Abstract
Intragastric balloons (IGBs), by occupying the stomach space and prolonging satiety, is a promising method to treat obesity and consequently improves its associated comorbidities, e.g. coronary heart disease, diabetes, and cancer. However, existing IGBs are often tethered with tubes for gas or liquid delivery or require endoscopic assistance for device delivery or removal, which are usually uncomfortable, costly, and may cause complications. This paper presents a novel tetherless, magnetically actuated capsule (EndoPil) which can deploy an IGB inside the stomach after being swallowed and being activated by an external magnet. The external magnet attracts a small magnet inside the EndoPil to open a valve, triggering the chemical reaction of citric acid and potassium bicarbonate to produce carbon dioxide gas, which inflates a biocompatible balloon (around 120 mL). A prototype, 13 mm in diameter and 35 mm in length, was developed. Simulations and bench-top tests were conducted to test the force capability of the magnetic actuation mechanism, the required force to activate the valve, and the repeatability of balloon inflation. Experiments on animal and human were successfully conducted to demonstrate the safety and feasibility of inflating a balloon inside the stomach by an external magnet.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Obesity is a medical condition that happens when a person accumulates an excessive amount of body fat. This is a major risk factor for many comorbidities, including hypertension, stroke, gallbladder and liver disease, cardiovascular morbidity and mortality, musculoskeletal disorders, type 2 diabetes mellitus, and certain types of cancer.6 The global prevalence of overweight and obesity is increasing rapidly. According to Non-communicable Disease Risk Factor Collaboration (NCD-RisC), the number of obese people nearly tripled during the period 1975–2016. In 2016, more than 2.3 billion people worldwide were overweight, and over 34% of this number were obese.1 A wide range of weight loss therapies, from lifestyle changing, to pharmacotherapy, to bariatric surgery and bariatric endoscopy, is being implemented to fight obesity. Multiple studies found that intensive lifestyle intervention such as physical activity, balanced diet, and social support is suitable to promote a modest weight loss.17 The combination of these modifications in lifestyle with drug therapies produces an additional weight loss ranging from 3 to 9%, but this medical treatment has significant side effects.27 Bariatric surgery, including sleeve gastrectomy, Roux-en-Y gastric bypass, and adjustable gastric band, has been proven to be the most effective treatment method for obesity and its comorbidities. However, these surgical procedures often involve high cost, risks of complication, reoperation, and surgical-associated mortality.5 The development of endoscopic bariatric therapies is an inevitable result to bridge a gap between lifestyle intervention with or without pharmaceutical treatment and bariatric surgery. These approaches offer various advantages such as less-invasiveness and repeatability, thus translate to a noticeable increase in the accessibility of a larger portion of the population with moderate obesity.2
Using intragastric balloons (IGBs) for obesity treatment is one of the most developed methods among the space-occupying branch of endoscopic bariatric therapies. IGBs, with gas or liquid filled, occupy the patient’s stomach, reduce available gastric volume, and present the sensation of satiety or fullness sooner, then lessen the amount of food eaten and promote weight loss as a result.22 By treating obesity, IGBs subsequently improve its associated morbidities.12,21 With more than three decades of development, IGBs have been attracted great attention from scientists and researchers all over the world, benefiting obese patients by offering a variety of effective and safe treatment options. The Garren-Edwards Gastric Bubble (GEGB)11 was the first commercialized IGB, which was obtained approval from the United States Food and Drug Administration (FDA) in 1985. The GEGB was endoscopically placed into the stomach of obese patients, then was insufflated with 200 mL of air and remained there for 3 months before being retrieved endoscopically. Because of multiple serious complications, it was then withdrawn from the market. The IGB field has rapidly grown with the presence of many noticeable products, including Heliosphere Bag,16 Orbera Intragastric Balloon2 (Apollo Endosurgery, Austin, TX, USA), ReShape Duo18 (ReShape Medical, San Clemente, CA, USA), Obalon Intragastric Balloon7 (Obalon Therapeutics Inc., Carlsbad, CA, USA), Spatz Adjustable Balloon System4 (Spatz Medical, Great Neck, NY, USA), TransPyloric Shuttle20 (BAROnova, San Carlos, CA, USA), Elipse Balloon3 (Allurion Technologies, Wellesley, MA, USA).
