Abstract
Carbon nanomaterials such as carbon nanotubes and graphene have gained significant interest in the fields of materials science, electronics and biomedicine due to their interesting physiochemical properties. Typically these carbon nanomaterials have been dispersed in polymeric matrices at low concentrations to improve the functional properties of nanocomposites employed as two-dimensional (2D) substrates or three-dimensional (3D) porous scaffolds for tissue engineering applications. There has been a growing interest in the assembly of these nanomaterials into 2D and 3D architectures without the use of polymeric matrices, surfactants or binders. In this article, we review recent advances in the development of 2D or 3D all-carbon assemblies using carbon nanotubes or graphene as nanoscale building-block biomaterials for tissue engineering and regenerative medicine applications.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
A central goal of the field of regenerative medicine is to develop strategies that repair or replace organ or tissue functionality lost due to trauma, disease, age or congenital defects.53 Tissue engineering, considered to be a sub-discipline of regenerative medicine, typically involves any combination of relevant cell types, engineered biomaterials and biochemical factors to (1) promote cell growth to restore normal physiological function, (2) stimulate regeneration of previously irreparable organs or (3) grow organs or tissues ex vivo for surgical implantation.24 Significant advances in the areas of tissue engineering and regenerative medicine hold the potential to address shortage of donor organs and limitations associated with well-established tissue grafting procedures.50
Nanotechnology-based approaches have been explored towards the development of multifunctional biomaterials for tissue engineering that, not only serve as scaffolds for cells and/or growth factors, but also allow monitoring the process of tissue regeneration, controlled delivery of therapeutic (drugs, gene materials or biologics) agents, and/or control over biological processes responsible for organogenesis.80 Carbon nanomaterials such as carbon nanotubes and graphene are one of the most widely researched nanomaterials, and exhibit some remarkable physiochemical properties. A large number of investigations have harnessed some of these properties and explored these nanomaterials for several biomedical imaging and therapeutic applications.10,18,19,29,31,36,38,39,41,42,48,59,79 These studies have been summarized in number of excellent reviews.2,14,31,65 Their potential for tissue engineering and regenerative medicine have also been extensively reviewed.22,26,34,72 These review articles have mainly focused on studies wherein these nanoparticles have been used as pristine or functionalized (covalent or non-covalent functionalization) formulations or when incorporated into polymeric matrices towards the development of composites (porous or non-porous) with enhanced functionalities (e.g. improvement in mechanical properties, stimulus responsive, or allow imaging).
There has been a growing interest in fabricating all-carbon 2D and 3D assemblies employing carbon nanomaterials as building blocks, but without the use of surfactants, polymeric matrices or secondary binder materials.40 Use of polymers or binder materials leads to the encapsulation or coating of nanomaterials that may diminish their interactions with cells or host tissue or change the topography of substrates leading to altered physiochemical properties. Several studies have explored the use of 2D and 3D all-carbon assemblies for materials science or biomedical applications.40,46,58,78 This review summarizes recent advances in the development of all-carbon 2D and 3D materials for tissue engineering and regenerative medicine. We first provide an overview of various methods used for the fabrication of these all carbon structures and discuss the pros and cons of these methods for manufacturing biomedical substrates or implants. Next, we discuss the in vitro and in vivo investigations of these assemblies for potential tissue engineering applications. Finally, we outline the future perspectives of these 2D and 3D all carbon assemblies as biomedical substrates and implants for tissue engineering and regenerative medicine.
Fabrication Methods
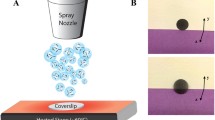
Chemical vapor deposition (CVD), vacuum filtration and dip and spray coating have all been explored in the development of 2D all carbon assemblies as thin films, coatings, and freestanding films for tissue engineering applications.1,55,58,75 CVD is one of the most commonly used techniques. The method involves one or more gaseous carbon precursors that undergoes a high temperature catalytic reaction and/or decomposition to generate carbon nanotubes or graphene.5,30 Although typically grown on metal catalysts, once synthesized these coatings can be transferred onto a variety of substrates. However, to transfer the coating onto other substrates, the substrate must be relatively flat.69 In the case of graphene, this method can produce extremely thin, large area coatings down to one-atom layer thick structures.45 CVD has several limitations such as the need for a thermally stable substrate capable of withstanding high temperatures, presence of metal catalysts that may act as impurities in the final product, and high operational costs. Vacuum filtration, another commonly used method to fabricate carbon nanomaterial coatings, involves filtering of a nanomaterial suspension through a membrane filter followed by air-drying. The film can then be made into a freestanding film by dissolving away the membrane in an organic solvent or peeling away the membrane filter. Vacuum filtration has several advantages such as: (1) the method is simpler than CVD and can create macroscopic free-standing films, (2) the surface roughness is self-correcting; as one region of the film becomes dense, the solvent will follow the path of least resistance and auto-corrects the thickness of the film,76 and (3) the method is flexible for creating films from various carbon nanomaterials based on the desired application.8,13,56 Disadvantages of vacuum filtration include limitations towards building 3D freestanding films due to a high-pressure drop across the filter membrane as a function of increasing film thickness. Additionally, use of organic solvents to degrade methylcellulose ester filters may lead to incorporation of impurities in the final product. Other methods of fabricating carbon nanomaterial coatings include dip- and spray-coating. Dip coating is a process of dipping a pre-treated substrate into a heated liquid dispersion of nanomaterial. This method produces graphene films of thicknesses as low as 30 nm74 or films of thicknesses as low as 12 nm with single walled carbon nanotubes.28 Thicker coatings can be generated by increasing the carbon nanomaterial concentration, repetitive dipping, and by decreasing the rate of removal of the substrate from the dipping solution.52 Dip coating requires the substrate surface to be continuous without bends, like a flat surface or rod, to enable assembly without aggregated nanoparticles in localized regions.68 There are disadvantages of dip coating carbon nanomaterials onto substrates.67 First, it requires the surface to be continuous without bends. Second, the uniformity of the coating depends on the precision of the rate at which the substrate is removed from the nanomaterial dispersion. Also, surface uniformity could be strongly influenced by the stability of the dispersion. If the dispersed nanomaterial begins to aggregate, the dispersion is no longer homogeneous and will not coat the target substrate uniformly.67 In spray coating, an aerosolized dispersion of carbon nanomaterials is sprayed onto a heated substrate to evaporate the carrier solvent and deposit a film of nanomaterials. Spray coating is the most scalable technique of the aforementioned techniques, however; it leads to the fabrication of highly sparse coatings.21 Spray coating can create coatings with highly tailorable thicknesses, conductivity, transmittance, and is versatile for various nanomaterials.21,60
3D all-carbon scaffolds have been fabricated using CVD, template etching, capillary-based self-assembly, and ice-segregation-induced-self-assembly (ISISA) processes for tissue engineering applications.11,12,47,49,64 The CVD method requires a thick (~ 1 mm) porous nickel or copper foil, placed inside the CVD chamber, used as a template for graphene synthesis. After deposition of graphene, the 3D template is etched using FeCl3 followed by treatment with strong acids leaving a 3D porous network of graphene sheets.9,45 In capillary based self-assembly approaches, a thin film of vertically aligned carbon nanotubes is subjected to capillary forces resulting in their assembly into an interlocked network with 3D sieve-like architecture.11 The unidirectional freezing and ISISA process involves the freezing of a solution of nanomaterial followed by freeze-drying to remove trapped ice crystals leaving 3D porous assemblies with shape and size of the freezing container.
