Abstract
This study analyzed the postural control of older adults on inclined surfaces, and was conducted in 17 elderly adults and 18 young adults of both genders. Ground reaction forces and moments were collected using two AMTI force platforms, one of which was in a horizontal position (HOR), while the other was inclined 14° in relation to the horizontal plane. Each participant executed three 70 s-trials of bipedal standing with their eyes open and eyes closed in three inclination conditions: the HOR, the inclined position at ankle dorsi-flexion (UP), and the inclined position at ankle plantar-flexion (DOWN). Spectral analysis, global (mean velocity-Velm, ellipse area-Area and F80), and structural stabilometric descriptors (sway density curve–SDC, detrended fluctuation analysis–DFA, sample entropy-SEn) were employed to assess the center of pressure sway. Velm and F80 were greater for the elderly, whereas SDC, DFA, and SEn were smaller for this group. Global, SDC and DFA variables were sensitive to visual deprivation, however the relative difference from the EO to EC condition was higher in young than in elderly. The DOWN condition was more stable than the UP condition for both young and older adults. With regard to the UP condition, the challenge observed is essentially associated with the corresponding biomechanical constraints. In conclusion, the elderly showed significant differences compared to the young, but age per se may not necessarily result in compromised postural control.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Aging is associated with a decreased ability to maintain body posture and a decline of the functions of the postural control system,13 leading to reduced functional mobility, social and physiological impairments and an increased risk of fall.9 The major causes of decreased postural stability in the elderly are a loss of sensory information and a decrease in muscle strength, although these constraints alone respond to a small decrease in stability.28
Inclined surfaces represent a common postural challenge in human daily activities, especially to elderly people. Some authors have employed inclined surfaces to study the effects on trunk kinematics,27 walking strategies,18 and postural control in a quiet standing task.11,19
Previous studies of postural control on inclined surfaces have found higher spectral amplitude of anterior-posterior (AP) center of pressure (COP) sway of young healthy individuals at lower frequencies in an upward condition (UP, ankle dorsi-flexion), an indicative of instability, whereas in a downward condition (DOWN, ankle plantar-flexion) higher amplitudes at higher frequencies.19 These results possibly indicate changes in both high frequency components superimposed on a low frequency components of COP sway power spectra.6 Furthermore, eyes closure provoked an increase in the entire COP power spectra in all inclination conditions, markedly in UP condition.19 However, Mezzarane and Kohn19 have enrolled only young adults and analyzed only AP COP sway, and Garkavenko et al. 11 have enrolled subjects in a wide range of age that, in some aspects, compromise the results. These studies have focused mainly on the spectral analysis of COP sway, and the interpretation of other variables extracted from COP time series can added valuable information about the postural control system in such conditions. Besides, Garkavenko et al. 11 have used relatively short trials (20 s) that may have not produced reliable results.33
The control of upright stance involves a complex integration of somatosensory, visual, and vestibular inputs, and can be assessed by center of pressure (COP) sway using different descriptors. In the present study, global COP descriptors, and structural2 stabilometric variables related to sway density curve (SDC), detrended fluctuation analysis (DFA), and entropy, were computed. Global COP descriptors, such as velocity, power spectrum, and area, quantify COP sway assuming that it is a stationary signal, and give few insights into the control of posture, since they are just summary statistics and ignore the dynamic characteristics of COP movement.20 However, they have been extensively used in postural control studies, and they were computed for a comparison with previous studies. On the other hand, SDC analysis extracts the time structure of COP sway and relies on the direct relation between COP position and ankle torque from the biomechanics of the inverted pendulum model,2 providing a physiological interpretation of COP movement. DFA and entropy are nonlinear techniques that quantify the regularity of a time series providing a measure of the complexity of the corresponding physiological system. System complexity has been considered as reflecting the ability to generate adaptive responses to perturbations.14 In this sense, aging has been associated to a progressive loss of complexity.20
The present study used inclined surfaces to assess the postural control of elderly people, changing the proprioceptive input by exposing flexor or extensor muscle spindles around the ankle joint to different lengths that result in different spindle output. Furthermore, visual condition was manipulated to assess the efficiency of feedback loops based on spindle afferents and sensory reweighting to compensate for the absence of vision,19 since older adults seem to have difficulties in sensory reweighting.10 Vision has a key role on postural control whose inputs accurately represent the orientation of the environment and provide a useful reference to central nervous system (CNS).16 Such combined manipulation provides useful information regarding the integration of different sensory inputs.
