changes in the baseline circuit resistances due to residual uncleaned microspheres in the connectors or oxygenator or due to the change in the oxygenator used. These differences may be seen as analogous to differences among patients and circuits used in the clinic. Therefore, in this study we focus on changes from baseline for the selected parameters (as would be done in the clinic).
Experiments with saline as flow medium. (a) Dose–response curves depicting the changes in pressure drop across the oxygenator as microspheres are injected into the ECMO circuit. Four experiments were done at 1000 mL/min and two experiments at a flow rate of 1500 mL/min. (b) All experiments show a roughly linear increase in shunt flow as the resistance (obstruction) within the oxygenator is increased and a clear offset in the trend as the pump flow rate is increased. (c) Calculated shunt fraction metric shows a measurable roughly linear increase with the increase in oxygenator obstruction/resistance. Unlike shunt flow, shunt fraction is less confounded by the pump flow rate.
Experiments with blood analog fluid as the flowing medium were similar to those with saline, but each experiment now consisted of simulating three pump target flow rates (1000, 1500, and 2000 mL/min). The measured values were expectedly different owing to its higher and blood-matching viscosity, but the trends and hence the findings were quite similar to experiments with saline as medium. The dose–response curves allowed us to document the effect of microsphere injections on oxygenator obstruction levels at all three flowrates. Figure 5a demonstrate that progressive injections of microspheres (quantified by its weight injected) causes increase in oxygenator resistance (quantified by pressure drop across it) and this relationship changes with pump flow rate. As with saline, the shunt flow (Fig. 5b) and shunt fraction (Fig. 5c) increased with increasing obstruction in the oxygenator. Shunt flow showed distinct linear relationships for each of the three pump target flowrates. Shunt fraction however showed much less dependence on pump target flow rates (Fig. 5c). The experiment—measurement of shunt flow and fraction at three pump target flow rates—was repeated five more times. Results of all six experiments were consistent with the representative experiment shown in Fig. 5. The results of all six experiments are shown in aggregate form in Fig. 6 and used for statistical test of our hypothesis.
Experiments using blood analog as the flow medium—(a) increase in pressure drop across the oxygenator (buildup of resistance) as the weight of microspheres creating an obstruction within the oxygenator is increased with repeated injections (dose–response). Experiments were done at flow rates 1000, 1500 and 2000 mL/min respectively. (b) Increase in shunt flow as the pressure drop across the oxygenator (resistance) is increased. (c) Change in shunt fraction (shunt flow normalized by pump flow rate) with respect to change in oxygenator resistance (calculated using pressure drop and flow).
The difference in shunt fraction between oxygenators obstructed at two different levels from their baseline values across six sets of experiments performed at three different flow rates—1000, 1500 and 2000 mL/min. [MO–Base] refers to values at moderate obstruction subtracted by values at no obstruction and [SO–Base] refers to values at severe obstruction subtracted by values at no obstruction. Boxes represent quartiles and the whiskers represent the maximum and minimum values with all points superimposed. A difference in shunt fraction of greater than 0 is observed in most cases with a higher median difference in severely obstructed oxygenators. Therefore, a clear and measurable difference in shunt fraction with increasing levels of oxygenator obstructions is observed. For all pump flow rates, the shunt fraction changed with statistical significance (p < 0.05, paired t-test; indicated with *) when the oxygenator was moderately and severely obstructed from a baseline (no obstruction) condition.
Statistical Analysis
The hypothesis that shunt fraction will be elevated due to oxygenator obstruction was tested for the observations made with blood analog experiments. Three oxygenator obstruction groups were defined:
-
No obstruction (Base) measurements at baseline/no microspheres injected.
-
Moderate obstruction (MO) measurements after the injection of 110 ± 10 mg of microspheres—corresponds to oxygenator pressure drop of approximately 60 mmHg—clinically, such a pressure drop would trigger significant corrective efforts.
-
Severe obstruction (SO) measurements after the injection of 210 ± 10 mg of microspheres—corresponds to oxygenator pressure drop of approximately 100 mmHg.
Paired Student-t tests were performed to compare the shunt fraction at the MO and SO obstruction levels against the Base levels under various pump flow rates to test the hypothesis that shunt fraction indicates oxygenator obstruction. The shunt fraction was elevated for both moderate (MO) and severe (SO) oxygenator obstructions compared to baseline with statistical significance (p < 0.05) as illustrated by Fig. 6. All these were repeated for shunt flow as a post hoc analysis and in all cases, the findings were the same as for shunt fraction—that is, it too was elevated by MO and SO levels with statistical significance.