While the above methods maybe suitable for deposition of the carbon nanomaterials, these methods do not by themselves ensure the structural integrity of the 2D and 3D assemblies, which is mainly dependent on physical entanglement of the nanomaterials, or weak van der Waals’ forces between the nanomaterials. Thus, these assemblies are prone to dissociation by compressive flexural or shear forces that substrates or implants experience under dynamic physiological conditions. Therefore, recent efforts have focused on strategies that can chemically crosslink these nanomaterials in conjugation with deposition methods.40,58 In this strategy, a radical initiator is mixed with carbon nanomaterials in the presence of a suitable solvent and is either deposited by spray coating or a slurry-mixture is poured into pre-fabricated Teflon molds and subjected to thermal crosslinking. This process leads to the formation of covalent bonds between carbon nanomaterials, which are assembled into macroscopic 2D films or 3D architectures with macro-, micro-, and nano-scale interconnected pores. The formation of covalent bonds between the nanomaterials may ensure good mechanical and structural stability of 3D all-carbon architectures under in vivo conditions. The porosity of 3D architectures can be tailored by varying the amount of radical initiator used during the crosslinking process.
Applications
In conjugation to assessing the suitability of the fabrication method for tissue engineering applications, various studies have also assessed the use of 2D and 3D all-carbon assemblies for tissue engineering applications. Below we summarize the salient results of these studies.
Two Dimensional All-Carbon Coatings or Films
Bone Tissue Engineering Studies
Bone tissue engineering is currently the most widely studied tissue engineering application for 2D carbon nanomaterial coatings. Many researchers have investigated the effects of two dimensional carbon nanomaterial substrates on mature osteoblast proliferation and mineralization as well as stem cell differentiation into osteogenic lineages. Osteoblasts are mature, bone matrix depositing cells responsible for rebuilding resorbed or damaged bone tissue.25 Interfacing native bone tissue with synthetic implant materials; including titanium, stainless steel, and ceramic materials is a key to successful clinical outcomes.63
Modifying the surface characteristics of the implant material by surface roughening and surface coatings can provide a better interface for bone cell adhesion to implant surfaces.4 Surface roughness, chemistry, and mechanical properties of implants have a pronounced effect on bone and stem cell attachment, integration, and differentiation on implant materials.3,15 Some examples of these techniques, which are used in clinic include (a) roughening the surface of the implant for better cell adhesion,4 (b) hydroxyapatite coatings for directing mesenchymal stem cell differentiation,7 and (c) BMP-2 coatings for increased bone formation in vivo. 51 While these techniques have proven to be effective, some of which are used in clinical settings, new materials can provide distinctive advantages to the current gold standards in implant surface characteristics. Carbon nanomaterials, compared to hydroxyapatite coatings and roughened titanium, have sp 2 bonded carbon networks, that could bind or adsorb molecules for osteoconduction or osteoinduction. Both carbon nanotubes and graphene coatings enhance preosteoblast and osteoblast adhesion and mineralization on implant surfaces. Additionally, both graphene and carbon nanotubes show innate osteoinductive properties. These studies are further discussed below.
Wojtek et al. have investigated the effects of carbon nanotube surface properties on preosteoblast attachment and growth in vitro.75 Single walled carbon nanotube (SWCNTs) films, fabricated via vacuum filtration, provided optimum preosteoblast growth when surface roughness of the film was ~100 nm and hydrophilic due to mild -COOH oxidation by nitric acid.75 The preosteoblasts grown on these coatings had >80% initial cell adhesion. Increasing or decreasing the surface roughness of the SWCNT films yielded lower (~10–70%) cell adhesion. Aryaei et al. have shown that graphene films, fabricated by CVD, and transferred onto various substrates (silicon, stainless steel, and soda lime glass), showed no observable toxicity to murine osteoblasts. Furthermore, the authors observed ~148% improvement in cell spreading on graphene coated stainless steel substrates compared to controls.1
Adult multipotent stem cells, sourced from bone marrow (MSCs)61 or adipose tissue (ADSCs),82 can differentiate into many lineages including osteoblasts, chondrocytes, and adipocytes. Both, carbon nanotubes and graphene coatings/films been investigated for their ability to increase osteogenesis in adult stem cells. Kroustalli et al. have studied the adhesion and biocompatibility of MSCs on multiwalled carbon nanotube (MWCNT) substrates fabricated by vacuum filtration.33 LDH assay, ALP quantification and immunofluorescence studies show that MWCNT substrates were cytocompatible and cell attachment was mediated by proteins adsorbing onto the surface allowing for integrin related attachment. MSCs grown on MWCNT substrates exhibit >1.5 fold increases in the expression of integrin β1 and β3, compared to tissue culture polystyrene (TCPS) after 1 day.33
Nayak et al. showed spray coated MWCNT coatings on glass coverslips can accelerate the differentiation of MSCs towards osteoblasts.55 Results show two fold greater expression of osteopontin and approximately five fold greater deposition of calcium (markers of osteogenic differentiation) on MWCNT coated coverslips compared to control glass coverslips. Furthermore, the MWCNT substrates induced equivalent stem cell differentiation, calcium deposition, and osteopontin expression compared to MSCs treated with bone morphogenetic protein-2 (BMP-2), a chemical stimulus for osteogenic differentiation of stem cells.55 In another study, Nayak et al. reported accelerated osteogenic differentiation of stem cells on substrates coated with CVD deposited graphene. Results show significantly greater expression of osteogenic markers and matrix calcium deposition by MSCs cultured on graphene deposited substrates compared to MSCs on substrates without graphene or in the presence of BMP-2.54 Immunofluorescence imaging revealed that MSCs cultured on graphene coated or BMP-2 treated Si/SiO2 substrates, both, expressed differentiation markers integrin-β1 and osteocalcin at Days 4 and 7 (Fig. 1).54 The results of these studies taken together suggest that graphene and carbon nanotube substrates are osteoinductive.
Immunostaining of human mesenchymal stem cells growing on Si/SiO2 substrates coated with graphene or in the presence of BMP-2. (Left) CD-44 expression (stem cell marker) decreased over time and completely disappeared at Day 7, (center) integrin expression (cell adhesion marker) increased over time with peak expression at Day 15, (right) osteocalcin (osteogenic differentiation marker) expressed at Day 4 and increased by Day 7. Scale bars are 100 μm. Adapted from Ref. 54 with permissions, copyright © American Chemical Society, 2011.