Therefore, we aimed to identify changes in the postural control of elderly people during a quiet standing task under a challenging postural condition, with and without visual feedback. Thus, stabilometric variables were computed from force platforms, such as global COP descriptors, SDC, DFA, and entropy. We hypothesized that the posture control of elderly adults on inclined surfaces would be less effective than on horizontal surfaces, especially in the UP condition, since this position imposes more pronounced biomechanical constraints than DOWN condition, such as increased and uncomfortable ankle plantar-flexion muscle stretching, and approximately the maximal ankle dorsi-flexion for older adults in the chosen inclination.4
Methods
Participants and Ethics Statement
Seventeen community-dwelling elderly adults of both genders (9 males, 8 females: age, 70.1 ± 3.2 years; weight, 66.41 ± 3.81 kg; height, 1.52 ± 0.21 m) and 18 young adults of both genders as a control group (10 males, 8 females: age, 24.7 ± 3.9 years; weight, 71.4 ± 14.2 kg; height, 1.71 ± 0.09 m) participated in the study. All participants were healthy, had no musculoskeletal injury or pain, were capable of walking independently, and had minimal hearing and visual impairments at the time of data collection. Prior to participation, they voluntarily signed an informed consent form approved by the institutional Ethics Committee for Human Research.
Experimental Procedure
Ground reaction forces and moments were collected using two AMTI force platforms (AccuGait model) operating at 100 Hz, one of which was in a horizontal position while the other was inclined 14° in relation to the horizontal plane, an inclination used in previous studies 19 and approximately the maximal ankle dorsi-flexion for older adults.4 Each participant executed three trials of bipedal standing with eyes open (EO) and eyes closed (EC) in three inclination conditions: the horizontal inclination (HOR), the inclined position at ankle dorsi-flexion (UP), and the inclined position at ankle plantar-flexion (DOWN).
During each trial, the participants stood barefoot on the force platform for 70 s with their feet positioned at a comfortable distance from one another (within shoulder width) and with their arms alongside their body, and looked at a fixed eye-level target at a distance of 1.5 m, adjusted for height. No specific instructions were given in the inclined conditions, meaning that the participants were free to find their most comfortable orientation with regard to gravity. The experimental conditions were randomly assigned to each participant, and a resting period of 30 s between trials was permitted.19
Data Analysis
The data were filtered using a fourth-order, zero-lag, low-pass Butterworth filter, with a cut-off frequency of 12.5 Hz. The first 10 s of each acquisition were discarded to avoid transients.19
Global stabilometric descriptors, and SDC, DFA, and sample entropy (SEn) were employed to compute features from the COP displacement, in both the anterior-posterior (AP) and medial–lateral (ML) directions, except in the case of SDC. The calculated values for each stabilometric descriptor were averaged from the three trials to improve reliability. A custom-written Matlab code was used to process the data and to calculate the descriptors.
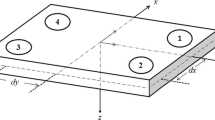
For the inclined force platform, the reference frame of the force platform itself was considered, rather than that associated with the gravitational vector. Thus, the COP was computed over the surface of the inclined force platform and not the projection of the COP sway on the horizontal plane (Fig. 1).19
Global Stabilometric Descriptors
The computed global stabilometric descriptors included the elliptical area (Area) that encompassed 95% of the COP samples in 60 s, which was calculated using the prediction ellipse area concept,26 and the COP mean velocity (Velm).31
The power spectrum of the COP displacement obtained in each trial was estimated using the Welch method, with 2000 samples per periodogram, which resulted in a spectral resolution of 0.05 Hz. The procedure also included the use of a Hann window, an overlap of 1000 samples, and the subtraction of the best linear regression on each data window.19 The F80 power frequency, which encompasses 80% of the area under the COP power spectrum, was then estimated.
Structural Stabilometric Descriptors
Sway Density Curve
The SDC2 is defined as the time-dependent curve that counts the number of consecutive COP samples falling within a circle with a 2.5 mm radius for each instant of time. The peaks of the resulting curve correspond to time instants of posture stabilization, while the valleys correspond to shifts between the stabilization events. The SDC analysis is an attempt to recognize anticipatory control strategies, by identifying data subsets within COP signal and interpreting them as instants in which the anticipatory command is stable.2
Three SDC-related stabilometric parameters quantitatively describe the process of generating sequences of postural commands: MP, the mean amplitude of the peaks, which is an estimate of the degree of postural stability; MD, the mean distance between one peak and another, which corresponds to the amplitude of torque required for stabilization; and MT, the mean time interval between one peak and another, which is related to the rate of torque production.2 Normalization of SDC with respect to the sampling frequency provides a time dimension to MP, which thus represents the mean time spent by the COP inside the defined circle.