Discussion
The monitoring of an ECMO circuit is extremely important but tedious. The presence of competing risks during ECMO—pathologic thrombosis and bleeding, makes monitoring especially vital—to manage anticoagulation treatment.12 An ideal monitoring technique would be one that does not interrupt the path of blood and can be measured continuously without the addition of cuvettes, connectors or extra tubing to the circuit. The current practice of measuring the pressure drop across the oxygenator, utilizes indwelling pressure lines which increase the risk of infection and air entrainment. In addition, the procedure requires frequent manual measurement and due to its non-continuous nature, may delay the identification of obstructions. Other proposed methods such as measuring the oxygenator blood volume at a given time to gauge the level of obstruction using saline injections,8 still comes with the caveat of being non-continuous and interferes with the circuit. Our proposed method1 of monitoring flow rate in the ECMO circuit shunt, if shown to be reliable, gives us the ability to address many of these limitations because flow measurement, unlike pressure measurement, is continuous, automated and entirely non-contact. In addition, pressure drop measurement though specific to the oxygenator, is confounded by flow rate of the pump. Hence at different flow rates, the same measured pressure drop would correspond to different levels of resistance within the oxygenator (as shown in Fig. 7).
In this study, we assessed whether and to what extent the flow in the shunt is sensitive to oxygenator obstruction under experimental conditions using a clinical VV ECMO circuit with blood analog fluid and microspheres to simulate oxygenator clotting and using a mathematical model. We found that the flow in the shunt is consistently elevated by measurable levels when the oxygenator is both moderately and severely obstructed (Figs. 4, 5, and 6). These findings are consistent for experiments with saline and blood analog. Though blood analog experiments are a more realistic simulation of common conditions, findings from saline experiments suggest that flow monitoring will likely be effective even for patients with low blood viscosity such as due to low hematocrit. We further found that the shunt fraction—the shunt flow normalized by the pump target flow rate—is a more consistent indicator of developing obstructions than just shunt flow because unlike shunt flow or even pressure drop across the oxygenator, the shunt fraction is less dependent on the pump target flow rate (compare Figs. 5b and 5c). This means, the threshold for safety may be defined independent of pump target flow rate and therefore easily adopted in clinical practice. This suggests that monitoring shunt fraction has the potential to serve as an early, continuous, automated and non-contact indicator of oxygenator obstructions and holds potential as an alternative to current practice of periodically manually measuring pressure drop across the oxygenator. Further, elevations in shunt fraction can also be an indicator of other circuit obstructions such as kinking in the cannula that may sometimes occur during movement of ECMO patients from, to or between hospital units. Alternatively, thrombosis may occur at other locations in the circuit such as at the entry to the shunt, although this is uncommon because all ECMO tubing have specialized heparin or plasma resistant coating. Still such obstructions too will be reflected in changes to shunt flow, albeit as a reduction in shunt flow. This means that shunt fraction, while sensitive to oxygenator obstructions, is not specific to it, but rather to any obstruction in the circuit. We believe that a significant increase in shunt fraction may serve as a highly sensitive, but less specific warning that something is amiss in the ECMO circuit which may then trigger more specific tests such as pressure drop measurements. Such a system offers the advantages of providing early warnings of obstructions and minimizing the need for risky pressure drop measurements unless when necessary.
Some limitations in our study are worth noting. The experimental conditions, while realistic, still did not include some clinical aspects such as actual blood flow, the inclusion of a patient and cannulas. We therefore could not study what effect, if any, that other confounding patient related factors such as changes in SVR, hematocrit, and venous pressures would have had on shunt flow measurements. We note for instance, in Fig. 5, at a pump flow rate of 2000 mL/min the sensitivity of shunt fraction to a change in obstruction is diminished in comparison to flow rates of 1000 and 1500 mL/min—the shunt fraction starts to perceptibly elevate only after a moderate obstruction is achieved. The slope or change in shunt fraction for small changes in oxygenator resistances need to be further assessed to understand the sensitivity of shunt flow to small changes in oxygenator resistances and how this sensitivity interplays with the accuracy of the ultrasound flow transducers. Further, by focusing on VV ECMO, we have not demonstrated that shunt fraction will be as effective for monitoring patients under VA ECMO. The mechanisms studied here for VV ECMO also exists for VA ECMO and therefore, shunt fraction is likely to remain an indicator of circuit obstructions. However, in VA ECMO, since the outflow cannula is in an artery, the patient’s blood pressure, SVR and arterial pressures will have an impact on shunt flow and this may affect its sensitivity to circuit obstructions. Therefore, the findings in this study under VV ECMO conditions should be studied under VA ECMO conditions. Our use of blood analog fluid, while less realistic than human blood, is a reasonable approximation as blood-specific phenomenon such as Fahraeus–Lindqvist effect only occur in conduits that are smaller than 0.3 mm in diameter, which is far smaller than the smallest tubing in our study—the 1.8 mm diameter shunt. Finally, the proposed shunt flow monitoring will need to be tested in human subjects before adoption in clinical practice.