For all well-known IGB devices mentioned above, it is clear that they are all tethered with tubes for gas or fluid delivery and also need endoscopic assistance for device delivery and/or removal in clinical practice, which may cause discomfort or complications to the patients. Several concepts have been developed to eliminate these limitations. The first approach is wireless communication between an internal capsule, which is inside the stomach and an external control unit. Wireless signal is utilized to activate an inflation mechanism, triggering acid-alkaline reaction to produce carbon dioxide gas inflating the balloon. This method poses some critical problems, including safety concerns related to electronic components and batteries inside the human stomach, and relatively large size at 57 mm in diameter and 157 mm in length (D57 × L157 mm)14 and D30 × L80 mm.26 The second option is the magnetic actuation of an internal magnetic mechanism by an external magnet. These magnetically actuated approaches have been generally developed for drug delivery and biopsy.25 Furthermore, Phee et al.24 and Do et al.8,9 developed a magnetic soft capsule for obesity treatment using an external magnetic field to control the inflation and deflation of an intragastric balloon. However, the complex inflation and deflation mechanism leads to complex fabrication, large size, and unreliable actuation. In addition, the actuation feasibility of these balloons in in-vivo scenarios is unknown.
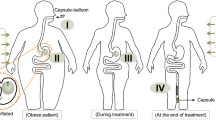
To overcome the limitations as mentioned above, we introduce a magnetically actuated capsule—EndoPil. This is a swallowable compact device (D13 × L35 mm) consisting of a simple inflation mechanism and an encapsulated intragastric balloon. Upon exposure under an external magnetic field, the inflation mechanism will trigger an acid-base reaction to produce carbon dioxide gas inflating the balloon to around 120 mL. The balloon is made from a thin-film, biocompatible, and low permeability material that enables the potential suitability and long-term treatment of the EndoPil. We targeted a treatment procedure starting with the patients swallowing the EndoPil, activating the balloon by an external magnet, the balloon will stay inside the stomach for a predefined period before being deflated by an external magnet or self-deflating by its biodegradable plug, and ending with the excretion of the deflated balloon out of the patients’ body. Animal and human trials were conducted to prove the safety and feasibility of the inflation mechanism and the balloon design of the proposed device.
Materials and Methods
Design Concept and Working Principle
It is highly desirable that an intragastric balloon device can be easily swallowed without tethers or tubes for gas or liquid delivery and the help of an endoscope or catheter for device delivery or removal. Thus, the device should comprise an embedded controllable inflation mechanism, a biocompatible and durable balloon to occupy gastric space, a mechanism to deflate the balloon after a treatment period. Subsequently, the balloon will be naturally evacuated out of the patients’ bodies. The magnetically actuated capsule—EndoPil (Fig. 1) consists of two chambers (for storage of the reactants), an inflation mechanism, an intragastric balloon, and a thin gelatin casing. The acid chamber carries citric acid, while potassium bicarbonate is held inside the base chamber. Both chemicals and their reaction products are safe for humans. Two chambers are separated by an inflation valve, which is connected with a membrane and an internal magnet via a rod to create the inflation mechanism. When attracted by a magnetic field, the internal magnet will move axially toward the capsule’s cap, which simultaneously opens the valve; this enables the acid to flow out to react with the base, resulting in the generation of carbon dioxide gas. Subsequently, the produced gas inflates the intragastric balloon occupying the stomach. The membrane retains the internal magnet from immersing into the acid and also assists in stabilizing the entire inflation mechanism. The gelatin casing at the outermost layer of the EndoPil is to form an integrated device, thus facilitating the swallow procedure. Once arriving the stomach, the gelatin casing will be quickly dissolved in 1 to 2 min.
We aim to use the EndoPil for the following treatment procedure (Fig. 1). The patient firstly swallows the capsule. Once the capsule has reached the stomach, as confirmed by an external magnetic sensor, an external magnet is introduced to open the inflation valve triggering chemical reaction to create carbon dioxide gas. The balloon is then inflated to the desired volume by the produced gas and occupied a certain space in the stomach to induce the sensation of satiety sooner, thus promoting weight loss. Multiple EndoPils can be swallowed successively over several days to gradually achieve the desired space-occupying effect while avoiding vomiting and discomfort due to sudden inflation. After a defined treatment period, the balloon will be deflated by either magnetic control or an integrated biodegradable plug. The deflated balloon is then excreted naturally through the gastrointestinal tract.