Detrended Fluctuation Analysis
DFA is based on a classic root-mean square analysis of a random walk process,30 and was used to assess the presence of long-range correlations in the COP time series. In brief, the integrated time series of length N is divided into intervals of length τ without overlapping, and its corresponding mean is subtracted. In each interval of length τ, a least squares line, fitted to the data, is subtracted. The root-mean-squared fluctuation of this integrated and detrended time series is calculated. This computation is repeated over all interval sizes to characterize the relationship between F(τ), the average fluctuation, and the interval size τ. In the present study, we have used intervals ranging from 12 to N/4 data points.14
A behavior F(τ) ≈ τ α is expected where the scaling exponent α can be extracted by the slope of the adjusted line in the graph log(F(τ)) vs. log(τ), indicating whether the signal is antipersistent, persistent, or white noise.12 When α < 0.5, the signal shows negative correlations (antipersistent), whereas α > 0.5 characterizes positive correlations (persistent). An exponent α = 0.5 characterizes a non-correlated random series (white noise).30
Sample Entropy
The SEn quantifies the regularity or predictability of a time series, and provides a complexity index, with typical values ranging between 0 and 214,22 Lower values indicate a more regular or predictable time series and smaller complexity of the corresponding system, whereas higher values indicate a less regular time series.22
The directionality of SEn and DFA values are opposed, i.e. lower SEn values indicate more regular behavior, whereas lower DFA values indicate more random behavior.
The SEn corresponds to the negative natural logarithm of the conditional probability that two m-dimensional delayed vectors that are close within a tolerance r, remain close in the (m + 1)-dimensional state space, without allowing self-matches.
In the present study, we estimated SEn for the standardized differenced data to account for the non-stationarity and correlations of COP data, and because SEn calculation from COP differenced data is insensitive to sampling frequency and noise.23 We used an adapted practical solution22 for choosing the best parameters of the SEn method. We found r = 0.2*data standard deviation and m = 3 for both AP and ML directions.
Statistical Analysis
A mixed repeated measures analysis of variance (GLM 5) was applied to assess the effects of group, position, and eyes conditions, followed by Bonferroni post hoc tests, for all variables with a normal distribution (Global, SDC, and DFA, Shapiro–Wilk test, p > 0.05). The variables that did not exhibit a normal distribution (SEn) were tested using a nonparametric Friedman design test, followed by a post hoc analysis using the Wilcoxon signed-rank test with a Bonferroni correction applied, and a nonparametric U-Mann–Whitney test for group comparisons.
All statistical analyses were performed using Statistical Package for the Social Sciences software, version 17 (SPSS Inc., Chicago, IL), with a significance level set at p < 0.05.
Results
Global Stabilometric descriptors
There was a significant main effect of the inclination for AP Velm (F(2,66) = 15.120, p < 0.001, η 2 = 0.314), ML Velm (F(2,66) = 15.814, p < 0.001, η 2 = 0.324), and AP F80 (F(2,66) = 11.953, p < 0.001, η 2 = 0.266). There was no significant interaction effect between inclination conditions and age (F(2,66) = 1.321, p = 0.273, η 2 = 0.038, F(2,66) = 1.199, p = 0.297, η 2 = 0.035, F(2,66) = 2.724, p = 0.073, η 2 = 0.076, for AP Velm, ML Velm and AP F80, respectively) indicating that both groups have similar behavior (Fig. 2).
The participants exhibited a dependence on vision with significant main effect for AP Velm (F(1,33) = 86.315, p < 0.001, η 2 = 0.723), ML Velm (F(1,33) = 28.611, p < 0.001, η 2 = 0.464) and AP F80(F(1,33) = 35.709, p < 0.001, η 2 = 0.52), with increased values in the EC condition. There was no significant interaction effect between eye conditions and age (F(1,33) = 1.120, p = 0.298, η 2 = 0.033, F(1,33) = 0.089, p = 0.768, η 2 = 0.003, F(1,33) = 0.298, p = 0.589, η 2 = 0.009, for AP Velm, ML Velm and AP F80, respectively) indicating that both groups have similar behavior (Fig. 2).
There was significant effect of age for AP Velm (F(1,33) = 10.938, p = 0.002, η 2 = 0.249) and AP F80 (F(1,33) = 30.806, p < 0.001, η 2 = 0.483) (Table 1, bold values; Fig. 2a, c) with increased values for older adults.
In the HOR condition, the Velm results in both the EO and EC conditions were very similar to those previously reported.7,32 AP Velm variables were higher in the DOWN position, whereas ML Velm variables in the UP position.
Spectral Analysis
The COP AP power spectral density (PSD) showed a steady decrease, starting at 0.05 Hz in both EO and EC conditions for all inclination conditions, with higher power values in the EC condition along the frequency range for all inclination conditions (Fig. 3). With regard to young adults, the COP AP PSD was very similar to that reported in previous studies,19 and we will focus on COP PSD characteristics of older adults.
