Abstract
Brain’s micro-structure plays a critical role in its macro-structure material properties. Since the structural anisotropy in the brain white matter has been introduced due to axonal fibers, considering the direction of axons in the continuum models has been mediated to improve the results of computational simulations. The aim of the current study was to investigate the role of fiber direction in the material properties of brain white matter and compare the mechanical behavior of the anisotropic white matter and the isotropic gray matter. Diffusion tensor imaging (DTI) was employed to detect the direction of axons in white matter samples, and tensile stress-relaxation loads up to 20% strains were applied on bovine gray and white matter samples. In order to calculate the nonlinear and time-dependent properties of white matter and gray matter, a visco-hyperelastic model was used. The results indicated that the mechanical behavior of white matter in two orthogonal directions, parallel and perpendicular to axonal fibers, are significantly different. This difference indicates that brain white matter could be assumed as an anisotropic material and axons have contribution in the mechanical properties. Also, up to 15% strain, white matter samples with axons parallel to the force direction are significantly stiffer than both the gray matter samples and white matter samples with axons perpendicular to the force direction. Moreover, the elastic moduli of white matter samples with axons both parallel and perpendicular to the loading direction and gray matter samples at 15–20% strain are not significantly different. According to these observations, it is suggested that axons have negligible roles in the material properties of white matter when it is loaded in the direction perpendicular to the axon direction. Finally, this observation showed that the anisotropy of brain tissue not only has effects on the elastic behavior, but also has effects on the viscoelastic behavior.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Traumatic brain injury is one of the primary causes of disability and death in the world.38 In order to reliably predict the mechanical response of brain tissue in different injury conditions such as car crashes46,51 sport,17,33 and blast,34,48 finite element (FE) models including accurate geometry and material properties of brain tissue are needed.18
The advent of new neuroimaging techniques such as diffusion tensor imaging (DTI) has provided anatomical details of brain microstructure. DTI is a magnetic resonance imaging (MRI) technique based on the motion of water molecules. In conventional MRI, the white and gray matter of brain tissue appear relatively homogeneous, while in DTI, white matter tracts are visualized. There is an analogy between the shape of an ink drop spreading on a paper and the motion of water molecules in a tissue. If the ink distribution is circular, it is an isotropic diffusion, and if it is spread in an elliptical shape with significant preference to one direction, the diffusion is considered anisotropic. In DTI, this anisotropic diffusion is attributed to the fibrous structure.40 Since the introduction of DTI in the mid-1990s,6 many studies have aimed to extract the axonal fiber orientation of brain tissue from both fresh and formalin-fixed specimens,4,8,12,31,49 and have shown that DTI of live and fixed brain tissue displays similar results.40
Many studies have attempted to determine the material properties of different brain regions through in-vivo and ex-vivo experiments.3,27,39,44 While most of these studies have shown that the gray matter of brain tissue has an isotropic structure,23,43,54 there is not a clear consensus about brain white matter.10,12,13,43,52 Arbogast and Margulies measured viscoelastic properties of the swine brainstem under shear loading in three axonal fiber directions and showed the brainstem displays transversely isotropic behavior.2 Feng et al. investigated the stiffness of the porcine corpus callosum in two orientations, parallel and perpendicular to the axons, using an indentation method. The results exhibited that the corpus callosum is a transversely isotropic material and the stiffness in the perpendicular direction is significantly higher than the parallel direction.22 Samadi-Dooki et al. showed that the stiffness of bovine white matter varies in the horizontal, sagittal, and coronal planes through indentation loading.47 Shuck and Advani studied the mechanical properties of human brain tissue under shear, and showed that white matter may be considered as an isotropic material.52 Budday et al. studied the effect of fiber direction on the mechanical behavior of human white matter under compression and tension up to 10% strain. The results revealed no significant direction-dependent responses,12 and suggested that although white matter is an anisotropic structure due to the presence of axons, this anisotropy did not present itself mechanically.10 However, this observation may be justified by the assumption proposed by Murphy that at infinitesimal strains, anisotropic materials could be considered as an isotropic material.41 As Bayly et al. using a tagged MRI method showed that human brain under impact condition could experience strains higher than 20%,7 it seems that investigating the effect of axonal fiber direction on the mechanical properties of brain tissue for strains higher than 10% is necessary.