Fabrication Procedure (Part Numbers are in Fig. 1)
Injection Molding Parts (Refer to Part 1 and 7)
The cap and acid chamber are made from a medical-grade polycarbonate named Makrolon® 2458 (Convestro AG, Germany) by injection molding. This work had been outsourced from X Plas Pte. Ltd., Singapore. To proceed for injection molding, two critical parameters need to be satisfied. Firstly, the smallest wall thickness of the parts is not less than 0.5 mm. Secondly, the draft angle for mold ejection is not less than 2o.
Internal Magnet and Its Connector (Refer to Part 2 and 3)
The internal magnet is a ring neodymium magnet, nickel plating, grade N35, axially magnetized with dimension OD8 × ID3 × H3 mm (outer diameter × inner diameter × height), and is obtained from MISUMI South East Asia Pte. Ltd. The connector is a cylindrical plastic bead (OD3 × ID0.7 × H3 mm). The connection between an internal magnet and its connector is achieved by the tight-fit assembly.
Central Rod (Refer to Part 4, 5, 6, 8, 9, and 10)
Fiber carbon rod (D0.7 × L14.5 mm) is the spine of the central rod subassembly. Top support (OD1.5 × ID0.7 × H0.5 mm), middle support (OD2.5 × ID0.7 × H0.5 mm), and bottom support (OD2.5 × ID0.7 × H0.5 mm) are made by cylindrical plastic beads. They are attached to the rod by medical-grade adhesive (LOCTITE® 4014TM, Henkel, USA). The inflation valve is made from polydimethylsiloxane (PDMS, Sylgard 184 Silicone Elastomer, Dow Corning, USA) by the molding process (Fig. 2a). Firstly, place the rod (with bottom support) stand at the center hole of the mold cavity. Secondly, mixing silicone base and curing agent with ratio 10:1 to produce a PDMS solution, subsequently pour PDMS solution into the mold cavity. Thirdly, place the mold in the vacuum drying chamber (BINDER, model VD23, Germany) at 80 °C for 2 h. Lastly, the curing valve with the rod is taken out of the mold. The membrane is made from ethylene-vinyl alcohol (EVOH) film (EVAL, Kuraray Co. Ltd., Japan), securing between the top and middle support.
Gelatin Parts (Refer to Part 11 and 13)
Gelatin (Sigma-Aldrich, USA) is used to produce the base chamber and the outermost casing by the dip molding process (Fig. 2b). Firstly, mixing gelatin powder and water with ratio 1:5 by weight, then stir them by the hotplate stirrer (Stuart, US152, UK) in 2 min under 150 °C. The solution is subsequently placed in the oven (BINDER) at 80 °C in 15 min to reduce air bubbles. Secondly, dip the metal pins into the prepared gelatin solution to create a thin layer of material outside the pins. There are two types of pin: large size pins (D13 × L70 mm) for the outer casing and small size pins (D11 × L70 mm) for the base chamber. Thirdly, hang the pins at room temperature for 6 h to cure the gelatin layer. One end of the pin is attached with a magnet to facilitate the hanging step. Lastly, carefully removing the gelatin layer out of the pins and trim them to an appropriate length, 35 and 14 mm for the outer casing and base chamber, respectively. Then, creating some tiny holes at the bottom of the outer casing for easy to assemble in later steps while a big hole around 6 mm in diameter is required for the base chamber.
Balloon (Refer to Part 12)
The balloon material is EVOH, which is suitable in medical and pharmaceutical applications. Its low gas permeability and thickness15 allow the balloon to retain the carbon dioxide while remaining compact. EVOH is an ideal material for the IGBs because it possesses the outstanding gas barrier properties compared to other popular polymers such as high-density polyethylene (HDPE), low-density polyethylene (LDPE), polyethylene terephthalate (PET), polyamide nylon 6 (PA 6), polypropylene (PP), and polystyrene (PS).19 The material is bought as EVALTM film (12 μm thickness) from Kuraray Co. Ltd., Japan, and folded into a rectangular pouch using origami techniques (Fig. 2c). Its dimension is 90 × 55 mm, and it can expand into a volume of 120 mL after the acid-base reaction.