At frequencies above 0.35 Hz, the power of the AP COP signal in position DOWN was higher than in HOR and UP in the EO condition, in accordance with the significantly higher AP F80 in the DOWN position (p < 0.05). In the DOWN-EO condition, AP F80 increased by 24.5% compared to the HOR-EO condition. The combined effect of force platform position and deprivation of vision added 26.5% to the AP F80 of the HOR-EO condition compared to the DOWN-EC condition. This was not an additive effect, since the increase observed when comparing HOR-EO to HOR-EC was 12.2%.
The AP COP power at frequencies below 0.2 Hz was higher in the HOR position in the EO condition, compared to the UP and DOWN positions. At a frequency of 0.1 Hz, participants deprived of visual input increased the power by approximately 36% compared to the EO condition. A corresponding 6.7% decrease was observed when comparing the HOR-EO to the UP-EO conditions. However, the cumulative effect of inclination and eye condition increased the power by 27% when comparing HOR-EO to UP-EC, which was again different from an additive effect.
The results presented above are qualitatively similar to those obtained with young adults.19 However, although the COP sway power spectra above 0.3 Hz and F80 were higher for older adults, as previously reported,32 especially in inclined positions, this was not the case at below 0.3 Hz: COP low frequencies components are higher for young adults. Furthermore, the COP power spectra differences among inclination conditions are lower in the EC condition for older than for younger adults.
In the ML direction, the COP PSD showed a steady decrease, starting at 0.05 Hz in both the EO and EC conditions for all inclination conditions, with higher power values in the EC condition at frequencies from 0.05 to 0.5 Hz (Fig. 4). For older adults, the COP power spectra differences between the EO and EC conditions were not so evident at frequencies ranging from 0.5 to 0.9 Hz. However, at the lowest frequencies, young adults exhibited a higher power spectrum than older adults. In the ML direction, the COP power was higher in position UP than in the HOR and DOWN positions in both the EO and EC conditions, especially for older adults. The COP power spectra in the ML direction was lower than in the AP direction for the entire frequency range, indicating lower COP sway amplitude, for both young and older adults.
SDC Descriptors
With regard to SDC descriptors, there was significant main effect of inclination for MD (F(2,66) = 8.744, p < 0.001, η 2 = 0.209) and MP (F(2,66) = 18.070, p < 0.001, η 2 = 0.354). There was significant interaction effect between inclination conditions and age only for MT (F(2,66) = 4.828, p = 0.017, η 2 = 0.128), with larger effect for young adults (Fig. 5).
With regard to eye conditions, there was significant main effect for MD (F(1,33) = 59.011, p < 0.001, η 2 = 0.641) and MP (F(1,33) = 29.768, p < 0.001, η 2 = 0.474). The increased MD values and decreased MP values in the EC conditions indicate a significant dependence on vision and more demands to postural control system. There was significant interaction effect between eyes conditions and age only for MP (F(1,33) = 6.911, p = 0.013, η 2 = 0.173), with larger effect for young adults (Fig. 5).
There was significant effect of age for MT (F(1,33) = 9.171, p = 0.005, η 2 = 0.217), MD (F(1,33) = 6.547, p = 0.048, η 2 = 0.187), and MP (F(1,33) = 7.668, p = 0.009, η 2 = 0.189) (Table 2, bold values; Fig. 5). The increased MD values and decreased MP values for older adults indicate decreased postural stability compared to young adults.
DFA and SEn
With regard to DFA, there was significant main effect of inclination for AP DFA (F(2,66) = 18.990, p < 0.001, η 2 = 0.365), but not for ML DFA (F(2,66) = 2.553, p = 0.086, η 2 = 0.072). There was significant interaction effect between inclination conditions and age only for AP DFA (F(2,66) = 6.543, p = 0.003, η 2 = 0.165), with larger effect for older adults (Fig. 6).
With regard to eye conditions, there was significant main effect for AP DFA (F(1,33) = 64.176, p < 0.001, η 2 = 0.660), but not for ML DFA (F(1,33) = 1.899, p = 0.178, η 2 = 0.054). AP DFA was sensitive to eyes conditions in all inclination conditions, with greater values in the EO condition (Table 3). There was no significant interaction effect between eyes conditions and age (F(1,33) = 0.006, p = 0.938, η 2 < 0.001; F(1,33) = 3.399, p = 0.074, η 2 = 0.093 for AP DFA and ML DFA, respectively), indicating that both groups have similar behavior (Fig. 6a, 6b).
There was significant effect of age for AP DFA (F(1,33) = 34.502, p < 0.001), η 2 = 0.511) (Table 3, bold values; Fig. 6). The AP DFA values were lower than the ML DFA values. All DFA α coefficients were greater than 1.0, characterizing a random walk behavior with positive correlations (persistent) in the COP time series.