In conclusion, we have shown that monitoring shunt flow during VV ECMO can serve as an effective, early, continuous, automated, and non-contact indicator of oxygenator obstructions. Shunt fraction—the shunt flow normalized to pump target flowrate—is an especially robust indicator that may be used to define simple safe thresholds in the clinic and monitored accordingly. These findings need to be tested in human subjects before this proposed monitoring method may be adopted.
References
Badheka, A., S. E. Stucker, J. W. Turek, and M. L. Raghavan. Efficacy of flow monitoring during ECMO. ASAIO J. 48:598–605, 2017.
Banfi, C., M. Pozzi, N. Siegenthaler, M. E. Brunner, D. Tassaux, J. F. Obadia, K. Bendjelid, and R. Giraud. Veno-venous extracorporeal membrane oxygenation: cannulation techniques. J. Thorac. Dis. 8:3762–3773, 2016.
Bartlett, R. H., D. M. McMullan, S. B. Horton, and L. Lequier. Extracorporeal membrane oxygenation circuitry. NIH Public Access. 14:1–10, 2014.
Bhavsar, S. S., T. Schmitz-Rode, and U. Steinseifer. Numerical modeling of anisotropic fiber bundle behavior in oxygenators. Artif. Organs. 35:1095–1102, 2011.
Butt, W., M. Heard, and G. J. Peek. Clinical management of the extracorporeal membrane oxygenation circuit. Pediatr. Crit. Care Med. 14:13–19, 2013.
Chung, M., A. L. Shiloh, and A. Carlese. Monitoring of the adult patient on venoarterial extracorporeal membrane oxygenation. Sci. World J. 2014:393258, 2014.
Dornia, C., A. Philipp, S. Bauer, M. Lubnow, T. Müller, K. Lehle, C. Schmid, R. M. Müller-Wille, P. Wiggermann, C. Stroszczynski, and A. G. Schreyer. Analysis of thrombotic deposits in extracorporeal membrane oxygenators by multidetector computed tomography. ASAIO J. 60:652–656, 2014.
Evans, T. C., E. Gavrilovich, R. C. Mihai, and I. Isbasescu. Measurement of fluid volume of a blood oxygenator in an extracorporeal circuit. E. L. Patent, 2015.
George Makdisi, I. W. Extra Corporal Membrane Oxygenation (ECMO) review of a lifesaving technology. J. Thorac. Dis. 7:166–176, 2015.
Lehle, K., A. Philipp, O. Gleich, A. Holzamer, T. Müller, T. Bein, and C. Schmid. Efficiency in extracorporeal membrane oxygenation-cellular deposits on polymethypentene membranes increase resistance to blood flow and reduce gas exchange capacity. ASAIO J. 54:612–617, 2008.
Marasco, S. F., G. Lukas, M. McDonald, J. McMillan, and B. Ihle. Review of ECMO (extra corporeal membrane oxygenation) support in critically ill adult patients. Heart Lung Circ. 17(Suppl 4):S41–S47, 2008.
Olson, S. R., C. R. Murphree, D. Zonies, A. D. Meyer, O. J. T. Mccarty, T. G. Deloughery, and J. J. Shatzel. Thrombosis and bleeding in extracorporeal membrane oxygenation (ECMO) without anticoagulation. ASAIO J. 67(3):290–296, 2020.
Schaadt, J. Oxygenator thrombosis: an international phenomenon. Perfusion. 14:425–435, 1999.
Sen, A., H. E. Callisen, C. M. Alwardt, J. S. Larson, A. A. Lowell, S. L. Libricz, P. Tarwade, B. M. Patel, and H. Ramakrishna. Adult venovenous extracorporeal membrane oxygenation for severe respiratory failure: current status and future perspectives. Ann. Card. Anaesth. 19:97–111, 2016.
Skinner, S. C., J. A. Iocono, H. O. Ballard, M. D. Turner, A. N. Ward, D. L. Davenport, M. L. Paden, and J. B. Zwischenberger. Improved survival in venovenous vs venoarterial extracorporeal membrane oxygenation for pediatric noncardiac sepsis patients: a study of the Extracorporeal Life Support Organization Registry. J. Pediatr. Surg. 47:63–67, 2012.
Acknowledgments
The authors are grateful to Jennifer Crumley and Kristina Rudolph in the ECMO Clinic, University of Iowa Hospitals and Clinics.
Funding
This study was partly funded by University of Iowa Office of the Vice President for Research.
Conflict of interest
The authors declare no competing interests.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Stefan M. Duma oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Sarathy, S., Turek, J.W., Chu, J. et al. Flow Monitoring of ECMO Circuit for Detecting Oxygenator Obstructions. Ann Biomed Eng 49, 3636–3646 (2021). https://doi.org/10.1007/s10439-021-02878-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-021-02878-w