Patel et al. have investigated the cytocompatibility of 2D all-carbon films fabricated using multiwalled carbon nanotubes (MWCNTs) as building blocks.58 MWCNTs were deposited on glass coverslips by adapting an air-pressure driven spray-coating method. Radical initiated thermal crosslinking induced the fabrication of covalent bonds between MWCNTs. Adipose derived stem cells (ADSC) cultured on MWCNT substrates for 1, 3 and 5 days showed good cytocompatibility compared to glass coverslip controls. Immunofluorescence studies showed expression of Ki-67 (cell proliferation marker, Figs. 2a and 2b) and scanning electron microscopy (SEM, Figs. 2c and 2f) imaging confirmed cell attachment. ADSCs extended numerous filopodia and cytoplasmic extensions to wrap onto the underlying nanotube mesh (red arrows, Figs. 2c and 2f). These results suggest that all-carbon MWCNT coatings are cytocompatible and can be used as nano-fibrous mats for tissue engineering applications.
(a, b) Representative immunohistochemistry images of adipose derived stem cells cultured on 2D multiwalled carbon nanotube substrates. Cells are stained for Actin (green) and Ki-67 (red, cell proliferation marker). (c–f) Representative scanning electron microscopy images of adipose derived stem cells attaching on multiwalled carbon nanotube substrates after 5 days. Red circles in (c) and (e) are magnified in (d) and (f), respectively with red arrows showing the presence of cytoplasmic extensions and protrusions extending underneath and wrapping the underlying nanotube mesh. Adapted from Ref. 58 with permission. Copyright © Patel et al.
Lee et al. have investigated several factors that may be responsible for the observed accelerated osteogenic differentiation of MSCs on carbon nanotube and graphene coated substrates.44 Results show that carbon nanotubes and graphene adsorb osteogenic factors such as β-glycerophosphate and dexamethasone, present in stem cell culture media. The adsorption of these factors on nanomaterial substrates would lead to a direct interaction of stem cells with osteogenic inducing factors due to increased local contact. In addition to osteogenic differentiation, MSCs and ADSCs can differentiate towards adipogenic lineages. However, carbon nanotubes and graphene coatings induce denaturation of insulin, a potent adipogenic differentiation factor, which may further accelerate osteogenic differentiation of stem cells cultured on carbon nanotube and graphene substrates.44
The electromagnetic properties of carbon nanomaterials can be exploited for tissue engineering applications. Green et al. have reported the use of carbon nanotube enhanced photoacoustic stimulus for osteogenic differentiation of MSCs.23 SWCNT coatings were used as substrates to generate photoacoustic waves, which were then interfaced with MSCs for 1, 5, and 16 days. At day 16, MSCs treated with SWCNT-enhanced photoacoustic stimulus showed a significant increase in calcium deposition (~396% increase) compared to MSCs treated with chemical stimulus.23 Lalwani et al. have shown that graphene nanoparticles can generate stronger photoacoustic signals compared to carbon nanotubes.36 Therefore, future studies should focus on investigating the comparative effect of graphene and carbon nanotube coatings on photoacoustic stimulus-responsive differentiation of stem cells.
Neural Tissue Engineering Studies
Neuronal tissue is one of the most complex ordered structures, and thus, neural tissue engineering is extremely challenging.24 Since neurons are highly organized and connected,20 controlled growth of neurons on carbon nanomaterial coatings is very important to repair damaged neurons and restore normal organizational complexity. Drop-casted MWCNT coatings have shown the ability to host organotypic slice cultures (sectioned, live spinal cord explants) and induce neurite outgrowth.16 Scanning electron microscopy (SEM) images of cells shows the formation of junctions between cell membrane and underlying MWCNT substrate. A 72% increase in glutamatergic postsynaptic currents were observed in spinal cord slice cultures on MWCNT coatings compared to control groups after stimulating the dorsal root ganglion. These results suggest an improved response in sensory neurons upon culture on MWCNT substrates.16 Cellot et al. have reported improvements in neuronal activity and nerve impulse conduction of hippocampal neurons cultured on carbon nanotube substrates.6 Results show that cultured neurons form tight membrane-nanotube contacts and may form nanotube-neuron hybrid units. Theoretical modeling results show that these tight nanotube-membrane contacts may favor electrical shortcuts between distal and proximal compartments of neurons.
Tu et al. studied the effects of surface charge of graphene oxide on neuronal growth and branching.71 Graphene oxide (GO) of various surface charges (negative, neutral, and positive) was spray coated onto polyethylene imine coated glass coverslips and used as substrates for neuronal culture. GO coatings with positive charge showed greater neurite outgrowth, neurite length, neurite count per neuron, neuron cell area, neuronal branches, and neurite branches compared to neurons cultured on neutral or negatively charged graphene oxide substrates.71 Park et al. have reported the ability of CVD deposited graphene films to differentiate neural stem cells into mature neurons.57 To observe if the CVD deposited graphene films would induce neural differentiation, neural stem cell maintaining factors in the cell culture media (βFGF and EGF) were removed after seeding the neural stem cells on the graphene substrate. A 17.4% greater expression of TUJ1 (a tubulin marker for neuronal differentiation) was observed upon culture of cells on graphene coated substrates compared to glass coverslip controls (Fig. 3). Furthermore, the percentage of stem cells differentiating into neurons (39.1%) compared to glial cells (22.9%) on graphene surfaces was greater than neurons (21.7%) to glial cells (34.5%) on glass coverslip controls.57 Immunofluorescence imaging showed that neural stem cells express GFAP, TUJ-1 (neuronal differentiation markers) after 1 month of culture on graphene coatings (Fig. 3b). These results suggest that graphene films/coatings can promote greater neuronal differentiation of neural stem cells compared to glial cells. The enhanced neural differentiation was attributed to increased cell adhesion and upregulated laminin receptors of the neurons on the graphene substrate compared the neurons on the glass substrate.
(a) Brightfield images of human neuronal stem cells differentiated on glass and graphene substrates for 3 days (left), two weeks (middle) and three weeks (right). Note that cells on glass substrates gradually declined in number after 2 weeks whereas on graphene substrates remained stable even after 3 weeks. (b) Brightfield (top) and immunofluorescence images (bottom) of stem cells after 1 month of differentiation. Cells are stained for GFAP (red) for astroglial cells, TUJ1 (green) for neural cells, and DAPI (blue) for nuclei. Note that more neuronal stem cells were adhered to graphene than glass. (c) Cell counting per area after 1 month of differentiation on graphene and glass substrates (n = 5, p < 0.001). (d) Percentage of immunopositive cells for GFAP (red) and TUJ1 (green) on glass and graphene. Note that glass regions show more GFAP-positive cells (glia) than TUJ1-positive ones (neurons), while graphene regions have more TUJ1-positive ones (neurons) than GFAP-positive ones (glia) (n = 5, p < 0.05). Adapted from Ref. 57 with permission, copyright © John Wiley & Sons, 2011.