The choice between material isotropy and anisotropy could lead to significantly different predictions by computational models. As some recent studies have used DTI results to implement fiber orientation of brain tissue into FE models,26,28,29,60 there is a growing interest in finding the effect of axonal fibers on the mechanical behavior of brain tissue. The present study aimed to investigate the mechanical anisotropy of brain white matter using a continuum approach, compare the mechanical behavior of white matter with that of gray matter, and suggest a microstructural explanation for the existing diversity about white matter anisotropy.
Materials and Methods
Diffusion Tensor Imaging
Three bovine brains were acquired from a local slaughterhouse and all the experimental tests took place within 5 h post-mortem. MRI including T1-weighted imaging and DTI was performed on fresh bovine brains to explore the orientations of axonal fibers. During imaging process, all brains were immersed in 0.01 M phosphate buffer saline (PBS) solution while kept in polypropylene (PP) containers to reduce noise artifacts. Images were obtained with a 3T scanner (Magnetom PRISMA, Siemens Healthcare, Erlangen, Germany) using a head coil array with 32 elements in 12 directions at National Brain Mapping Lab (NBML) in Iran, and the setting was similar to Budday et al.12 The results of DTI were processed and visualized using ExploreDTI software package,36 and orientation distribution of axons in brainstem was characterized by OrientationJ plugin (ImageJ, National Institute of Health, MD).
Sample Preparation for Mechanical Testing
Based on the results of DTI, brainstem samples were extracted in two orthogonal directions, with axons parallel (θ = 0°) and perpendicular (θ = 90°) to the loading direction (Fig. 1a). Fifteen cylindrical bovine brain samples, with diameter of 12.73 ± 1.04 mm and height of 10.23 ± 1.41 mm, were extracted using a metal punch from fresh brain specimens from cerebral cortex (gray matter) and brainstem (white matter), respectively. The samples were extracted in such an order that there was at least one sample from each brain specimen in each group. The samples were kept in PBS solution at room temperature (~ 23 °C).
Experimental Setup
Each excised sample has gone under uniaxial tensile stress-relaxation loading using a uniaxial universal testing machine (Instron 5566, Instron Co., Norwood, MA, USA) up to 20% strain with loading rate of 150 mm/min and 30 s hold time (Fig. 1b). The samples were attached to the upper and lower plates of the test device using cyanoacrylate adhesive (Fig. 1c). This would ensure the confined boundary conditions during the loading period.
Constitutive Modeling
The mechanical behavior of gray matter and white matter was modeled using quasi-linear viscoelastic (QLV) theory defined as25:
where Pe(λ1) is the instantaneous elastic function, λ1 is the stretch ratio, and G(t) is the reduced relaxation function in the form of a Prony series:
where ηi is the decay rate and three decay rates of 0.1, 1, and 10 s−1 were chosen with respect to ramp and relaxation time.35 The instantaneous elastic function for Pe(λ1) in Eq. (1) was calculated using an Ogden-type hyperelastic model including isotropic and anisotropic parts for describing the mechanical behavior of extra-fibrillar matrix and fibers, respectively37:
where Ψ is the strain energy density functions (SEDF), λ1, λ2, λ3 are the principal stretch ratios, μ is the shear modulus of isotropic material, k is a coefficient which indicates the increase of material stiffness in the fiber direction, and α and β are material parameters associated with the material nonlinearity. Previous publications suggested that the nonlinear behavior of the material is not sensitive to the direction, therefore we can assume α = β.37I4 is a pseudo-invariant that is related to fiber direction (θ)32:
Under uniaxial tension in one direction (λ1), deformation gradient tensor can be described as:
Assuming white matter as an incompressible material and due to symmetry (λ2 = λ3), the determinant of tensor F is equal to 1:
Based on the SEDF, first Piola–Kirchhoff stress tensor P was derived from54:
where u(i) and v(i) are eigenvectors of the right and left stretch tensors, respectively.54
The visco-hyperelastic model contains eight material parameters, of which the fiber directions (θ) in white matter samples were determined using the DTI. According to the range of – 5 < α < 5 proposed by Velardi et al. for brain tissue,54α can be determined using trial-and-error approach.61 The remaining parameters, i.e., μ, k, and G’s can be obtained by curve fitting. To obtain the best fit to the experimental data, the least-squares method as a minimization algorithm was used. This method minimized the sum of squared errors (SSE):
where n is the number of sets of pairs i.e. the total number of measurements in the corresponding time series.
