Abstract
18F-FDG-PET is increasingly used to assess pulmonary inflammatory cell activity. However, current models of pulmonary 18F-FDG kinetics do not account for delays in 18F-FDG transport between the plasma sampling site and the lungs. We developed a three-compartment model of 18F-FDG kinetics that includes a delay between the right heart and the local capillary blood pool, and used this model to estimate regional pulmonary perfusion. We acquired dynamic 18F-FDG scans in 12 mechanically ventilated sheep divided into control and lung injury groups (n = 6 each). The model was fit to tracer kinetics in three isogravitational regions-of-interest to estimate regional lung transport delays and regional perfusion. 13NN bolus infusion scans were acquired during a period of apnea to measure regional perfusion using an established reference method. The delayed input function model improved description of 18F-FDG kinetics (lower Akaike Information Criterion) in 98% of studied regions. Local transport delays ranged from 2.0 to 13.6 s, averaging 6.4 ± 2.9 s, and were highest in non-dependent regions. Estimates of regional perfusion derived from model parameters were highly correlated with perfusion measurements based on 13NN-PET (R 2 = 0.92, p < 0.001). By incorporating local vascular transports delays, this model of pulmonary 18F-FDG kinetics allows for simultaneous assessment of regional lung perfusion, transit times, and inflammation.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Positron emission tomography (PET) imaging of 2-deoxy-2-[18F]-fluoro-d-glucose (18F-FDG) in the lungs has been increasingly used to investigate changes in glucose metabolism underlying various lung diseases. In the acute respiratory distress syndrome (ARDS), 18F-FDG-PET is used to assess the metabolic activation associated with lung inflammation, which reflects primarily the infiltration and activation of neutrophils.6,15,18,23,31 Several analytical methods, including the Patlak analysis,25 Sokoloff model,36 and four-compartment model,33 have been applied to quantify 18F-FDG transport and trapping within lung tissue. Current techniques for defining the lung plasma input function that is required for these models include manual sampling of blood in the pulmonary artery4 or systemic artery,26 and imaging of a region-of-interest characterizing the right heart blood pool with calibration using manual samples.32
A major assumption underlying those approaches is that the plasma activity assessed at the sampling site (e.g., the arteries or right heart) is an accurate representation of the plasma activity within the pulmonary capillary blood pool, the site of tracer entry into the tissue. However, the vascular transport of intravenously infused 18F-FDG gives rise to a delay between the sampling site and the pulmonary capillary blood pool. In the lungs, transport delays are likely heterogeneous, given the previously described heterogeneity of pulmonary capillary transit times.41 Such transport delays have long been recognized and modeled in other applications of PET such as myocardial perfusion imaging,16,24 assessment of cerebral blood flow37 and metabolism,12,45 and 15O-water imaging of pulmonary blood flow.29 In contrast, analyses of pulmonary 18F-FDG kinetics have not yet accounted for delays in the plasma input function,4,8,33 potentially biasing estimates of parameters describing pulmonary 18F-FDG uptake. To date, no study has examined the magnitude of this delay or its effect on estimates of pulmonary 18F-FDG kinetics parameters. Moreover, the ability to assess delays in 18F-FDG transport may allow for regional quantification of pulmonary capillary transit times and perfusion, key physiologic parameters which may be altered in disease.35,39
In this study, we incorporate an input function delay into the standard three-compartment model of pulmonary 18F-FDG kinetics. This new model—hereafter referred to as the delayed input function model—includes a delay of the plasma function to account for tracer transport time between the right heart and the local capillary blood pool. The model is compared with the standard three-compartment model without a delay—herein referred to as the right heart input function model. In addition, we demonstrate a novel method to estimate local pulmonary perfusion using the delayed input function model parameters. In two groups of mechanically ventilated sheep with healthy or acutely injured lungs, we aimed to: (1) examine distributions of the regional input function delay between the right heart and lung regions-of-interest; (2) compare values of 18F-FDG kinetics parameters and measures of model goodness-of-fit between the right heart and delayed input function models; and (3) compare estimates of regional perfusion derived from parameters of the new model with those of a reference 13NN-PET technique.
Materials and Methods
Animal Preparation
The protocols of this study were approved by the Subcommittee on Research Animal Care of the Massachusetts General Hospital. Twelve sheep (21.7 ± 2.0 kg) were anesthetized, intubated, and mechanically ventilated. Femoral artery, internal jugular vein, and pulmonary artery catheters were inserted. Anesthesia was maintained with a continuous infusion of ketamine and propofol titrated to heart rate and blood pressure, and intermittent boluses of fentanyl. Paralysis was established with a bolus of pancuronium (0.1 mg/kg) at induction and repeated every 90 min (0.02–0.04 mg/kg).