To further control neuronal growth directionality, several studies have utilized various lithography and patterning methods to fabricate nanomaterial linear or complex patterns of carbon nanotubes or graphene. Hong et al.. have patterned CVD deposited single-layer graphene using a PDMS stamping method to create linear and checkered line patterns of graphene.27 Results show that neurons followed the patterned graphene substrate to create controlled neural networks on 30 μm line patterns without bridging over the non-coated regions. The neurons could controllably and selectively grow on line patterns as low as 5 μm in size.27 These results show that using patterned graphene coated substrates controlled neural networks can be developed. Fan et al. have used super-aligned carbon nanotube yarns, fabricated from CVD grown vertically aligned carbon nanotubes substrates, to directionally guide neurite outgrowth.17 The nanotube yarns (2–5 μm diameter strings) were spaced 30–50 μm apart for controlled neuronal growth. These structures supported average cell density of 140/mm2, similar to cell densities on TCPS dishes. Cultured neurons on carbon nanotube yarns show consistent branching and directionality up to 7 days post seeding (Fig. 4).17 Some challenges faced with patterned carbon nanomaterial substrates includes the lack of neurite branching in regions where carbon nanomaterials are absent.17 Patterning and controlling neuron growth on carbon nanomaterial substrates opens avenues for repair of the highly organized central nervous system.
Fluorescence image showing directional alignment of neurite outgrowth on super aligned carbon nanotube yarn patterned substrates. Adapted from Ref. 17 with permission, copyright © American Chemical Society, 2012.
Other Tissue Engineering Studies
Graphene oxide substrates fabricated by dip coating have shown the ability to differentiate murine C2C12 skeletal myoblasts.35 Skeletal myoblasts cultured on GO coated glass substrates exhibit significantly greater proliferation after 2 and 4 days compared to cells cultured on glass (controls) and reduced GO coated glass coverslips. Myoblasts on GO substrates also showed greater cell area, myotube length, myosin heavy chain staining, and myogenin positive cells compared to other groups (Fig. 5).35 In addition to adult stem cells, carbon nanomaterial substrates have also been studied for their effects on pluripotent stem cell fate.62 Pluripotent stem cells are either collected from embryotic sources73 or induced from adult fibroblasts (iPSCs) by chemical and molecular biology methods.70 Pryzhkova et al. studied the differentiation potential of pluripotent stem cells cultured on carbon nanotubes arrays of varying surface roughness, mechanical properties, and topography. Results show that altering these physiochemical properties resulted in differentiation and growth of the stem cells into different germ layers (ectoderm, mesoderm and endoderm) depending on the surface properties of the nanotubes.73
Myotube formation on unmodified, graphene oxide and reduced graphene oxide coated glass substrates. (a) Immunofluorescence images showing the expression of myosin heavy chain (MHC). (b) Quantitative analysis of total MHC positive area. (c) Quantitative analysis of fusion and maturation index. (d) Quantification of cell area (d), myotube length (e) and myotube diameter (f). Significant differences are marked ‘*’, compared with unmodified glass (p < 0.05). Adapted from Ref. 35 with permission, copyright © Elsevier, 2013.
3D All-Carbon Scaffolds for Tissue Engineering
Few studies have investigated the cytocompatibility and biocompatibility of 3D all-carbon scaffolds for tissue engineering applications. In this section, only articles that report the use of all-carbon (no polymer) macroscopic (>1 mm all three-dimensions) carbon nanotube or graphene scaffolds are included. Lalwani et al. have investigated the cytocompatibility of macroscopic 3D all-carbon scaffolds fabricated using single- and multi- walled carbon nanotubes (SWCNTs and MWCNTs). SWCNTs and MWCNTs were assembled in 3D porous (>80% porosity, 5–8 mm height, 4–6 mm diameter) cylinders using a radical initiated thermal crosslinking method.37 MC3T3 pre-osteoblast cells and NIH3T3 dermal fibroblast cells show good cell viability on SWCNT and MWCNT scaffolds (Compared to 3D poly(lactic-co-glycolic) acid (PLGA) controls) after 1, 3, and 5 days of culture. Confocal live cells imaging (calcein-AM staining) confirm the presence of live, metabolically active cells on SWCNT and MWCNT scaffolds. Immunofluorescence imaging shows expression of vinculin (cell adhesion protein) and Ki-67 (cell proliferation marker) suggesting that cells can adhere to SWCNT and MWCNT scaffolds via focal adhesion complexes and are proliferating. SEM imaging showed the presence of numerous cytoplasmic extensions and filopodia for cell attachment to the underlying nanotube architecture (Figs. 6a–6f). Furthermore, confocal live cell imaging showed that the cells were able to penetrate into the scaffolds up to depths of ~300 μm (Figs. 6g–6i). Due to limitations associated with laser penetration through 3D scaffold, cells at greater depths were not visualized. These results show that 3D macroscopic SWCNT and MWCNT scaffolds, fabricated via radical initiated thermal crosslinking of carbon nanotubes, are cytocompatible (allow cell attachment, proliferation and infiltration) and opens avenues for their application in bone tissue engineering.
Representative SEM images showing adhesion of MC3T3 cells on (a and d) poly(lactic-co-glycolic) acid, (b and e) 3D multiwalled carbon nanotube, and (c and f) single-walled carbon nanotube scaffolds. Formation of cytoplasmic extensions (filopodia and pseudopodia) can be observed for each scaffold group (insets in d–f). (g–i) Representative spectrally color coded images of calcein-AM–stained MC3T3 cells a function of confocal Z-depth (i.e., cellular infiltration) after 5 days of culture on (g) poly(lactic-co-glycolic) acid, (h) multiwalled carbon nanotube, and (i) single-walled carbon nanotube scaffolds. Presence of cells can be detected up to a depth of 200–300 μm for each scaffold group. Adapted from Ref. 37 with permission, copyright © John Wiley & Sons, 2015.
Crowder et al. have investigated the cytocompatibility of 3D graphene foams synthesized on Ni-templates using CVD.12 Multilayered graphene was deposited on porous 3D nickel templates (1.2 mm height) and etched using FeCl3 to remove Ni. The resulting 3D graphene foam was seeded with MSCs and cell viability was analyzed after 1, 4, 7, and 14 days by staining cells with calcein-AM. Results show good cell viability of MSCs at all time points. SEM imaging showed MSC attachment to underlying graphene substrate (Figs. 7a–7c). Immunofluorescence staining showed that cells on graphene scaffolds showed greater expression of osteogenic (osteocalcin and osteopontin) markers compared to cells cultured on TCPS (Figs. 7d and 7e). These results show that 3D graphene substrates are cytocompatible and promote osteogenic differentiation of MSCs.