In comparison with other commercial IGBs such as Orbera or Elipse, the EndoPil has a much lower balloon volume translating into three crucial advancements: (1) eliminating the side effects of immediate inflation of a big balloon; (2) customizing the number of treated balloons for each individual patient; (3) reducing the overall size of the final product.
Reactants
Citric acid (C6H8O7) 60% of concentration and potassium bicarbonate (KHCO3) powder are selected to produce CO2 because they are both biocompatible effervescent chemicals with the most efficient combination in terms of fast reaction and high rate of gas production.9 Citric acid (Sigma-Aldrich, USA) is available in powder form. To produce an acid solution with 60% of concentration, stir acid powder and water with ratio 3:2 by the hotplate stirrer (Stuart, US152, UK) in 3 hours under 100°C. Potassium bicarbonate (Sigma-Aldrich, USA) is ready to use in powder form. The acid-base reaction is as follow,
The required volume of citric acid solution and the mass of potassium bicarbonate are calculated,
In which, the molar mass of the citric acid \(M_{\text{acid}}\) = 192.123 g/mol; the molar mass of carbon dioxide \(M_{{{\text{CO}}_{2} }}\) = 44.01 g/mol; the molar mass of potassium bicarbonate \(M_{\text{base}}\) = 100.115 g/mol; the density of carbon dioxide \(\rho_{{{\text{CO}}_{2} }}\) = 0.001977 g/cm3; the density of citric acid solution 60% \(\rho_{\text{sol}}\) = 1.399 g/cm3; the concentration of the citric acid solution \(c\) = 60%; the estimated chemical reaction efficiency \(\eta\) = 85%. To generate 120 mL of carbon dioxide gas, an amount of around 0.48 mL of citric acid solution 60% and 0.64 g of potassium bicarbonate are required.
In the assembly procedure, the air inside the balloon is expected to be squeezed out as much as possible before sealing the balloon’s last edge. Figure 3 shows the components required for the assembly procedure, a final EndoPil prototype, and its fully inflated state.
Magnetic Tracking Device
Since the detail of the tracking approach will be reported in another article, we only briefly summarize the approach here. The localization of the capsule is performed based on a magnet tracking method utilizing the actuation magnet embedded inside the capsule.23 We employ two ROHM BM1422AGMV 3 axis magnetic sensors (ROHM Co. Ltd., Japan) placed in front and back of the chest to sense the magnetic field generated by the magnet. The sensor information is processed in a grid search based algorithm to estimate the position of the magnet. The tracking method with the given sensors can achieve a tracking range from the throat to the top of the stomach (Fig. 4).
Magnet Actuation Forces at Different Distances
The external magnet is a cylindrical neodymium magnet, nickel plating, grade N42, axially magnetized with dimension D50 × L120 mm (K&J Magnetics Inc., USA). To find the relationship between attractive force and distance of the interaction between the internal magnet and external magnet, we ran a simulation in Wolfram Mathematica, Radia library, which is constructed by the Boundary Integral Method (BIM).10 Input parameters are as follow: internal magnet with dimension OD8 × ID3 × H3 mm, grade N35, remanence Bri = 1.2 T; external magnet with dimension D50 × L120 mm, grade N42, remanence Bre = 1.3 T. While the distance between two magnets is varied from 10 to 100 mm with 5 mm increment, the software outputs the attractive force between them. We also carried an experiment to verify the simulation result. The internal magnet is connected to a load cell (LTH 300, FUTEK, USA) through a fiber carbon rod. The external magnet is mounted on a rack-pinion mechanism, which is driven by a DC-motor (FAULHABER, Germany) (Fig. 5). When the distance between two magnets varied by the motor, the attractive force of the external magnet on the internal magnet is recorded by the load cell and is illustrated in Fig. 7. Both motor and load cell are controlled by a QPIDe controller (Quanser, Canada).