Results
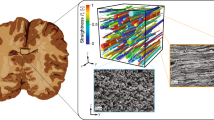
Figure 2 is the Red–Green–Blue (RGB) color-coded fractional anisotropy (FA) image of a bovine brain tissue. Green color shows nerve fiber tracts running in the anterior–posterior direction, red color shows fiber tracts running in the left–right direction, and blue color shows the superior–inferior direction. The analysis of the DTI scans showed that most of axonal fibers in the sagittal plane of brainstem are aligned with the angle of 0.5°, and the median of the axonal direction is 0° (Fig. 2).
The results of the mechanical testing showed that all white matter and gray matter samples displayed strain-softening behavior, shown in Fig. 3. In the range of 0–15% strains, the incremental elastic moduli (E1, E2, and E3) of white matter samples with axons parallel (WM0) to the loading direction are significantly more than gray matter (GM) and white matter samples with axons perpendicular (WM90) to the loading direction. Figure 3 also reveals that the incremental elastic moduli from 15 to 20% strains (E4) did not show significant differences between gray and white matter samples.
A representative experimental stress time history and the fitted model to the white matter samples with axons parallel and perpendicular to the loading direction and gray matter samples are shown in Fig. 4. The corresponding parameters of the Ogden-type visco-hyperelastic constitutive model are reported in Table 1. The results showed that the shear moduli of isotropic material (μ) for white matter samples with axons parallel and perpendicular to the loading direction and gray matter samples are not significantly different (p < 0.05). The value of parameter k is significantly higher for samples with axonal fibers parallel to the loading direction compared with those perpendicular to the loading direction (p < 0.05), which means axonal fibers in parallel direction are stiffer than in perpendicular direction (kμWM0 > kμWM90). Also, the nonlinearity (α) of white matter samples with axons parallel and perpendicular to the loading direction is less than that of the gray matter samples (Table 1).
Discussion
The mechanical anisotropy of brain white matter has been subjected to discussion for a long time and several studies. The aim of the present study was to investigate the effect of axons directions on the mechanical behavior of brain white matter, and define a correlation between the structural anisotropy and mechanical anisotropy. Also, this study aimed to shed light on the existent variations about the mechanical properties of white and gray matters, and propose a new description for the relationship between material properties and the microstructure of white matter and gray matter.
While many previous studies have observed that white matter shows anisotropic behavior,2,22,54 there has been suggestions that the axons orientation has no significant contribution in the mechanical behavior of the white matter.10,12,52 In this study, the analysis of DTI scans showed that most of axonal fibers in brainstem are aligned in one direction (Fig. 2). Therefore, the brainstem was considered as a transversely isotropic material and tested in parallel and perpendicular directions to the axonal fibers.