(a–c) Representative scanning electron microscopy images of human mesenchymal stem cells cultured on 3D graphene foams for four days. Images show cells forming protrusions up to 100 mm in length (yellow arrowheads) that extended from small cell bodies (black arrows). Immunostaining images of (d) neuronal and (e) osteogenic markers for human mesenchymal stem cells cultured on 3D graphene foams or tissue culture polystyrene (TCPS) control for 7 days. Culture on graphene foams did not affect the expression of neuronal markers, but stimulated de novo expression of the osteogenic marker osteocalcin and upregulated the expression of osteopontin. (d) Scale bars are 100 mm and (e) scale bars are 50 mm. Adapted from Ref. 12 with permission, copyright © Royal Society of Chemistry, 2013.
Serrano et al. have fabricated 3D graphene oxide (GO) scaffolds using ice-segregation-induced-self-assembly (ISISA) process—a procedure of freezing and lyophilization to produce porous monoliths.64 GO scaffolds (4.5 mm diameter, 3 mm height) were seeded with rat neural progenitor cells and cell viability was assessed via Live/Dead assay. Fluorescence imaging showed presence of live cells (green fluorescence). Immunofluorescence imaging of cells after 14 days showed the presence of interconnected neural networks of neurons and glial cells rich in axons, dendrites and synaptic connections (Fig. 8). These results suggest that 3D graphene oxide scaffolds may be suitable for neuronal tissue engineering. Li et al. have fabricated 3D graphene foams using a CVD based sacrificial transfer method and shown that these scaffolds are cytocompatible substrates for neuronal stem cells.47 3D graphene scaffolds permit attachment and proliferation of neural stem cells; robust expression of Ki-67 (cell proliferation marker) was observed. After 5 days of culture, expression of Tuj-1, nestin, O4, and GFAP were observed suggesting successful neuronal differentiation of neural stem cells (Fig. 9). Additionally, under external electrical stimulus, differentiated neural stem cells showed an increase in the levels of intracellular calcium suggesting that 3D graphene foams may be used as a conductive scaffold to electrically stimulate neuronal cells.
Embryonic neural progenitor cell differentiation on 2D and 3D graphene oxide films and scaffolds after 1, 7, and 14 days. Cells are stained for GFAP (green), TAU (red) and cell nuclei (blue, DAPI). Scale bars represent 50 μm for all images except zoom panel (25 μm). Adapted from Ref. 64 with permission, copyright © Royal Society of Chemistry, 2014.
(a; b) Representative fluorescence images of differentiated neuronal stem cells stained with Tuj-1 for neuron (green), GFAP for astrocyte (red, a; b), O4 for oligodendrocytes (green, b) and DAPI for cell nuclei (blue, a; b). (c) Western blot analysis of nestin, Tuj-1, GFAP, and RIP protein expression of differentiated neuronal stem cells on 2D and 3D graphene substrates. (d) Relative optical densities of markers depicted in (c). The data is represented as mean ± standard error, *p < 0.05, **p < 0.01. Adapted from Ref. 47 with permission, copyright © Li et al.
López-Dolado et al. have investigated the in vivo biocompatibility of 3D reduced graphene oxide (rGO) scaffolds in adult male Wistar rats.49 rGO monoliths (4.5 mm diameter, 2 mm height) were implanted in C6 segment of spinal cord for 10 days and tissue response was assessed via immunofluorescence, histology, and histomorphometric analysis. No local or systemic toxicity was observed; 3D rGO scaffolds facilitate tissue integrity after injury and prevent the extension of lesion. Histological analysis of lesion site shows the presence of cells and collagen fibers in the interface tissue. Immunofluorescence analysis showed the expression of ED1 (expressed by macrophages) and platelet derived growth factor receptor (PDGF-β) at the injury area suggesting early hematopoiesis and blood vessel formation. CD80 and CD163 macrophages (M2 macrophages, reparative cells) were present at the interface tissue and were observed infiltrating the scaffolds. Furthermore, a high percentage of actively proliferating cells were also present at the injury site. No significant damage in other systemic organs (brain, lung, kidney, heart, intestine, liver) was observed. These results suggest that 3D rGO scaffolds are biocompatible and may potentially be used as efficient platforms for the treatment of spinal cord injuries.
Summary and Future Perspective
Recent advances in synthesis methods have enabled the fabrication of 2D and 3D all-carbon coatings/films and porous scaffolds using carbon nanotubes or graphene as starting material. These all-carbon assemblies have been explored for various tissue engineering applications. Studies indicate that coatings and scaffolds fabricating using carbon nanotube or graphene can enhance osteogenesis by accelerating the differentiation of mesenchymal stem cells towards osteoblasts and promote extracellular calcium deposition in the matrix. Several reports also show enhanced neuronal impulse conduction, neural network formation, and neurite outgrowth on carbon nanotube and graphene substrates. Studies have also exploited the physiochemical properties (electrical conductivity, strong photoacoustic response) of carbon nanotube and graphene assemblies to develop stimulus-responsive strategies that induce and enhance progenitor cell differentiation. The results till date suggest that, harnessing the multifunctional capabilities of all-carbon assemblies offers exciting opportunities towards the development of next-generation technologies of tissue engineering and regenerative medicine.
Several challenges need to be addressed to translate the promise of these all-carbon assemblies into clinic. These barriers can be broadly related to manufacturing and safety.
Manufacturing
-
(i)
Novel strategies need to be developed and existing strategies need to be optimized to chemically connect these nanoparticles to each other or their substrates to improve the structural and mechanical robustness of these assemblies. Current fabrication techniques that are unable to tightly ‘trap’ the individual nanoparticles in a confined space in these assemblies are not suitable for many implant applications as loosening of nanomaterials and their eventual separation from the assemblies can lead to failure of the medical device or implant. Furthermore, improvements in fabrication technology would also enable finer control of the porosities and pore geometry of 3D architectures, important features for tissue engineering constructs.
-
(ii)
The scalability issues for fabricating 3D all-carbon scaffolds need to be addressed and more efforts are needed to identify cost efficient processes. Several fabrication methods are associated with high operational costs, and/or scalability issues that may present a practical challenge to develop macroscopic (>1 mm in all three dimensions) 3D all-carbon architectures. For example, 3D graphene foams made by CVD require a sacrificial metal catalysis substrate, poly-methyl methacrylate reinforcing agents, hydrochloric acid to etch away the metal substrate, and heated acetone to dissolve the poly-methyl methacrylate.9 This technique, although robust, is process intensive and may lead to metal/polymer residues that may serve confound factors in biocompatibility studies.
-
(iii)
Eventually, for each implant or device manufactured for clinical use, current good manufacturing practice (cGMP) requirements for medical devices would have to be strictly followed. Thus, related quality system protocols and procedures would have to be developed that comply with regulatory guidelines.
Safety
-
(i)
Comprehensive in vivo investigations are needed to adequately assess the biocompatibility of 2D and 3D all-carbon assemblies. Majority of the studies reported till date have been performed in vitro.
-
(ii)
The confounding effects of the various impurities that could be incorporated during the manufacturing process needs to be elucidated. The initial starting materials and the fabrication methods used in the production of the assemblies can result in the presence of metallic and/or organic impurities in the final product, which could result in variable toxicity effects.