The SEn results were similar to the DFA results. There were significant differences among inclination conditions in AP SEn (Young: χ 2 = 24.111, p < 0.001; χ 2 = 16.333, p < 0.001; Elderly: χ 2 = 12.824, p = 0.002; χ 2 = 13.059, p = 0.001, EO and EC, respectively) and ML SEn (Elderly: χ 2 = 9.882, p = 0.007, only EC).
AP SEn was sensitive to eyes conditions in the HOR (p = 0.039) and DOWN (p = 0.025) conditions, whereas ML SEn was sensitive to eyes conditions in the UP (p = 0.031) and DOWN (p = 0.044) conditions. The SEn values were greater in the ML direction than in the AP direction, and were greater in the EC condition than in the EO condition.
Furthermore, for older adults, the values were highest in the UP condition, followed by the DOWN condition and then the HOR condition. Significant differences between groups (Table 3, bold values) were found for AP SEn in HOR/EO (U = 87.00, p = 0.029, r = 0.368), DOWN/EO (U = 66.00, p = 0.003, r = 0.485), and DOWN/EC (U = 75.00, p = 0.009, r = 0.435) conditions.
Qualitatively, the same results were found for the young adults. However, AP DFA and AP SEn were higher for the younger than the older adults (Table 3, bold values), whereas ML DFA was higher for the older adults.
Discussion
The present study assessed the effects of inclined surfaces and vision deprivation on the postural control of elderly adults, using global and structural stabilometric descriptors. Global descriptors numerically express the magnitude of sway patterns, either in a time or frequency domain, whereas structural descriptors identify sub-units in the stabilometric data and attempt to relate these to the underlying motor control process.2 In the latter descriptors, we included SDC parameterization and nonlinear descriptors as DFA and SEn.
Effects of Inclined Surfaces
When comparing the different surface conditions, Velm_AP, Velm_ML, and F80_AP showed significant differences, with higher values on inclined surfaces. Moreover, Velm_AP and F80_AP were higher for elderly adults than for young adults. Similar results have previously been found with regard to the HOR condition.7,19 For elderly adults, the Velm value was higher in the UP position, followed by the DOWN position, whereas for young adults, DOWN position values were higher than UP condition values. According to Prieto et al.,21 this variable can quantify the relationship between the activity of the postural control system and the stability achieved by the individual. Therefore, the fact that the highest values were found in the UP position suggests that this condition may be challenging for elderly adults, although not for young adults. The stretching of the calf muscle in the UP condition appears to be more uncomfortable for elderly adults, possibly as a result of increased ankle stiffness in dorsiflexion. Indeed, older adults present reduced range of motion and muscle strength at ankle joint compared to young adults. In UP condition, the chosen inclination, for example, is approximately the maximal ankle dorsi-flexion for older adults (~15°), but, in DOWN condition, it is less than half of maximal ankle plantar flexion.4
However, the F80_AP results were similar in the two groups, with higher values in the DOWN condition, although higher values were shown with the elderly adults compared to the young adults. The elderly adults explored their base of support faster in the UP condition (increased Velm), but at higher frequencies in the DOWN condition (increased F80). Higher values of F80_AP for elderly adults likely relates to increased ankle stiffness, since changes in energy distribution toward higher frequencies reflect changes in the ankle torque pattern.2 The increased ankle stiffness may have resulted from ankle muscle modulations rather than a co-contraction strategy.32
Mezzarane and Kohn19 found different spectral characteristics among inclination conditions in young adults. They suggested that such differences could be related to altered sensory inputs, such as muscle and cutaneous mechanoceptors. Other factors that could also potentially contribute are the biomechanical constraints imposed by the inclined surface. In our study, we obtained qualitatively similar results. With the exception of frequencies below 0.15 Hz, the COP sway power spectra and F80 were higher for elderly than for young adults, as previously reported,32 especially in inclined positions, reinforcing the hypothesis of increased ankle stiffness. The lower COP power spectra below 0.15 Hz for elderly adults indicates a decrease of the low frequency component with age, and we did not observe an increase in postural sway in such frequencies in the UP condition, as has previously been reported for young adults.19 Mezzarane and Kohn19 simulated a simple biomechanical model based on Maurer and Peterka,17 including the representation of passive muscle stiffness, and showed that increases in passive stiffness led to a decrease in low frequency postural sway, as observed for older adults in the present study. Again, such findings reinforce the hypothesis of increased ankle stiffness with age.
The sway features in the DOWN position are different from those in the UP condition in a particular manner. Supposing the existence of two processes of postural control, the lower spectral data at frequencies below 0.3 Hz in the DOWN condition, compared to the UP condition, suggest that long-term mechanisms acting on the control of slow postural sway would be more efficient in the DOWN position, which is similar to what has been found for young adults.19
The increase of AP COP sway high frequency band in the DOWN and EO conditions (but not in the EC condition) can be attributed to increased soleus activation that, in turn, may represent more frequent postural corrections, leading to a decrease in the slow postural sway.19
In the ML direction, the COP power spectra were very similar in the elderly and the young adults. However, the differences between EO × EC conditions were smaller for elderly adults. This suggests that elderly adults use proprioception rather than visual cues for postural control, as previously reported.34 even on inclines.