The results showed that up to 15% strain, the stiffness values of white matter samples with axons parallel to the loading direction are more than samples perpendicular to the fibers (Fig. 3), which is in agreement with the study of Yousefsani et al.58 On the other hand, Dennerll et al. showed that the lateral stiffness of axons is less than the axial stiffness by applying lateral and axial tensile displacement using a needle to measure mechanical properties of a single axon (Fig. 5).16 Therefore, the higher stiffness of white matter tissue parallel to axonal fibers in comparison to the samples perpendicular to the axons could be attributed to the differences between lateral and axial stiffness of the axonal fibers (Fig. 6). In future studies, multiscale assessments should be followed by more multiscale modeling to improve our knowledge about the contribution of axonal fibers in the mechanical behavior of nervous tissue.
Displacement methods and viscoelastic responses of the axons to the (a) lateral and (b) axial tensions.16
Also, our results showed that up to 15% strain, the stiffness values of gray matter samples and white matter samples perpendicular to the axons are not significantly different. Wu et al. have expressed that the material properties of the isotropic ground substrate of white matter are the same as gray matter.57 Therefore, it could be suggested that axons in perpendicular direction have negligible roles in the material properties of white matter. On the other hand, brain tissue in comparison to collagenous tissues has an insignificant amount of extracellular matrix (ECM), and most of the volume of brain tissue is composed of different cell types (glial cells and neurons).11 As white matter has a high cell-to-ECM volume ratio42 and the vast majority of glial cells in white matter are astrocytes,62 it could be hypothesized that the white matter stiffness calculated in perpendicular direction is mostly affected by the stiffness of astrocytes.
The results of elastic moduli calculated using strass-strain data showed that with increasing the strain levels, the stiffness values of gray matter samples and white matter samples with axons parallel and perpendicular to the loading direction decrease (Fig. 3), which is in agreement with the results presented by previous works. They have reported that brain tissue exhibits strain-softening under tension and strain-stiffening under compression.19,53 Moreover, some previous studies showed that the stiffness of white matter is higher than gray matter9,24,55 and this difference is attributed to the contribution of axons in the mechanical properties of the tissue,47,59 but few studies have shown that gray matter is stiffer than white matter15,30 or their stiffness is equal.14,20
The results of the present study indicated that white matter samples with axons parallel to the loading direction were significantly stiffer than gray matter, while the stiffness of white matter samples with axons perpendicular to the loading direction and gray matter samples was not significantly different. Most previous studies have not been determined the axonal orientations using precise methods such as DTI or histological staining, and they have tested white matter samples randomly without considering axonal fiber directions. Therefore, our findings may explain the diversity observed among the previous works.
The results of this study have also showed that the stiffness of gray matter and white matter samples decreases as the strain increases and at about 15–20% strains, the values are not significantly different (Fig. 3). As it has been shown that the injury threshold for the brain tissue is ~ 18% strain,5,13,56 and the injury alters the material properties of the brain tissue,1,21,45,50 this similarity between the stiffness of gray matter and white matter samples could be attributed to the effect of injury on the changes in the mechanical properties of the tissue. However, investigating the recoverability of this softening behavior could help to verify the suggested hypothesis. While Budday et al. examined the recoverability of the softening behavior for brain tissue samples under cyclic loading with 10% strain, and concluded that the mechanical behavior of brain tissue after 60 min is recoverable,12 they did not study the recoverability of brain tissue for strains close to the injury threshold that is highly recommended for future works.
Moreover, the results displayed that relaxation parameters of white matter samples with axons parallel and perpendicular to the loading direction are significantly different (Table 1). This observation showed that the anisotropy of brain tissue not only has effects on the elastic behavior, but also has effects on the viscoelastic behavior. According to these results, it could be suggested that different fiber orientation may lead to different behaviors in sub-structural components such as sliding fibers on each other, and in turn alter the viscoelastic responses. However, these results are obtained at a certain strain and strain rate, and a general conclusion requires more data from different applied strain and strain rate. Also, using real-time microscopy methods to detect the changes in the microstructure caused by mechanical loading and its effect on the viscoelastic response is recommended for future researches.