-
(iii)
Studies that directly compare the safety and efficacy of 2D or 3D assemblies fabricated using various carbon nanomaterials for specific tissue engineering applications would be beneficial. Carbon nanotubes and graphene possess different nanoscale architectures and physiochemical properties. Therefore, 2D and 3D assemblies of carbon nanotubes and graphene may exhibit distinct cellular and molecular responses when interfaced with biological systems.
-
(iv)
Studies that provide information on the long-term effects of degradation products are required. It has been reported that hydrogen peroxide and other peroxidase enzymes present in the body can degrade carbon nanotubes and graphene.32,43,77,81 However, the biodegradation process is slow and the effects of the degradation products on surrounding cells and tissues are unknown.66
-
(v)
Eventually, the preclinical regulatory pathway needs to be established for any implant or device intended for use in humans. The good laboratory practice (GLP) and current good manufacturing practice (cGMP) guidelines would have to be followed while establishing this pathway.
References
Aryaei, A., A. H. Jayatissa, and A. C. Jayasuriya. The effect of graphene substrate on osteoblast cell adhesion and proliferation. J. Biomed. Mater. Res. Part A 102(9):3282–3290, 2014.
Bhunia, S. K., A. Saha, A. R. Maity, S. C. Ray, and N. R. Jana. Carbon nanoparticle-based fluorescent bioimaging probes. Sci. Rep. 3:1473, 2013.
Boyan, B. D., T. W. Hummert, D. D. Dean, and Z. Schwartz. Role of material surfaces in regulating bone and cartilage cell response. Biomaterials 17(2):137–146, 1996.
Buser, D., R. Schenk, S. Steinemann, J. Fiorellini, C. Fox, and H. Stich. Influence of surface characteristics on bone integration of titanium implants. A histomorphometric study in miniature pigs. J. Biomed. Mater. Res. 25(7):889–902, 1991.
Cassell, A. M., J. A. Raymakers, J. Kong, and H. Dai. Large scale CVD synthesis of single-walled carbon nanotubes. J. Phys. Chem. B 103(31):6484–6492, 1999.
Cellot, G., E. Cilia, S. Cipollone, V. Rancic, A. Sucapane, S. Giordani, L. Gambazzi, H. Markram, M. Grandolfo, and D. Scaini. Carbon nanotubes might improve neuronal performance by favouring electrical shortcuts. Nat. Nanotechnol. 4(2):126–133, 2009.
Chen, F., W. Lam, C. Lin, G. Qiu, Z. Wu, K. Luk, and W. Lu. Biocompatibility of electrophoretical deposition of nanostructured hydroxyapatite coating on roughen titanium surface: in vitro evaluation using mesenchymal stem cells. J. Biomed. Mater. Res. B 82(1):183–191, 2007.
Chen, H., M. B. Müller, K. J. Gilmore, G. G. Wallace, and D. Li. Mechanically strong, electrically conductive, and biocompatible graphene paper. Adv. Mater. 20(18):3557–3561, 2008.
Chen, Z., W. Ren, L. Gao, B. Liu, S. Pei, and H.-M. Cheng. Three-dimensional flexible and conductive interconnected graphene networks grown by chemical vapour deposition. Nat. Mater. 10(6):424–428, 2011.
Chowdhury, S. M., G. Lalwani, K. Zhang, J. Y. Yang, K. Neville, and B. Sitharaman. Cell specific cytotoxicity and uptake of graphene nanoribbons. Biomaterials 34(1):283–293, 2013.
Correa-Duarte, M. A., N. Wagner, J. Rojas-Chapana, C. Morsczeck, M. Thie, and M. Giersig. Fabrication and biocompatibility of carbon nanotube-based 3D networks as scaffolds for cell seeding and growth. Nano Lett. 4(11):2233–2236, 2004.
Crowder, S. W., D. Prasai, R. Rath, D. A. Balikov, H. Bae, K. I. Bolotin, and H.-J. Sung. Three-dimensional graphene foams promote osteogenic differentiation of human mesenchymal stem cells. Nanoscale 5(10):4171–4176, 2013.
Dikin, D. A., S. Stankovich, E. J. Zimney, R. D. Piner, G. H. Dommett, G. Evmenenko, S. T. Nguyen, and R. S. Ruoff. Preparation and characterization of graphene oxide paper. Nature 448(7152):457–460, 2007.
Du, D., Y. Yang, and Y. Lin. Graphene-based materials for biosensing and bioimaging. MRS Bull. 37(12):1290–1296, 2012.
Engler, A. J., S. Sen, H. L. Sweeney, and D. E. Discher. Matrix elasticity directs stem cell lineage specification. Cell 126(4):677–689, 2006.
Fabbro, A., A. Villari, J. Laishram, D. Scaini, F. M. Toma, A. Turco, M. Prato, and L. Ballerini. Spinal cord explants use carbon nanotube interfaces to enhance neurite outgrowth and to fortify synaptic inputs. ACS Nano 6(3):2041–2055, 2012.
Fan, L., C. Feng, W. Zhao, L. Qian, Y. Wang, and Y. Li. Directional neurite outgrowth on superaligned carbon nanotube yarn patterned substrate. Nano Lett. 12(7):3668–3673, 2012.
Farshid, B., G. Lalwani, and B. Sitharaman. In vitro cytocompatibility of one-dimensional and two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites. J. Biomed. Mater. Res. Part A 103(7):2309–2321, 2015.
Feng, L., S. Zhang, and Z. Liu. Graphene based gene transfection. Nanoscale 3(3):1252–1257, 2011.
Gähwiler, B. Organotypic cultures of neural tissue. Trends Neurosci. 11(11):484–489, 1988.
Geng, H.-Z., K. K. Kim, K. P. So, Y. S. Lee, Y. Chang, and Y. H. Lee. Effect of acid treatment on carbon nanotube-based flexible transparent conducting films. J. Am. Chem. Soc. 129(25):7758–7759, 2007.
Goenka, S., V. Sant, and S. Sant. Graphene-based nanomaterials for drug delivery and tissue engineering. J. Control. Release 173:75–88, 2014.
Green, D. E., J. P. Longtin, and B. Sitharaman. The effect of nanoparticle-enhanced photoacoustic stimulation on multipotent marrow stromal cells. ACS Nano 3(8):2065–2072, 2009.
Griffith, L. G., and G. Naughton. Tissue engineering–current challenges and expanding opportunities. Science 295(5557):1009–1014, 2002.
Harada, S.-I., and G. A. Rodan. Control of osteoblast function and regulation of bone mass. Nature 423(6937):349–355, 2003.
Harrison, B. S., and A. Atala. Carbon nanotube applications for tissue engineering. Biomaterials 28(2):344–353, 2007.
Hong, D., K. Bae, S. Yoo, K. Kang, B. Jang, J. Kim, S. Kim, S. Jeon, Y. Nam, and Y. G. Kim. Generation of cellular micropatterns on a single-layered graphene film. Macromol. Biosci. 14(3):314–319, 2014.