Valve Opening Forces
One of the most important features of the EndoPil is the inflation valve, which separates citric acid and potassium bicarbonate apart. The function of the inflation valve is characterized by the valve opening force. This value depends on several factors, including the friction between the inflation valve and its gate, the stiffness of the membrane, fluid pressure of the acid, and gravity. An experiment (Fig. 6) was conducted to measure the required valve opening force of EndoPil. The inner capsule of the EndoPil is placed on top of the load cell (LTH 300, FUTEK Inc., USA) by a 3D-print frame (made by 3D printer SLM® 500 HL, SLM Solutions Group AG Germany). The external magnet is attached with a manual slider in the vertical direction. We slowly moved the external magnet closer to the inner capsule. When the inflation valve opens, we fix the position of the external magnet, record the distance between two magnets, and the valve opening force recorded by the load cell. The experiment was run on 5 different inner capsules, 5 times for each of them.
Activation Repeatability Tests
Because the EndoPil is manually fabricated and assembled, we had conducted a series of 20 inflation trials on the final EndoPil assembly to examine the repeatability of the inflation mechanism, the quality of the balloon as well as the overall function of the device. The experimental setup was similar to those in Fig. 6, but the inner capsule was replaced with a virtual stomach (EMS, The Chamberlain Group, USA), which contained around 50 mL of water. A batch of 20 EndoPils was prepared following the steps mentioned above. The EndoPil had been placed into the virtual stomach for 1 min to soften the outer casing, then the external magnet was slowly moved downward via the manual slider. When the balloon started inflation, the external magnet was stopped; the distance between the external magnet and the EndoPil, as well as inflation time, was recorded.
Animal and Human Studies
To further validate the actuation mechanism of the capsule in vivo, we conducted an animal study on a pig carcass first and then the first-in-human trial on a human subject. Since the goal was only to test the actuation mechanism of the balloon, we completed the procedures with endoscopy assistance for the safety and quick validation of results, although the capsule is designed to be swallowable.
In the animal study conducted at the Advanced Surgery Training Center, National University Hospital, Singapore, a pig carcass was selected and prepared for the experiment. The EndoPil was endoscopically delivered to the stomach of the euthanized pig with the help of a snare. After 1 min required to soften the gelatin casing, an external magnet (D50 × L120 mm; K&J Magnetics, Inc., PA, USA) was applied over the pig’s abdomen to initialize acid-base reaction generating carbon dioxide gas. This gas then inflated the balloon. Upon the balloon was fully inflated, it was retrieved by postmortem to measure the volume as well as visual evaluation.
We further tested the inflation feature of the EndoPil in the first-in-human trial. A healthy volunteer (41 years old, female) with no history of gastrointestinal surgery was recruited for the experiment performed at the Endoscopy Centre, National University Hospital, Singapore. The subject was sedated before the EndoPil was endoscopically inserted to the stomach by a gastroscope (GIF-HQ 190; Olympus Medical System, Tokyo, Japan) and the assistance of a Roth Net retriever (US Endoscopy, Mentor, OH, USA). When the gelatin casing was softened, an external magnet (K&J Magnetics, Inc., PA, USA) was used to trigger the chemical reaction inflating the balloon. The subject was observed throughout the procedure to recognize any side effects. The fully inflated balloon was then punctured by an injection needle (21G NeedleMaster; Olympus Medical System, Tokyo, Japan) and was retrieved by a snare (Olympus Medical System, Tokyo, Japan) through the upper gastrointestinal tract.
The animal study was conducted with prior approval of the Institutional Animal Care and Use Committee (IACUC) of the National University of Singapore. The participant voluntarily signed the informed consent form to take part in the first-in-human trial. The human study protocol was approved by the National Healthcare Group – Domain Specific Review Board (NHG-DSRB, reference number: 2018/00018). The clinical trial was registered at ClinicalTrials.gov (identification number: NCT03760861). Detail experimental procedures can be found in the reference.13
Results
Magnetic Interaction and Valve Opening Force
The relationship between magnetic force and distance of the attraction between the internal magnet and external magnet is illustrated in Fig. 7a. The results of the experiment and simulation are almost identical. When the distance between two magnet increases, the magnetic force between them decreases significantly.