In the current study, the orientation of axonal fibers in bovine white matter was determined precisely using DTI method. The uniaxial tensile stress-relaxation tests were applied to bovine white matter samples with axons parallel and perpendicular to the loading direction and gray matter samples, and the mechanical properties were examined using an Ogden-type visco-hyperelastic model. The results showed that the mechanical properties of white matter is highly dependent to the direction of the fibers, and tissue stiffness in the parallel direction is significantly higher than perpendicular direction. Also, the results indicated that the mechanical behavior of white matter samples with axons perpendicular to the loading direction and gray matter samples are similar. According to these observations, it is suggested that structural anisotropy caused by axons is similar to other biological fibers such as collagen and elastin, and could contribute in mechanical properties of the tissue. Therefore, it could be concluded that structural anisotropy caused by axons may lead to the mechanical anisotropy, and considering brain white matter as an anisotropic tissue in future brain modeling is strongly recommended. Moreover, this study has investigated the relaxation behavior of the tissue under 20% tensile strain, therefore the changes in the value of different parameters of the visco-hyperelastic model according to the changes of strain could not be calculated. Accordingly, we propose that the variation in the visco-hyperelastic parameters with respect to the strain value should be examined in the future studies.
References
Alfasi, A. M., A. V. Shulyakov, and M. R. Del Bigio. Intracranial biomechanics following cortical contusion in live rats. J. Neurosurg. 119:1255–1262, 2013.
Arbogast, K. B., and S. S. Margulies. Material characterization of the brainstem from oscillatory shear tests. J. Biomech. 31:801–807, 1998.
Atay, S. M., C. D. Kroenke, A. Sabet, and P. V. Bayly. Measurement of the dynamic shear modulus of mouse brain tissue in vivo by magnetic resonance elastography. J. Biomech. Eng. 130:021013, 2008.
Auhustinack, J. Direct visualization of the perforant pathway in the human brain with ex vivo diffusion tensor imaging. Front. Hum. Neurosci. 4:1–13, 2010.
Bain, A. C., and D. F. Meaney. Tissue-level thresholds for axonal damage in an experimental model of central nervous system white matter injury. J. Biomech. Eng. 122:615–622, 2000.
Basser, P. J., J. Mattiello, and D. LeBihan. MR diffusion tensor spectroscopy and imaging. Biophys. J. 66:259–267, 1994.
Bayly, P. V., E. E. Black, R. C. Pedersen, E. P. Leister, and G. M. Genin. In vivo imaging of rapid deformation and strain in an animal model of traumatic brain injury. J. Biomech. 39:1086–1095, 2006.
Berns, G. S., P. F. Cook, S. Foxley, S. Jbabdi, K. L. Miller, and L. Marino. Diffusion tensor imaging of dolphin brains reveals direct auditory pathway to temporal lobe. Proc. R. Soc. B Biol. Sci. 282:20151203, 2015.
Budday, S., R. Nay, R. de Rooij, P. Steinmann, T. Wyrobek, T. C. Ovaert, and E. Kuhl. Mechanical properties of gray and white matter brain tissue by indentation. J. Mech. Behav. Biomed. Mater. 46:318–330, 2015.
Budday, S., T. C. Ovaert, G. A. Holzapfel, P. Steinmann, and E. Kuhl. Fifty shades of brain: a review on the mechanical testing and modeling of brain tissue. Arch. Comput. Methods Eng. 2019. https://doi.org/10.1007/s11831-019-09352-w.
Budday, S., M. Sarem, L. Starck, G. Sommer, J. Pfefferle, N. Phunchago, E. Kuhl, F. Paulsen, P. Steinmann, V. P. Shastri, and G. A. Holzapfel. Towards microstructure-informed material models for human brain tissue. Acta Biomater. 104:53–65, 2020.
Budday, S., G. Sommer, C. Birkl, C. Langkammer, J. Haybaeck, J. Kohnert, M. Bauer, F. Paulsen, P. Steinmann, E. Kuhl, and G. A. Holzapfel. Mechanical characterization of human brain tissue. Acta Biomater. 48:319–340, 2017.