Jang, E. Y., T. J. Kang, H. W. Im, D. W. Kim, and Y. H. Kim. Single-walled carbon-nanotube networks on large-area glass substrate by the dip-coating method. Small 4(12):2255–2261, 2008.
Kanakia, S., J. D. Toussaint, S. M. Chowdhury, G. Lalwani, T. Tembulkar, T. Button, K. R. Shroyer, W. Moore, and B. Sitharaman. Physicochemical characterization of a novel graphene-based magnetic resonance imaging contrast agent. Int. J. Nanomed. 8:2821, 2013.
Kim, K. S., Y. Zhao, H. Jang, S. Y. Lee, J. M. Kim, K. S. Kim, J.-H. Ahn, P. Kim, J.-Y. Choi, and B. H. Hong. Large-scale pattern growth of graphene films for stretchable transparent electrodes. Nature 457(7230):706–710, 2009.
Kostarelos, K., A. Bianco, and M. Prato. Promises, facts and challenges for carbon nanotubes in imaging and therapeutics. Nat. Nanotechnol. 4(10):627–633, 2009.
Kotchey, G. P., B. L. Allen, H. Vedala, N. Yanamala, A. A. Kapralov, Y. Y. Tyurina, J. Klein-Seetharaman, V. E. Kagan, and A. Star. The enzymatic oxidation of graphene oxide. ACS Nano 5(3):2098–2108, 2011.
Kroustalli, A. A., S. N. Kourkouli, and D. D. Deligianni. Cellular function and adhesion mechanisms of human bone marrow mesenchymal stem cells on multi-walled carbon nanotubes. Ann. Biomed. Eng. 41(12):2655–2665, 2013.
Ku, S. H., M. Lee, and C. B. Park. Carbon-based nanomaterials for tissue engineering. Adv. Healthc. Mater. 2(2):244–260, 2013.
Ku, S. H., and C. B. Park. Myoblast differentiation on graphene oxide. Biomaterials 34(8):2017–2023, 2013.
Lalwani, G., X. Cai, L. Nie, L. V. Wang, and B. Sitharaman. Graphene-based contrast agents for photoacoustic and thermoacoustic tomography. Photoacoustics 1(3):62–67, 2013.
Lalwani, G., A. Gopalan, M. D’Agati, J. Srinivas Sankaran, S. Judex, Y. X. Qin, and B. Sitharaman. Porous three dimensional carbon nanotube scaffolds for tissue engineering. J. Biomed. Mater. Res. Part A 103(10):3212–3225, 2015.
Lalwani, G., A. M. Henslee, B. Farshid, L. Lin, F. K. Kasper, Y.-X. Qin, A. G. Mikos, and B. Sitharaman. Two-dimensional nanostructure-reinforced biodegradable polymeric nanocomposites for bone tissue engineering. Biomacromolecules 14(3):900–909, 2013.
Lalwani, G., A. M. Henslee, B. Farshid, P. Parmar, L. Lin, Y.-X. Qin, F. K. Kasper, A. G. Mikos, and B. Sitharaman. Tungsten disulfide nanotubes reinforced biodegradable polymers for bone tissue engineering. Acta Biomater. 9(9):8365–8373, 2013.
Lalwani, G., A. T. Kwaczala, S. Kanakia, S. C. Patel, S. Judex, and B. Sitharaman. Fabrication and characterization of three-dimensional macroscopic all-carbon scaffolds. Carbon 2013(53):90–100, 2013.
Lalwani, G., and B. Sitharaman. Multifunctional fullerene-and metallofullerene-based nanobiomaterials. Nano LIFE 3(3):1342003, 2013.
Lalwani, G., J. L. Sundararaj, K. Schaefer, T. Button, and B. Sitharaman. Synthesis, characterization, in vitro phantom imaging, and cytotoxicity of a novel graphene-based multimodal magnetic resonance imaging-X-ray computed tomography contrast agent. J. Mater. Chem. B 2(22):3519–3530, 2014.
Lalwani, G., W. Xing, and B. Sitharaman. Enzymatic degradation of oxidized and reduced graphene nanoribbons by lignin peroxidase. J. Mater. Chem. B 2(37):6354–6362, 2014.
Lee, W. C., C. H. Y. X. Lim, H. Shi, L. A. L. Tang, Y. Wang, C. T. Lim, and K. P. Loh. Origin of enhanced stem cell growth and differentiation on graphene and graphene oxide. ACS Nano 5(9):7334–7341, 2011.
Li, X., W. Cai, J. An, S. Kim, J. Nah, D. Yang, R. Piner, A. Velamakanni, I. Jung, and E. Tutuc. Large-area synthesis of high-quality and uniform graphene films on copper foils. Science 324(5932):1312–1314, 2009.
Li, C., and G. Shi. Three-dimensional graphene architectures. Nanoscale 4(18):5549–5563, 2012.
Li, N., Q. Zhang, S. Gao, Q. Song, R. Huang, L. Wang, L. Liu, J. Dai, M. Tang, and G. Cheng. Three-dimensional graphene foam as a biocompatible and conductive scaffold for neural stem cells. Sci. Rep. 3:1604, 2013.
Liu, J., L. Cui, and D. Losic. Graphene and graphene oxide as new nanocarriers for drug delivery applications. Acta Biomater. 9(12):9243–9257, 2013.
López-Dolado, E., A. González-Mayorga, M. T. Portolés, M. J. Feito, M. L. Ferrer, F. del Monte, M. C. Gutiérrez, and M. C. Serrano. Subacute tissue response to 3D graphene oxide scaffolds implanted in the injured rat spinal cord. Adv. Healthc. Mater. 4(12):1861–1868, 2015.
Lysaght, M. J., and J. Reyes. The growth of tissue engineering. Tissue Eng. 7(5):485–493, 2001.
McKay, W. F., S. M. Peckham, and J. M. Badura. A comprehensive clinical review of recombinant human bone morphogenetic protein-2 (INFUSE® Bone Graft). Int. Orthop. 31(6):729–734, 2007.
Mirri, F., A. W. Ma, T. T. Hsu, N. Behabtu, S. L. Eichmann, C. C. Young, D. E. Tsentalovich, and M. Pasquali. High-performance carbon nanotube transparent conductive films by scalable dip coating. ACS Nano 6(11):9737–9744, 2012.
National Institutes of Health (NIH). Fact Sheet—Regenerative Medicine. 2010.
Nayak, T. R., H. Andersen, V. S. Makam, C. Khaw, S. Bae, X. Xu, P.-L. R. Ee, J.-H. Ahn, B. H. Hong, G. Pastorin, and B. Özyilmaz. Graphene for controlled and accelerated osteogenic differentiation of human mesenchymal stem cells. ACS Nano 5(6):4670–4678, 2011.
Nayak, T. R., L. Jian, L. C. Phua, H. K. Ho, Y. Ren, and G. Pastorin. Thin films of functionalized multiwalled carbon nanotubes as suitable scaffold materials for stem cells proliferation and bone formation. ACS Nano 4(12):7717–7725, 2010.