There were lower MP values in the UP < DOWN < HOR positions, indicating poorer stability of both groups in the UP position, with lower values observed for the elderly adults. The capacity of this variable to identify differences between different situations was similar to that found previously,2 and it was also capable of identifying differences between groups.
Baratto et al. 2 associated SDC results to two physiological mechanisms working together: feedback due to the intrinsic mechanical properties of ankle muscles, and anticipatory muscle activations determined by an internal inverted pendulum model. The SDC attempts to identify the motor control actions hidden in the COP signals. On the basis of the interpretations of Baratto et al., 2 higher MP values and lower MD values indicate improved stability and smaller amplitudes of posturographic commands, respectively. Thus, our results indicate that UP was the most challenging position, and that the elderly exhibit less postural stability than the young, requiring larger torques (higher MD) and a higher rate of torque production (lower MT). Again, besides the hypothesis of increased ankle stiffness with age,32 the reduced ankle range of motion and muscle strength for older adults compared to younger adults can explain our findings.4
Significant differences were observed using DFA when comparing different inclination conditions. DFA modified its scale properties, and the results are very similar to those obtained using SEn. Amoud et al. 1 identified differences in postural stability between young and old individuals using DFA, whereby older adults exhibited lower DFA values in the AP direction and higher DFA values in ML direction than the young adults. Similarly, Norris et al. 20 found a significantly lower α exponent in the ML direction in a group of elderly people compared to young people, but no differences in AP direction. However, DFA was capable of identifying significant differences among the three inclination conditions in the present study, with the highest values being observed for the HOR position, followed by the UP condition, and then the DOWN condition. Older adults exhibited smaller DFA_AP values, and, although not significant, greater DFA_ML values.
The DFA coefficient α > 0.5 indicates long-term correlations. Values of α larger than 0.5 indicate a persistent time series with a low variability.1 All DFA values observed in both the AP and the ML directions for all inclination conditions showed α > 1.0. This may be indicative of smoother signals with high correlation. Our results are consistent with previous studies7,30 in which were found exponents α > 1.0 for the AP and ML directions.
Higher SEn values were observed for the young compared to the elderly in DOWN condition, and SEn was sensitive to both inclination and eyes conditions, with increased values for the UP > DOWN > HOR conditions and for EC x EO for the elderly. Moreover, the ML values were greater than the AP values. The elderly exhibited a discrete loss of COP signal complexity. These results can be interpreted as showing that there is more effective postural control in DOWN position, possible due to less biomechanical constraints.
Effects of Visual Deprivation
In general, Velm_AP, Velm_ML, and F80_AP showed higher values in the EC condition. The relative increase of Velm_AP from the EO to EC condition was higher in young (~41%) than in older adults (~34%) in all inclination conditions, in accordance with previous findings.3,32 Contrasting results were found for F80_AP: the relative increase from the EO to EC condition was lower in young adults (~8%) than in older adults (~17%) in the HOR and UP conditions, but not in the DOWN condition (37% for the young adults and 4% for the older adults). Together, these findings suggest increased ankle stiffness in older adults in the EC condition, possibly as a result of ankle muscle modulations, since the results were observed in all inclination conditions. However, similar changes in F80 from EO to EC were also observed in the young adults, ruling out the exclusive contribution of age-dependent mechanisms to compensate for visual deprivation.
Furthermore, the COP power spectra differences between the EO and EC conditions were similar in the AP direction in both groups, and smaller in the ML direction for the elderly adults than for the young adults. Moreover, the increase of Velm and MD in the EC condition indicates higher torque level and neuromuscular activity. A possible explanation for this is the diminished accuracy of modulations in the ankle torque for both groups when standing with eyes closed.15
There was an increase in the MD when comparing EO x EC conditions, whereas the MP values were lower in the EC x EO conditions. These results are in accordance with those of previous studies.32 However, the relative decrease/increase of MP/MD from the EO to EC condition was higher in young (~−41 and ~48%, respectively) than in older adults (~−25 and ~29%, respectively) in all inclination conditions. These results ruled out again the exclusive contribution of age-dependent mechanisms to compensate for visual deprivation. Vieira et al. 32 suggested that these findings indicate a slight decrease in postural stability in the elderly, and high levels of torque, which could be a result of the reduction of modulation accuracy at the ankle torque when the individual remains standing with EC.