Study Groups
We studied two groups of six supine mechanically ventilated sheep. In the Control group, we aimed to investigate normal physiological conditions; sheep were mechanically ventilated with a volume controlled mode, with tidal volume (V T) set to 8 mL/kg, positive end-expiratory pressure (PEEP) of 0 cmH2O, inspired oxygen fraction (FIO2) initially set to 0.3 and adjusted to maintain arterial oxygen saturation >90%, inspiratory-to-expiratory ratio I:E = 1:2, and respiratory rate initially at 28 breaths/min and adjusted to maintain the arterial carbon dioxide partial pressure (PaCO2) between 32 and 45 mmHg. Animals were imaged after 20 min of mechanical ventilation to ensure steady-state conditions. For the Lung Injury group, we studied an ARDS model combining unilateral surfactant depletion8 with mild endotoxemia6 in order to produce varying degrees of regional inflammation as well as redistribution of perfusion. In each animal, a tracheotomy was performed and a left-sided double-lumen endobronchial tube was placed. While ventilating the right lung with FIO2 = 1, left lung surfactant depletion was produced by alveolar saline lavage. Starting from the supine position, warm saline (~400 mL) was instilled in the left bronchus at a pressure of ~30 cmH2O and then drained by gravity. After three aliquots, animals were switched to the prone position for another three aliquots to homogenize lavage of ventral and dorsal lung. The double-lumen endobronchial tube was then replaced by a regular endotracheal tube and ventilation of both lungs was performed for 4 h with PEEP = 10 cmH2O, FIO2 = 0.6, V T adjusted to a plateau pressure of 30 cmH2O (V T = 13.4 ± 3.5 mL/kg), I:E = 1:2, and respiratory rate adjusted to normocapnia. During this ventilation period, sheep received a continuous intravenous infusion of endotoxin (10 ng/kg/min, Escherichia coli O55:B5, List Biological Laboratories Inc, California).
PET Imaging Protocols
For all PET scans, sheep were positioned supine in the camera (Scanditronix PC4096, GE Healthcare, Milwaukee, WI) with the most caudal slice adjacent to the diaphragm dome. The camera collected 15 transverse slices of 6.5 mm thickness over a 9.7-cm-long axial field. The following PET scans were acquired after 20 min of mechanical ventilation in the Control group or after 4 h ventilation in the Lung Injury group.
-
1.
Transmission scans: obtained during 10 min of continuous breathing using a rotating pin-source of 68Ge to correct PET emission scans for tissue attenuation and to measure lung density.28 These scans were processed as previously described to construct images of average fractional gas content (F GAS).14
-
2.
13 NN bolus infusion emission scans: obtained as previously described to measure regional perfusion.38,43 Images consisted of 22 frames (8 × 2.5, 10 × 10, and 4 × 30 s). In brief, simultaneous with the start of imaging, a bolus of 13NN (~1000 MBq) dissolved in 40 mL saline was injected into the jugular vein at the beginning of 60 s of apnea. Due to its low solubility in water (partition coefficient water/air = 0.018 at 37 °C), virtually all 13NN in the blood diffuses into alveoli at first pass in aerated lung units, and 13NN activity accumulates in proportion to regional blood flow. After 60 s, mechanical ventilation was resumed to remove 13NN from the lungs over 10 min of washout.
-
3.
18 F-FDG emission scans: acquired immediately subsequent to 13NN washout. 18F-FDG (~200 MBq dissolved in 8 mL saline) was infused at a constant rate over 60 s through the jugular catheter. Simultaneous with the start of infusion, a dynamic PET scan was started, consisting of 37 sequential frames (9 × 10, 4 × 15, 1 × 30, 7 × 60, 15 × 120, 1 × 300 s) over 45 min. Pulmonary arterial blood was sampled at 5.5, 9.5, 25, 37, and 42.5 min, and plasma 18F-FDG concentration was measured in a well-counter cross-calibrated with the PET camera.32
PET images were reconstructed with voxel size of 2 × 2 × 6.5 mm using a convolution backprojection algorithm. Images were decay corrected to the beginning of tracer infusion and filtered in-plane with a circular moving average filter of diameter 12 mm and along the z-axis with a 2-point moving average filter. Each frame consisted of a 128 × 128 × 14 matrix with an effective volumetric resolution of 1.66 cm3.