Ng, S., J. Wang, Z. Guo, J. Chen, G. Wang, and H. K. Liu. Single wall carbon nanotube paper as anode for lithium-ion battery. Electrochim. Acta 51(1):23–28, 2005.
Park, S. Y., J. Park, S. H. Sim, M. G. Sung, K. S. Kim, B. H. Hong, and S. Hong. Enhanced differentiation of human neural stem cells into neurons on graphene. Adv. Mater. 23(36):H263–H267, 2011.
Patel, S. C., G. Lalwani, K. Grover, Y.-X. Qin, and B. Sitharaman. Fabrication and cytocompatibility of in situ crosslinked carbon nanomaterial films. Sci. Rep. 5:10261, 2015.
Patel, S. C., S. Lee, G. Lalwani, C. Suhrland, S. M. Chowdhury, and B. Sitharaman. Graphene-based platforms for cancer therapeutics. Ther. Deliv. 7(2):101–116, 2016.
Pham, V. H., T. V. Cuong, S. H. Hur, E. W. Shin, J. S. Kim, J. S. Chung, and E. J. Kim. Fast and simple fabrication of a large transparent chemically-converted graphene film by spray-coating. Carbon 48(7):1945–1951, 2010.
Pittenger, M. F., A. M. Mackay, S. C. Beck, R. K. Jaiswal, R. Douglas, J. D. Mosca, M. A. Moorman, D. W. Simonetti, S. Craig, and D. R. Marshak. Multilineage potential of adult human mesenchymal stem cells. Science 284(5411):143–147, 1999.
Pryzhkova, M. V., I. Aria, Q. Cheng, G. M. Harris, X. Zan, M. Gharib, and E. Jabbarzadeh. Carbon nanotube-based substrates for modulation of human pluripotent stem cell fate. Biomaterials 35(19):5098–5109, 2014.
Puleo, D., and A. Nanci. Understanding and controlling the bone–implant interface. Biomaterials 20(23):2311–2321, 1999.
Serrano, M. C., J. Patiño, C. García-Rama, M. L. Ferrer, J. Fierro, A. Tamayo, J. E. Collazos-Castro, F. del Monte, and M. C. Gutierrez. 3D free-standing porous scaffolds made of graphene oxide as substrates for neural cell growth. J. Mater. Chem. B 2(34):5698–5706, 2014.
Shen, H., L. Zhang, M. Liu, and Z. Zhang. Biomedical applications of graphene. Theranostics 2(3):283, 2012.
Shvedova, A. A., A. A. Kapralov, W. H. Feng, E. R. Kisin, A. R. Murray, R. R. Mercer, C. M. St Croix, M. A. Lang, S. C. Watkins, and N. V. Konduru. Impaired clearance and enhanced pulmonary inflammatory/fibrotic response to carbon nanotubes in myeloperoxidase-deficient mice. PLoS ONE 7(3):e30923, 2012.
Song, Y. I., G. Y. Kim, H. K. Choi, H. J. Jeong, K. K. Kim, C. M. Yang, S. C. Lim, K. H. An, K. T. Jung, and Y. H. Lee. Fabrication of carbon nanotube field emitters using a dip-coating method. Chem. Vap. Depos. 12(6):375–379, 2006.
Spotnitz, M. E., D. Ryan, and H. A. Stone. Dip coating for the alignment of carbon nanotubes on curved surfaces. J. Mater. Chem. 14(8):1299–1302, 2004.
Suk, J. W., A. Kitt, C. W. Magnuson, Y. Hao, S. Ahmed, J. An, A. K. Swan, B. B. Goldberg, and R. S. Ruoff. Transfer of CVD-grown monolayer graphene onto arbitrary substrates. ACS Nano 5(9):6916–6924, 2011.
Takahashi, K., K. Tanabe, M. Ohnuki, M. Narita, T. Ichisaka, K. Tomoda, and S. Yamanaka. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell 131(5):861–872, 2007.
Tu, Q., L. Pang, Y. Chen, Y. Zhang, R. Zhang, B. Lu, and J. Wang. Effects of surface charges of graphene oxide on neuronal outgrowth and branching. Analyst 139(1):105–115, 2014.
Veetil, J. V., and K. Ye. Tailored carbon nanotubes for tissue engineering applications. Biotechnol. Prog. 25(3):709–721, 2009.
Verfaillie, C. Pluripotent stem cells. Transfus. Clin. Biol. 16(2):65–69, 2009.
Wang, X., L. Zhi, and K. Müllen. Transparent, conductive graphene electrodes for dye-sensitized solar cells. Nano Lett. 8(1):323–327, 2008.
Wojtek, T., C. Manish, and S. Federico. The chemical and physical characteristics of single-walled carbon nanotube film impact on osteoblastic cell response. Nanotechnology 21(31):315102, 2010.
Wu, Z., Z. Chen, X. Du, J. M. Logan, J. Sippel, M. Nikolou, K. Kamaras, J. R. Reynolds, D. B. Tanner, and A. F. Hebard. Transparent, conductive carbon nanotube films. Science 305(5688):1273–1276, 2004.
Xing, W., G. Lalwani, I. Rusakova, and B. Sitharaman. Degradation of graphene by hydrogen peroxide. Part. Part. Syst. Charact. 31(7):745–750, 2014.
Xu, Y., G. Shi, and X. Duan. Self-assembled three-dimensional graphene macrostructures: synthesis and applications in supercapacitors. Acc. Chem. Res. 48(6):1666–1675, 2015.
Yang, K., S. Zhang, G. Zhang, X. Sun, S.-T. Lee, and Z. Liu. Graphene in mice: ultrahigh in vivo tumor uptake and efficient photothermal therapy. Nano Lett. 10(9):3318–3323, 2010.
Zhang, L., and T. J. Webster. Nanotechnology and nanomaterials: promises for improved tissue regeneration. Nano Today 4(1):66–80, 2009.
Zhao, Y., B. L. Allen, and A. Star. Enzymatic degradation of multiwalled carbon nanotubes. J. Phys. Chem. A 115(34):9536–9544, 2011.
Zuk, P. A., M. Zhu, P. Ashjian, D. A. De Ugarte, J. I. Huang, H. Mizuno, Z. C. Alfonso, J. K. Fraser, P. Benhaim, and M. H. Hedrick. Human adipose tissue is a source of multipotent stem cells. Mol. Biol. Cell 13(12):4279–4295, 2002.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Gaurav Lalwani and Sunny C. Patel have contributed equally to this work.
Rights and permissions
About this article
Cite this article
Lalwani, G., Patel, S.C. & Sitharaman, B. Two- and Three-Dimensional All-Carbon Nanomaterial Assemblies for Tissue Engineering and Regenerative Medicine. Ann Biomed Eng 44, 2020–2035 (2016). https://doi.org/10.1007/s10439-016-1623-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-016-1623-5