Carlsen, R. W., and N. P. Daphalapurkar. The importance of structural anisotropy in computational models of traumatic brain injury. Front. Neurol. 6:1–6, 2015.
Chatelin, S., J. Vappou, S. Roth, J. S. Raul, and R. Willinger. Towards child versus adult brain mechanical properties. J. Mech. Behav. Biomed. Mater. 6:166–173, 2012.
Christ, A. F., K. Franze, H. Gautier, P. Moshayedi, J. Fawcett, R. J. M. Franklin, R. T. Karadottir, and J. Guck. Mechanical difference between white and gray matter in the rat cerebellum measured by scanning force microscopy. J. Biomech. 43:2986–2992, 2010.
Dennerll, T. J., P. Lamoureux, R. E. Buxbaum, and S. R. Heidemann. The cytomechanics of axonal elongation and retraction. J. Cell Biol. 109:3073–3083, 1989.
Elkin, B. S., L. F. Gabler, M. B. Panzer, and G. P. Siegmund. Brain tissue strains vary with head impact location: a possible explanation for increased concussion risk in struck versus striking football players. Clin. Biomech. 64:49–57, 2019.
Eskandari, F., M. Shafieian, M. M. Aghdam, and K. Laksari. A knowledge map analysis of brain biomechanics: current evidence and future directions. Clin. Biomech. 75:105000, 2020.
Eskandari, F., M. Shafieian, M. M. Aghdam, and K. Laksari. Tension strain-softening and compression strain-stiffening behavior of brain white matter. Ann. Biomed. Eng. 2020. https://doi.org/10.1007/s10439-020-02541-w.
Feng, Y., E. H. Clayton, Y. Chang, R. J. Okamoto, and P. V. Bayly. Viscoelastic properties of the ferret brain measured in vivo at multiple frequencies by magnetic resonance elastography. J. Biomech. 46:863–870, 2013.
Feng, Y., Y. Gao, T. Wang, L. Tao, S. Qiu, and X. Zhao. A longitudinal study of the mechanical properties of injured brain tissue in a mouse model. J. Mech. Behav. Biomed. Mater. 71:407–415, 2017.
Feng, Y., C. H. Lee, L. Sun, S. Ji, and X. Zhao. Characterizing white matter tissue in large strain via asymmetric indentation and inverse finite element modeling. J. Mech. Behav. Biomed. Mater. 65:490–501, 2017.
Feng, Y., R. J. Okamoto, R. Namani, G. M. Genin, and P. V. Bayly. Measurements of mechanical anisotropy in brain tissue and implications for transversely isotropic material models of white matter. J. Mech. Behav. Biomed. Mater. 23:117–132, 2013.
Finan, J. D., S. N. Sundaresh, B. S. Elkin, G. M. McKhann, and B. Morrison. Regional mechanical properties of human brain tissue for computational models of traumatic brain injury. Acta Biomater. 55:333–339, 2017.
Fung, Y.-C. Biomechanics: Mechanical Properties of Living Tissues. New York: Springer, 1993. https://doi.org/10.1007/978-1-4757-2257-4.
Garimella, H. T., and R. H. Kraft. Modeling the mechanics of axonal fiber tracts using the embedded finite element method. Int. J. Numer. Methods Biomed. Eng. 33:e2823, 2017.
Gefen, A., and S. S. Margulies. Are in vivo and in situ brain tissues mechanically similar? J. Biomech. 37:1339–1352, 2004.
Giordano, C., and S. Kleiven. Connecting fractional anisotropy from medical images with mechanical anisotropy of a hyperviscoelastic fibre-reinforced constitutive model for brain tissue. J. R. Soc. Interfaces 11:20130914, 2014.
Giordano, C., S. Zappalà, and S. Kleiven. Anisotropic finite element models for brain injury prediction: the sensitivity of axonal strain to white matter tract inter-subject variability. Biomech. Model. Mechanobiol. 16:1269–1293, 2017.