Definition of Lung Fields for Analysis
Volumetric masks of the imaged lung field were delineated by: (1) including all voxels with F GAS > 0.5; (2) adding perfused but poorly aerated lung regions viewed in the 13NN infusion scan; and (3) manually refining masks to exclude the trachea, two main bronchi, and major blood vessels. Regions of interest (ROIs) were defined by dividing the lung field along the gravitational (i.e., ventral–dorsal) axis into three regions of equal height.
Delayed Input Function Model of Pulmonary 18F-FDG Kinetics
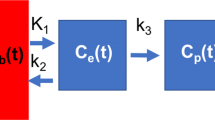
In previous applications where the three-compartment model was used to describe pulmonary 18F-FDG kinetics, the activity in the plasma compartment of the lungs was assumed to be equal to that at the blood sampling site.4,26,32 We developed a modified version of this model (i.e., the delayed input function model; Fig. 1) wherein the local lung plasma concentration (C p[t]) is represented by a delayed version of the image-derived plasma function measured in the right heart (C RH[t − t delay]) and calibrated with manual samples, as previously described.32 For a given value of t delay, C RH[t − t delay] was computed by linear interpolation between measured points. This delay represents the time required for the tracer to travel from the right heart to a lung ROI, and is a function of the regional vascular volume between the right heart and the pulmonary capillaries, as well as the corresponding blood flow. Thus, we allowed this parameter to vary regionally within the lungs, and in each ROI, t delay, k 1, k 2, k 3, and F B were simultaneously estimated by fitting the model to the regional 18F-FDG kinetics using an iterative optimization technique.17 In order to compare parameter estimates between the models with and without the delay, we also fit each ROI’s tracer kinetics with the conventional three-compartment model (i.e., the right heart input function model), which assumes the regional lung plasma input function to be equal to the right heart plasma function (C p[t] = C RH[t], t delay = 0).
The delayed input function model used to describe regional lung 18F-FDG kinetics. Rate constants k 1 and k 2 describe the transfer of 18F-FDG between blood in the pulmonary capillary blood pool, whose activity is represented by a time delay of the right heart plasma concentration (C RH[t − t delay]), and the extravascular substrate compartment (C s[t]). The parameter t delay, which is unique to this model, represents the time required for 18F-FDG to travel from the right heart to the local pulmonary capillary blood pool, while k 3 describes the rate of transfer into the metabolized compartment (C m[t]), representing intracellular phosphorylation of 18F-FDG. The fractional blood volume (F B, not shown) is an additional model parameter.
Estimation of Regional Perfusion from 18F-FDG Kinetics Parameters
In order to estimate regional perfusion from the parameters of the delayed input function model, we devised a multi-compartment model to describe tracer transport times between the right heart and distinct lung regions (Fig. 2). According to this model, the total delay between the right heart and a given lung ROI i (t delay,i ) represents the sum of a common pulmonary artery delay (t PA) and a local delay specific to ROI i (t ROI,i ). The pulmonary artery and each ROI’s blood pool were modeled as compartments whose transit dynamics were assumed to be described by the central volume principle, which states that the mean transit time (\(\bar{t}_{\text{transit}}\)) equals the ratio of blood volume (V B) to blood flow (\(\dot{Q}\)).
In order to estimate the delay specific to a given ROI, we first calculated the common pulmonary artery delay t PA using Eq. (1) and subtracted it from the total regional delay. The perfusion of the pulmonary artery was assumed to be equal to the measured cardiac output (CO), and its blood volume (V PA) was estimated from animal mass using morphometric data on mammalian vascular dimensions in the literature.30 Thus,
For each ROI i, t PA was subtracted from the total delay measured from local 18F-FDG kinetics (t delay,i ) to obtain the ROI-specific delay (t ROI,i ):
Rearranging Eq. (1), the perfusion of ROI i (\(\dot{Q}_{{{\text{ROI}},i}}\)) can be computed from the ROI’s delay (t ROI,i ) and its blood volume (V B,i ):
By substituting \(V_{{{\text{B}},i}} = F_{{{\text{B}},i}} \cdot V_{{{\text{ROI}},i}}\), where F B,i is the fractional blood volume of ROI i (estimated from tracer kinetics) and V ROI,i is the total volume of ROI i, we obtain specific perfusion, an index of perfusion per unit volume:
Thus, regional specific perfusion was calculated in each ROI as the ratio of F B to t ROI. In each animal, values of specific perfusion were mean normalized, providing measurements of relative regional perfusion derived with the delayed input function model (\(\dot{Q}_{\text{DIF}}\)).