The results of 25 trials (5 inner capsules × 5 times each) provide that the valve opening force of the EndoPil is 0.3537 ± 0.0143 N corresponding with the distance between two magnets is 47.7320 ± 0.7109 mm (Fig. 7b, 7c). In comparison with data from Fig. 7c, extracting data points at 0.3537 N of force, the distance is around 40.7726 mm (14.6% of different) and 41.9582 mm (12.1%) for experiment and simulation, respectively. The reliability testing result showed that 95% (19 out of 20) of the final EndoPil was successfully inflated and fully deployed the balloon to about 120 mL within approximately 6 min. The average inflation distance between the two magnets is 47.37 mm. Observation showed that all inflated balloons had no abnormal appearance, and no air leakage was found during the under-water test.
Animal and Human Study
In the non-survival animal study, the EndoPil was successfully placed in the porcine stomach. The gelatin casing was softened after 1 min, enabling the folded balloon to be inflated easily. The chemical reaction occurred almost instantly after the external magnet was applied, and about 6 min was required to obtain the fully inflated balloon. The retrieved balloon had a volume of 120 mL and was examined with no leakage. In the clinical human trial, the endoscopic procedure was successfully carried out to deliver the EndoPil into the subject’s stomach. The balloon was fully inflated within 3 min from activation (Fig. 8). Observation had shown that there is no abnormal symptom happened, and vital signs of the subject were normal during the experiment. The subject did not report any discomfort or adverse event during and after the procedure.
Discussion
Existing intragastric balloons normally require endoscopic or catheter assistance, which refrains its wide acceptance by the patients. The ideal device would be swallowable, actuated without intervention, effectively deploy an IGB inside patients’ stomach with no limit on the power supply, automatically deflated by biodegradable material or manually by magnetic control, and naturally excreted out of the patients’ body. Follow that treatment procedure, we proposed a new magnetically actuated capsule—EndoPil that is comprised of a hard-sell capsule containing two biocompatible reactants to produce carbon dioxide gas when triggering by an external magnetic field and a new balloon made by a food-grade, low permeability material. The whole device is encapsulated inside an outer gelatin casing to form a final product with an overall dimension of 13 mm in diameter and 35 mm in length. The EndoPil has a simple structure, most of the components are easy to produce massively by industrial processing, resulting in high reliability (95%) and low cost, which are the two key selling points for any devices to pursue further development and commercialization. Biocompatibility is a critical aspect of a medical device, and it always is our first consideration when developing the EndoPil. The inner capsule is made by a medical-grade polycarbonate—Makrolon® 2458, the two reactants are used in the EndoPil also the biocompatible chemicals both before and after the reaction, the balloon is fabricated from a thin film EVOH which is a food-grade material with excellent low permeability to retain CO2 for a relatively long period, and the outer casing is produced from gelatin—a popular material for commercial medical capsule productions.
The assistant of endoscope or catheter in the IGB treatment procedure can be eliminated thanks to the magnetically actuated mechanism inside the EndoPil. Inflating the balloon inside the patient’s stomach by an external magnet is simple, easy, reliable, and straightforward without the limitation of power supply and safety concerns related to on-board electronic components. This type of remote control without intervention offers the comfort treatment alternate and minimize the risks of complication to the patient. The inner magnet of the EndoPil also plays the role of a signal emitter allowing its position can be tracked in real-time by a magnetic tracking system. This will provide the physician with information on where the device is inside the patient body, then the inflation process will be occurred at the desire location—the stomach, thus increasing the safety aspect of the treatment procedure. The current EndoPil required a magnetic force around 0.3537 N to open the inflation valve to inflate the balloon. Our available external magnet achieved this value at approximately 47.7320 mm. The distance requirement may vary for each patient, but it should not be a problem because larger external magnets can be used if needed. The activation mechanism EndoPil has been validated through the bench-top tests, in-vivo animal and human trials. The magnetic-actuated inflation mechanism can desirably generate a 120 mL balloon within 3 to 6 min. Furthermore, the safety of inflating a balloon inside the human stomach by an external magnet was demonstrated by the fact that the human subject did not suffer any adverse event during and after the experiment.