Green, M. A., L. E. Bilston, and R. Sinkus. In vivo brain viscoelastic properties measured by magnetic resonance elastography. NMR Biomed. 21:755–764, 2008.
Guilfoyle, D. N., J. A. Helpern, and K. O. Lim. Diffusion tensor imaging in fixed brain tissue at 7.0 T. NMR Biomed. 16:77–78164, 2003.
Holzapfel, G. A. Nonlinear Solid Mechanics II. New York: Wiley, 2000.
Ji, S., W. Zhao, Z. Li, and T. W. McAllister. Head impact accelerations for brain strain-related responses in contact sports: a model-based investigation. Biomech. Model. Mechanobiol. 13:1121–1136, 2014.
Laksari, K., S. Assari, B. Seibold, K. Sadeghipour, and K. Darvish. Computational simulation of the mechanical response of brain tissue under blast loading. Biomech. Model. Mechanobiol. 14:459–472, 2015.
Laksari, K., M. Shafieian, and K. Darvish. Constitutive model for brain tissue under finite compression. J. Biomech. 45:642–646, 2012.
Leemans, A., B. Jeurissen, J. Sijbers, and D. K. Jones. ExploreDTI: a graphical toolbox for processing, analyzing, and visualizing diffusion MR data. Proc. Int. Soc. Magn. Reson. Med. 17:3537, 2009.
Meaney, D. F. Relationship between structural modeling and hyperelastic material behavior: application to CNS white matter. Biomech. Model. Mechanobiol. 1:279–293, 2003.
Meaney, D. F., B. Morrison, and C. Dale BassDale Bass. The mechanics of traumatic brain injury: a review of what we know and what we need to know for reducing its societal burden. J. Biomech. Eng. 2014. https://doi.org/10.1115/1.4026364.
Miller, K., K. Chinzei, G. Orssengo, and P. Bednarz. Mechanical properties of brain tissue in-vivo: experiment and computer simulation. J. Biomech. 33:1369–1376, 2000.
Mori, S., and J. Zhang. Principles of diffusion tensor imaging and its applications to basic neuroscience research. Neuron 51:527–539, 2006.
Murphy, J. G. Evolution of anisotropy in soft tissue. Proc. R. Soc. A Math. Phys. Eng. Sci. 2014. https://doi.org/10.1098/rspa.2013.0548.
Perepelyuk, M., L. Chin, X. Cao, A. van Oosten, V. B. Shenoy, P. A. Janmey, and R. G. Wells. Normal and fibrotic rat livers demonstrate shear strain softening and compression stiffening: a model for soft tissue mechanics. PLoS ONE 11:e0146588, 2016.
Prange, M. T., and S. S. Margulies. Regional, directional, and age-dependent properties of the brain undergoing large deformation. J. Biomech. Eng. 124:244–252, 2002.
Prevost, T. P., G. Jin, M. A. de Moya, H. B. Alam, S. Suresh, and S. Socrate. Dynamic mechanical response of brain tissue in indentation in vivo, in situ and in vitro. Acta Biomater. 7:4090–4101, 2011.
Qiu, S., W. Jiang, M. S. Alam, S. Chen, C. Lai, T. Wang, X. Li, J. Liu, M. Gao, Y. Tang, X. Li, J. Zeng, and Y. Feng. Viscoelastic characterization of injured brain tissue after controlled cortical impact (CCI) using a mouse model. J. Neurosci. Methods 330:108463, 2020.
Sahoo, D., C. Deck, and R. Willinger. Brain injury tolerance limit based on computation of axonal strain. Accid. Anal. Prev. 92:53–70, 2016.
Samadi-Dooki, A., G. Z. Voyiadjis, and R. W. Stout. An indirect indentation method for evaluating the linear viscoelastic properties of the brain tissue. J. Biomech. Eng. 139:061007, 2017.