Schematic of time delays in 18F-FDG transport between the right heart and the local lung microcirculation (i.e., capillary blood pool). 18F-FDG activity vs. time in the right heart, C RH(t), is measured using dynamic imaging of a region-of-interest (ROI) drawn over the right heart.32 We included a common delay in 18F-FDG transport associated with transit through the pulmonary artery (t PA), as well as local delays specific to each ROI i (t ROI,i ), the sum of which determined the total local delay i (t delay,i = t PA + t ROI,i ). For each ROI, t delay,i was estimated from local 18F-FDG kinetics with the delayed input function model (Fig. 1).
Measurement of Regional Perfusion from 13NN Kinetics
In order to compare our estimates of \(\dot{Q}_{\text{DIF}}\) with those of a reference method, regional perfusion was also estimated in each ROI from the kinetics of infused 13NN. During the apnea period of imaging, 13NN accumulates in aerated regions but passes through non-aerated alveoli. Thus, peak 13NN activity may be used as a measure of the relative perfusion to a lung region.11,38 Measurements of peak regional 13NN concentration were mean normalized in each animal to obtain 13NN-based measurements of relative perfusion (\(\dot{Q}_{\text{REF}}\)).
Statistical Analysis
Data are presented as mean ± standard deviation unless otherwise noted. Physiological variables were compared between groups using a Student’s t test for normally distributed data or a Wilcoxon signed rank test otherwise. Delay estimates were compared using a two-way ANOVA with group and region-of-interest as independent variables and Tukey–Kramer correction for multiple comparisons. Linear correlations were quantified with the Pearson coefficient. Model goodness-of-fit for the delayed input function and right heart input function models was quantified with the Akaike Information Criterion (AIC), which provides a measure of the balance between accurate description of data and minimization of the number of model parameters.1,19,33 AIC values for the two models were compared using a Student’s t test. Parameter estimates for the two models were compared by examining the percent differences between delayed input function model parameter estimates (P DIF) and those of the right heart input function model (P RHIF), where P denotes a given 18F-FDG kinetics parameter, using the equation:
All statistical analyses were performed with Matlab (MATLAB R2012a Statistics Toolbox, The Mathworks, Natick, MA). Significance was set at p < 0.05.
Results
Physiology and Animal Model
As per design, the Control group was ventilated with significantly lower tidal volumes, PEEP, and FIO2, and higher respiratory rates than the Lung Injury group (Table 1). The Lung Injury group showed oxygenation within the range defined for ARDS (PaO2/FiO2 < 200), while the Control group tended to have higher oxygenation. Cardiac output was significantly higher in the Control group.
Estimates of Regional Delay in 18F-FDG Kinetics
Comparison of the early (0–2 min) right heart tracer kinetics with pulmonary kinetics in distinct isogravitational ROIs (Fig. 3) revealed clear temporal differences between tracer arrival in the right heart and subsequent arrival in the lung. By accounting for this delay at the regional level, the delayed input function model allowed for improved description of the early kinetics, with the predicted local tracer arrival in the plasma coinciding much more closely with the initial increase in tracer activity observed in the lung ROIs (Figs. 3c and 3d).
(a) Representative image of regional gas fraction in the lung, with non-dependent (ND), middle (M), and dependent (D) regions-of-interest shown within the imaged lung field (outlined in green). (b) 18F-FDG activity within the first minute of tracer infusion: 18F-FDG is clearly visible within the right heart (RH, outlined in blue) but not yet distinguishable in the lung. (c, d) The delay required for tracer transport from the right heart to the lungs was evident in the early (<2 min) tracer kinetics, as the activity in the right heart (blue dashed lines) began to rise earlier than the activity in non-dependent (c) or dependent (d) lung regions (green lines). By accounting for tracer transport delays through inclusion of a delay of the right heart plasma function as a model parameter (t delay), the delayed plasma function in the ROIs (red lines) coincided much more closely with the initial rise in activity of those regions.