There were no differences between the EO × EC conditions for the young in SEn, which is in accordance with the results of a previous study.25 However, with regard to the elderly, our results contrast with those found in some previous studies,22 in which SEn was lower in the EC condition, but are in accordance with those observed in others.5,24 There is some evidence to suggest that the elderly favor proprioceptive over visual and vestibular information, compared to the young.34 This could explain our findings. Furthermore, although visual deprivation does not change the complexity of the postural control system in the young,25 it increases the level of muscle activity across the joints of the lower limbs, which produces an increase in the stochastic activity of control mechanisms,8 that, in turn, increases the stiffness in the musculoskeletal system. This effect could be exacerbated with age and explain our findings.
Therefore, there is evidence of a task-dependent change of COP complexity14,29 that is counter to the hypothesis of a universal loss of complexity with aging, supporting the hypothesis that there is a loss of adaptive change in complexity driven by the COP dynamics.14
Limitations of the Study
This study has some limitations that should be noted. Because the data were collected using only force platforms, we could not infer the characteristics of muscle activation in postural control on inclined surfaces. Therefore, it would be of interest for further studies to collect kinematic and electromyography data to analyze the correlation between body segments and muscle activity, as well as to implement biomechanical models to validate the propositions presented here in a theoretical basis.
Conclusions
Increased ankle stiffness and reduced ankle range of motion and muscle strength can explain our findings for older adults. In accordance with previous studies,19 the DOWN condition appears to be more stable and comfortable than the UP condition for both young and older adults. Biomechanical and structural characteristics of the lower limb, such as the posterior location of the ankle joint relative to the foot, may have contributed to this result. With regard to the UP condition, the observed challenge is essentially associated with the corresponding biomechanical constraints, since the chosen inclination is approximately the maximal ankle dorsi-flexion for older adults (~15°) and close to it for young adults.4 Several descriptors were sensitive to visual deprivation, whose changes may be related to increased muscle activity and increased stochastic activity of postural control mechanisms in such condition8,25 and not exclusively to age-dependent mechanisms.
Elderly adults presented significant differences compared to the young, but age per se may not necessarily result in compromised postural control. Such differences can be interpreted as compensation strategies to preserve postural stability.
References
Amoud, H., M. Abadi, D. J. Hewson, V. Michel-Pellegrino, M. Doussot, and J. Duchêne. Fractal time series analysis of postural stability in elderly and control subjects. J. Neuroeng. Rehabil. 4:12, 2007.
Baratto, L., P. G. Morasso, C. Re, and G. Spada. A new look at posturographic analysis in the clinical context: sway-density versus other parameterization techniques. Motor Control 6:246–270, 2002.
Benjuya, N., I. Melzer, and J. Kaplanski. Aging-induced shifts from a reliance on sensory input to muscle cocontraction during balanced standing. J. Gerontol. A. Biol. Sci. Med. Sci. 59:166–171, 2004.
Bok, S. K., T. H. Lee, and S. S. Lee. The effects of changes of ankle strength and range of motion according to aging on balance. Ann. Rehabil. Med. 37:10–16, 2013.
Borg, F. G., and G. Laxåback. Entropy of balance–some recent results. J. Neuroeng. Rehabil. 7:38, 2010.
Carpenter, M. G., J. S. Frank, D. A. Winter, and G. W. Peysar. Sampling duration effects on centre of pressure summary measures. Gait Posture 13:35–40, 2001.
Cavalheiro, G. L., and M. F. S. Almeida. A. a Pereira, and A. O. Andrade. Study of age-related changes in postural control during quiet standing through linear discriminant analysis. Biomed. Eng. Online 8:35, 2009.
Collins, J. J., and C. J. De Luca. The effects of visual input on open-loop and closed-loop postural control mechanisms. Exp. brain Res. 103:151–163, 1995.
Daley, M. J., and W. L. Spinks. Exercise, mobility and aging. Sports Med. 29:1–12, 2000.
Eikema, D. J. A., V. Hatzitaki, D. Tzovaras, and C. Papaxanthis. Age-dependent modulation of sensory reweighting for controlling posture in a dynamic virtual environment. Age (Omaha). 34:1381–1392, 2012.
Garkavenko, V. V., A. V. Gorkovenko, E. V. Kolosova, and V. V. Korneyev. A. V. Mel’nichouk, and D. A. Vasilenko. Modifcations of the stabilogram during upright standing posture under conditions of inclines of the support surface. Neurophysiology 44:131–137, 2012.
Ihlen, E. A. F. Introduction to multifractal detrended fluctuation analysis in matlab. Front. Physiol. 3:141, 2012.
Iosa, M., A. Fusco, G. Morone, and S. Paolucci. Development and decline of upright gait stability. Front. Aging Neurosci. 6:1–12, 2014.
Ko, J. H., and K. M. Newell. Aging and the complexity of center of pressure in static and dynamic postural tasks. Neurosci. Lett. 610:104–109, 2016.