Sarvghad-Moghaddam, H., A. Rezaei, M. Ziejewski, and G. Karami. Correlative analysis of head kinematics and brain’s tissue response: a computational approach toward understanding the mechanisms of blast TBI. Shock Waves 27:919–927, 2017.
Schmierer, K., C. A. M. Wheeler-Kingshott, P. A. Boulby, F. Scaravilli, D. R. Altmann, G. J. Barker, P. S. Tofts, and D. H. Miller. Diffusion tensor imaging of post mortem multiple sclerosis brain. Neuroimage 35:467–477, 2007.
Shafieian, M., K. K. Darvish, and J. R. Stone. Changes to the viscoelastic properties of brain tissue after traumatic axonal injury. J. Biomech. 42:2136–2142, 2009.
Shi, L., Y. Han, H. Huang, J. Davidsson, and R. Thomson. Evaluation of injury thresholds for predicting severe head injuries in vulnerable road users resulting from ground impact via detailed accident reconstructions. Biomech. Model. Mechanobiol. 2020. https://doi.org/10.1007/s10237-020-01312-9.
Shuck, L. Z., and S. H. Advani. Rheological response of human brain tissue in shear. J. Basic Eng. 94:905–911, 1972.
van Oosten, A. S. G., X. Chen, L. Chin, K. Cruz, A. E. Patteson, K. Pogoda, V. B. Shenoy, and P. A. Janmey. Emergence of tissue-like mechanics from fibrous networks confined by close-packed cells. Nature 573:96–101, 2019.
Velardi, F., F. Fraternali, and M. Angelillo. Anisotropic constitutive equations and experimental tensile behavior of brain tissue. Biomech. Model. Mechanobiol. 5:53–61, 2006.
Weickenmeier, J., R. de Rooij, S. Budday, P. Steinmann, T. C. Ovaert, and E. Kuhl. Brain stiffness increases with myelin content. Acta Biomater. 42:265–272, 2016.
Wright, R. M., and K. T. Ramesh. An axonal strain injury criterion for traumatic brain injury. Biomech. Model. Mechanobiol. 11:245–260, 2012.
Wu, T., A. Alshareef, J. S. Giudice, and M. B. Panzer. Explicit modeling of white matter axonal fiber tracts in a finite element brain model. Ann. Biomed. Eng. 47:1908–1922, 2019.
Yousefsani, S. A., A. Shamloo, and F. Farahmand. Nonlinear mechanics of soft composites: hyperelastic characterization of white matter tissue components. Biomech. Model. Mechanobiol. 19:1143–1153, 2020.
Zhang, W., L. Liu, Y. Xiong, Y. Liu, S. Yu, C. Wu, and W. Guo. Effect of in vitro storage duration on measured mechanical properties of brain tissue. Sci. Rep. 8:1247, 2018.
Zhao, W., and S. Ji. White Matter Anisotropy for Impact Simulation and Response Sampling in Traumatic Brain Injury. J. Neurotrauma 36:250–263, 2019.
Zhu, Z., C. Jiang, and H. Jiang. A visco-hyperelastic model of brain tissue incorporating both tension/compression asymmetry and volume compressibility. Acta Mech. 230:2125–2135, 2019.
Zlomuzica, A., S. Binder, and E. Dere. Gap Junctions in the Brain. New York: Elsevier, 2013. https://doi.org/10.1016/b978-0-12-415901-3.00001-3.
Acknowledgments
The authors wish to acknowledge the National Brain Mapping Lab (NBML), Tehran, Iran, for providing services for DTI acquisition and thank Fargol R. Araghi and Farzaneh Eskandari for assistance in mechanical testing.
Funding
Authors have not received any funding for this research.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Joel Stitzel oversaw the review of this article.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Eskandari, F., Shafieian, M., Aghdam, M.M. et al. Structural Anisotropy vs. Mechanical Anisotropy: The Contribution of Axonal Fibers to the Material Properties of Brain White Matter. Ann Biomed Eng 49, 991–999 (2021). https://doi.org/10.1007/s10439-020-02643-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-020-02643-5