Delays between the right heart and the lung ROIs estimated with the delayed input function model (t delay) ranged from 2.0 to 13.6 s, averaging 6.4 ± 2.9 s. The component of this delay attributed to the pulmonary artery transit time (t PA) averaged 0.90 ± 0.22 s for all animals studied, and was slightly higher (p = 0.049) in the Lung Injury group (0.97 ± 0.27 s) than in the Control group (0.82 ± 0.15 s), likely due to hemodynamic differences between the groups (see Table 1). After subtracting the pulmonary artery component from the total delays, we found ROI-specific delays (t ROI) ranging from 1.2 to 12.8 s for all ROIs studied, with an average of 5.5 ± 2.9 s. These delays showed a significant dependence (p < 0.001) on ROI gravitational position (Fig. 4), with non-dependent regions having higher delays than middle or dependent regions in all three studied lung conditions. There were no significant differences in t ROI between the control (4.8 ± 2.4 s), LPS (5.6 ± 3.3 s), or Lavage + LPS (6.1 ± 2.9 s) conditions.
Distributions of ROI-specific delays derived with the delayed input function model. ROI delays (t ROI) were computed by subtracting the pulmonary artery delay (t PA) from the total delay estimated from regional 18F-FDG kinetics (t delay), according to Eq. (3). A significant dependence of t ROI on gravitational position was found (p < 0.001), with the non-dependent (ND) regions showing higher delays than the middle (M) or dependent (D) regions in post hoc tests. No significant differences were found between the Control, LPS, or Lavage + LPS conditions. *p < 0.05, **p < 0.01, ***p < 0.001.
Model Goodness-of-Fit
The delayed input function model yielded lower AIC values than the right heart input function model (p < 0.001) in 98.1% (53 out of 54) of the studied ROIs (Fig. 5a). The difference in AIC between the models (AICRHIF–AICDIF) was positively correlated (r = 0.44, p < 0.001) with the magnitude of the estimated delay (Fig. 5b). These findings imply a substantial improvement in description of the data by including t delay as a model parameter, particularly in regions where a large delay was observed.
(a) Comparison of the Akaike Information Criterion (AIC) for the delayed input function (AICDIF) and right heart input function (AICRHIF) models. Lower AIC values imply better description of the data for the number of model parameters. AICDIF was lower than AICRHIF (i.e., below the identity line) in 53 of 54 studied regions-of-interest. (b) Model improvement with the delayed input function, defined as the difference in AIC between the two models, was significantly correlated with the magnitude of ROI delays (t ROI).
Differences in 18F-FDG Kinetics Parameters Between Models
Parameters describing the transport and trapping of 18F-FDG in the lung demonstrated significant differences between the delayed input function model and the right heart input function model (Fig. 6). Linear regressions between parameters of the two models revealed significant deviations from the line of identity, particularly for parameters k 1, k 2, and F B, implying systematic differences in parameter estimates between the models (see Table S1, Online Resource 1 for details). This was confirmed by the percent differences in right heart input function model parameter estimates relative to those of the delayed input function model, which were dependent on the parameter and specific lung condition (Table 2). The right heart input function model yielded substantially higher estimates of parameters k 1 and k 2 relative to the delayed input function model, which resulted in slightly higher values of F e and K i in most conditions (Table 2). The right heart input function model also yielded lower estimates of 18F-FDG phosphorylation rate k 3 in the Control group and higher estimates of k 3 in both Lung Injury conditions, while fractional blood volume F B was lower in all conditions with this model. The parameters k 1, k 2, and F B, which are particularly relevant to the description of the early (<5 min) 18F-FDG kinetics, showed the largest discrepancies between the models, whereas k 3, F e, and K i , which determine mainly the later (>5 min) 18F-FDG kinetics, showed smaller differences between models.
Comparison of 18F-FDG kinetics parameters estimated with the delayed input function and right heart input function models. Substantial differences between the models were observed for the parameters k 1, k 2, and F B, which are primarily determined by the early phase of 18F-FDG kinetics. The 18F-FDG net uptake rate K i , phosphorylation rate k 3, and volume of distribution F e, were quite similar between the models, as data fell close to the line of identity.
Blood Flow Parameters Derived from 18F-FDG Kinetics
Measurements of relative regional perfusion derived from parameter estimates of the delayed input function model were strongly correlated with measurements of perfusion obtained from 13NN imaging (Fig. 7a). The best-fit lines relating the two sets of perfusion estimates had slopes that were not significantly different from 1, and intercepts not different from 0, in all three studied conditions. Both techniques detected a significant dependence of perfusion on ROI gravitational position (p < 0.001), with highest perfusion in dependent regions according to both techniques. Considering the 13NN perfusion estimates as a reference, there was no systematic relation between error in \(\dot{Q}_{\text{DIF}}\) and perfusion magnitude in any group (see Fig. S1, Online Resource 2).