Lakie, M., and I. D. Loram. Manually controlled human balancing using visual, vestibular and proprioceptive senses involves a common, low frequency neural process. J. Physiol. 577:403–416, 2006.
Laurens, J., L. Awai, C. J. Bockisch, S. Hegemann, H. J. A. van Hedel, V. Dietz, and D. Straumann. Visual contribution to postural stability: Interaction between target fixation or tracking and static or dynamic large-field stimulus. Gait Posture 31:37–41, 2010.
Maurer, C., and R. J. Peterka. A new interpretation of spontaneous sway measures based on a simple model of human postural control. J. Neurophysiol. 93:189–200, 2005.
McIntosh, A. S., K. T. Beatty, L. N. Dwan, and D. R. Vickers. A. S. M. Ã, K. T. Beatty, L. N. Dwan, and D. R. Vickers. Gait dynamics on an inclined walkway. J. Biomech. 39:2491–2502, 2006.
Mezzarane, R. A., and A. F. Kohn. Control of upright stance over inclined surfaces. Exp. Brain Res. 180:377–388, 2007.
Norris, J. A., A. P. Marsh, I. J. Smith, R. I. Kohut, and M. E. Miller. Ability of static and statistical mechanics posturographic measures to distinguish between age and fall risk. J. Biomech. 38:1263–1272, 2005.
Prieto, T. E., J. B. Myklebust, R. G. Hoffmann, E. G. Lovett, and B. M. Myklebust. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans. Biomed. Eng. 43:956–966, 1996.
Ramdani, S., B. Seigle, J. Lagarde, F. Bouchara, and P. L. Bernard. On the use of sample entropy to analyze human postural sway data. Med. Eng. Phys. 31:1023–1031, 2009.
Rhea, C. K., T. A. Silver, S. L. Hong, J. H. Ryu, B. E. Studenka, C. M. L. Hughes, and J. M. Haddad. Noise and complexity in human postural control: Interpreting the different estimations of entropy. PLoS One 6:1–9, 2011.
Roerdink, M., M. De Haart, A. Daffertshofer, S. F. Donker, A. C. H. Geurts, and P. J. Beek. Dynamical structure of center-of-pressure trajectories in patients recovering from stroke. Exp. Brain Res. 174:256–269, 2006.
Sasaki, O., S. Usami, P.-M. Gagey, J. Martinerie, M. Le Van Quyen, and P. Arranz. Role of visual input in nonlinear postural control system. Exp. Brain Res. 147:1–7, 2002.
Schubert, P., and M. Kirchner. Ellipse area calculations and their applicability in posturography. Gait Posture 39:518–522, 2014.
Shin, G., and G. Mirka. The effects of a sloped ground surface on trunk kinematics and L5/S1 moment during lifting. Ergonomics 47:646–659, 2004.
Shupert, C. L., and F. B. Horak. Adaptation of postural control in normal and pathologic aging: Implications for fall prevention programs. J. Appl. Biomech. 15:64–74, 1999.
Stins, J. F., M. E. Michielsen, M. Roerdink, and P. J. Beek. Sway regularity reflects attentional involvement in postural control: Effects of expertise, vision and cognition. Gait Posture 30:106–109, 2009.
Teresa Blázquez, M., M. Anguiano, F. A. de Saavedra, A. M. Lallena, and P. Carpena. Study of the human postural control system during quiet standing using detrended fluctuation analysis. Phys. A Stat. Mech. its Appl. 388:1857–1866, 2009.
Vieira, M. F., I. S. de Avelar, M. S. Silva, V. Soares, and P. H. Lobo da Costa. Effects of four days hiking on postural control. PLoS One 10:e0123214, 2015.
Vieira, T. D. M. M., L. F. De Oliveira, and J. Nadal. An overview of age-related changes in postural control during quiet standing tasks using classical and modern stabilometric descriptors. J. Electromyogr. Kinesiol. 19:e513–e519, 2009.
Vieira, T. M. M., L. F. Oliveira, and J. Nadal. Estimation procedures affect the center of pressure frequency analysis. Brazilian J. Med. Biol. Res. 42:665–673, 2009.
Wiesmeier, I. K., D. Dalin, and C. Maurer. Elderly use proprioception rather than visual and vestibular cues for postural motor control. Front. Aging Neurosci. 7:97, 2015.
Acknowledgments
The authors are thankful to governmental agencies CAPES and FAPEG for supporting this study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Thurmon E. Lockhart oversaw the review of this article.
Rights and permissions
About this article
Cite this article
da Costa Barbosa, R., Vieira, M.F. Postural Control of Elderly Adults on Inclined Surfaces. Ann Biomed Eng 45, 726–738 (2017). https://doi.org/10.1007/s10439-016-1718-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1718-z