The placement of IGBs to treat obesity has several limitations, including the risk of bowel obstruction, complications, or even death; the weight loss effect reduces after balloon removal; and the treatment procedure expenditures. This study has not investigated the potential risks of esophageal obstruction on patients with medically complicated obesity when swallowing the EndoPil, and the possible complications of bowel obstruction caused by the deflated balloon. Our next steps will focus on reducing the device’s dimension, integrating deflation features into the capsule either by magnetic control or a biodegradable plug, adding safety indicators (detect by urine color) to detect undesirable balloon deflation for timely retrieval, developing the magnetic tracking device to detect the device position, and investigating a new method to manufacturing the balloon. We will also study the potential risks of esophageal and bowel obstruction. Subsequently, we will demonstrate the entire weight loss treatment procedure from swallowing to excreting on humans without endoscope or catheter intervention. Long-term trials also need to be conducted to prove the safety and effectiveness of the EndoPil.
In conclusion, we have introduced the design concept and prototype of the EndoPil—a magnetically actuated capsule that can be swallowed, thus eliminate the discomfort and complication of endoscopic or catheter assistance to the patient, and provide a tether-free intragastric balloon for obesity treatment. The experiments were successfully conducted on both animal and human to demonstrate the safety and feasibility of inflating a balloon inside the biological stomach by an external magnet. With further development, we believe that this device will gain widespread acceptance, consequently alleviate the global epidemic of obesity.
References
Abarca-Gómez, L. Worldwide trends in body-mass index, underweight, overweight, and obesity from 1975 to 2016: a pooled analysis of 2416 population-based measurement studies in 128.9 million children, adolescents, and adults. The Lancet 390:2627–2642, 2017.
Abu Dayyeh, B. K., S. A. Edmundowicz, S. Jonnalagadda, N. Kumar, M. Larsen, S. Sullivan, C. C. Thompson, and S. Banerjee. Endoscopic bariatric therapies. Gastrointest. Endosc. 81:1073–1086, 2015.
Alsabah, S., E. Al Haddad, S. Ekrouf, A. Almulla, S. Al-Subaie, and M. Al Kendari. The safety and efficacy of the procedureless intragastric balloon. Surg. Obes. Relat. Dis. 14:311–317, 2018.
Brooks, J., E. D. Srivastava, and E. M. H. Mathus-Vliegen. One-year adjustable intragastric balloons: results in 73 consecutive patients in the UK. Obes. Surg. 24:813–819, 2014.
Chang, S.-H., C. R. T. Stoll, J. Song, J. E. Varela, C. J. Eagon, and G. A. Colditz. The effectiveness and risks of bariatric surgery: an updated systematic review and meta-analysis, 2003-2012. JAMA Surg. 149:275–287, 2014.
Consideration of the evidence on childhood obesity for the Commission on Ending Childhood Obesity: report of the ad hoc Working Group on Science and Evidence for Ending Childhood Obesity, Geneva, Switzerland. Geneva: World Health Organization, 2016, p. 218.
De Peppo, F., R. Caccamo, O. Adorisio, E. Ceriati, P. Marchetti, A. Contursi, A. Alterio, C. Della Corte, M. Manco, and V. Nobili. The Obalon swallowable intragastric balloon in pediatric and adolescent morbid obesity. Endosc. Int. Open 5:E59–E63, 2017.
Do, T. N., P. T. Phan, K. Y. Ho, and S. J. Phee. A magnetic soft endoscopic capsule for non-surgical overweight and obese treatments. In: 2016 IEEE/RSJ International Conference on Intelligent Robots and Systems (IROS), pp. 2388–2393, 2016.
Do, T. N., T. E. Seah, K. Y. Ho, and S. J. Phee. Development and testing of a magnetically actuated capsule endoscopy for obesity treatment. PLoS ONE 11:e0148035, 2016.
Elleaume, P., O. Chubar, and J. Chavanne. Computing 3D magnetic fields from insertion devices. In: Proceedings of the 1997 Particle Accelerator Conference (Cat. No. 97CH36167), 1997, pp. 3509–3511 vol. 3503.
Fernandes, M. A. P., Á. Atallah, B. Soares, H. Saconato, S. M. Guimarães, D. Matos, L. R. Carneiro Monteiro, and B. Richter. Intragastric balloon for obesity. Cochrane Database Syst. Rev., 2007.
Fuller, N. R., S. Pearson, N. S. Lau, J. Wlodarczyk, M. B. Halstead, H.-P. Tee, R. Chettiar, and A. J. Kaffes. An intragastric balloon in the treatment of obese individuals with metabolic syndrome: a randomized controlled study. Obesity 21:1561–1570, 2013.
Kaan, H. L., P. T. Phan, A. M. H. Tiong, M. Miyasaka, S. J. Phee, and K. Y. Ho. First-in-man feasibility study of a novel ingestible magnetically inflated balloon capsule for treatment of obesity. Endosc. Int. Open 8:E607–E610, 2020.
Kencana, A. P., M. Rasouli, V. A. Huynh, E. K. Ting, J. C. Y. Lai, Q. D. Q. Huy, S. L. Tan, K. J. Wong, and S. J. Phee. An ingestible wireless capsule for treatment of obesity. In: 2010 Annual International Conference of the IEEE Engineering in Medicine and Biology, 2010, pp. 963–966.
Kuraray. Brochures / Kuraray - EVAL™.
Lecumberri, E., W. Krekshi, P. Matía, C. Hermida, N. G. de la Torre, L. Cabrerizo, and M. Á. Rubio. Effectiveness and safety of air-filled balloon heliosphere BAG® in 82 consecutive obese patients. Obes. Surg. 21:1508–1512, 2011.
Look AHEAD Research Group. Eight-year weight losses with an intensive lifestyle intervention: the look AHEAD study. Obesity 22:5–13, 2014.
Lopez-Nava, G., I. Bautista-Castaño, A. Jimenez-Baños, and J. P. Fernandez-Corbelle. Dual intragastric balloon: single ambulatory center spanish experience with 60 patients in endoscopic weight loss management. Obes. Surg. 25:2263–2267, 2015.
Maes, C., W. Luyten, G. Herremans, R. Peeters, R. Carleer, and M. Buntinx. Recent updates on the barrier properties of ethylene vinyl alcohol copolymer (EVOH): a review. Polym. Rev. 58:209–246, 2018.
Marinos, G., C. Eliades, V. Raman Muthusamy, and F. Greenway. Weight loss and improved quality of life with a nonsurgical endoscopic treatment for obesity: clinical results from a 3- and 6-month study. Surg. Obes. Relat. Dis. 10:929–934, 2014.
Mui, W. L.-M., E. K.-W. Ng, B. Y.-S. Tsung, C. H. Lam, and M. Y. Yung. Impact on obesity-related illnesses and quality of life following intragastric balloon. Obes. Surg. 20:1128–1132, 2010.
Palmisano, S., M. Silvestri, B. Melchioretto, M. Giuricin, F. Giudici, A. Lucchetta, V. P. Barbieri, E. Osenda, F. Urban, C. Simeth, F. Monica, and N. de Manzini. Intragastric balloon device: weight loss and satisfaction degree. Obes. Surg. 26:2131–2137, 2016.
Pham, D. M., and S. M. Aziz. A real-time localization system for an endoscopic capsule using magnetic sensors. Sensors 14:20910–20929, 2014.
Phee, S. J. L., T. N. Do, T. E. T. Seah, and K. Y. Ho. Intragastric device for weight management. 2018.
Sehyuk, Y., and M. Sitti. Shape-programmable soft capsule robots for semi-implantable drug delivery. IEEE Trans. Robot. 28:1198–1202, 2012.
Yan, L., T. Wang, D. Liu, J. Peng, Z. Jiao, and C.-Y. Chen. Capsule robot for obesity treatment with wireless powering and communication. IEEE Trans. Ind. Electron. 62:1125–1133, 2015.
Yanovski, S. Z., and J. A. Yanovski. Long-term drug treatment for obesity: a systematic and clinical review. JAMA 311:74–86, 2014.
Acknowledgments
The study is funded by the Gastroenterology Research Fund in Singapore (NUS WBS No. R-172-000-004-720) and the Startup Grant from Nanyang Technological University, Singapore (NTU WBS No. M4081419).
Conflict of interest
The authors have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Jane Grande-Allen oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 1 (MOV 38224 kb)
Rights and permissions
About this article
Cite this article
Phan, P.T., Tiong, A.M.H., Miyasaka, M. et al. EndoPil: A Magnetically Actuated Swallowable Capsule for Weight Management: Development and Trials. Ann Biomed Eng 49, 1391–1401 (2021). https://doi.org/10.1007/s10439-020-02692-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02692-w