(a) Regional perfusion estimated using the delayed input function model parameters (\(\dot{Q}_{\text{DIF}}\)) compared with the 13NN-saline reference method (\(\dot{Q}_{\text{REF}}\)). Data within each animal were mean-normalized, and are shown with distinct symbols. In all three groups, we found strong correlations between the two measurements, with regression lines not significantly different from the line of identity either in terms of slope or intercept. According to both techniques, perfusion was dependent on ROI gravitational position (p < 0.001), with non-dependent regions (empty symbols) showing lower perfusion than middle (gray) or dependent regions (black). (b) Mean-normalized fractional blood volume (F B) was also correlated with \(\dot{Q}_{\text{REF}}\), though the regression lines differed from the line of identity in terms of both slope and intercept for all three conditions. Of note, the positive intercepts in all groups imply the presence of a residual blood volume when perfusion is equal to zero. (c) Local lung delays (t ROI) were inversely associated with \(\dot{Q}_{\text{REF}}\) in all groups, with regions of higher perfusion showing shorter delays. Curves show regression of t ROI onto \(\dot{Q}_{\text{REF}}\).
Regional estimates of mean-normalized fractional blood volume as well as the transport delay were also associated with the reference perfusion measurements (Figs. 7b and 7c). Interestingly, while blood volume showed a strong linear association with perfusion, the intercept of this relation was significantly greater than 0, implying the presence of a positive lung blood volume in the absence of flow (Fig. 7b). The slopes of the F B vs. \(\dot{Q}_{\text{REF}}\) regression lines were smaller than 1 in all conditions. In addition, we found significant associations between the inverse of the local delays (t −1ROI ) and \(\dot{Q}_{\text{REF}}\), as would be expected from Eq. (4). * p < 0.05, ** p < 0.01, *** p < 0.001 for slope m different from 1 and intercept b different from 0.
When smaller regions were studied by dividing the lungs along the gravitational axis into a greater number of ROIs, the correlation coefficient between perfusion estimates of the two techniques decreased for all three studied lung conditions (see Fig. S2, Online Resource 2). Thus, for smaller ROIs, perfusion estimates obtained with the delayed input function model showed a weaker but still acceptable agreement with the reference method, implying a slight reduction in perfusion measurement accuracy with decreasing ROI size. The smallest ROI size studied was close to 50 mL, below which regional 18F-FDG kinetics became noisier and the model did not reliably converge.
Discussion
In this study, we propose an approach for modeling pulmonary 18F-FDG kinetics that includes an input function delay to account for tracer transport between the site of plasma function measurement (i.e., the right heart) and the plasma pool of the model ROI (i.e., the regional pulmonary capillary blood pool). By applying this technique in sheep models of healthy and injured lung conditions, we found that: (1) the delayed input function model provided significant improvements in description of 18F-FDG kinetics, with lower AIC values justifying the increased model complexity in 98% of studied regions; (2) vascular transport delays between the right heart and the lung range from 2.0 to 13.6 s and average 6.4 s, with highest values in gravitationally non-dependent regions; (3) 18F-FDG kinetics parameters k 1, k 2, and F B, which derive mainly from the early phase of kinetics, were different between the two models in all conditions studied; and (4) parameters derived with the delayed input function model provided measurements of relative regional perfusion that were highly correlated with those of an established 13NN perfusion imaging technique.
The calibration of image-derived input functions to account for temporal delays and dispersion is a topic that has received considerable attention in PET imaging of myocardial perfusion16,24 and cerebral perfusion37 and metabolism.12,45 However, applications in pulmonary imaging have been limited to 15O-H2O-PET of pulmonary perfusion, and input function delays have not been considered in pulmonary 18F-FDG-PET studies.4,23,32–34 In this study, we demonstrate clear differences in tracer arrival time between the right heart and the lungs following the start of 18F-FDG infusion, which emphasize the need for an input function delay in modeling pulmonary 18F-FDG kinetics. Thus, we modified the three-compartment model to include a unique input function delay in each lung region.
Lung-specific delays estimated with our model are closely related to pulmonary capillary transit times. In gravitationally distinct regions, we found lung-specific delays ranging from 1.2 to 12.8 s, with longest delays in non-dependent regions. Interestingly, Wagner et al. found a similar range of vertically dependent pulmonary capillary transit times in dogs of similar weight to our studied sheep, averaging 12.3 s in non-dependent regions and 1.6 s in dependent regions.41 In rats, capillary transit times showed a comparable range between 1.3 and 12.3 s, and were also dependent on blood flow.27 These consistent magnitudes, vertical trends, and flow dependence of pulmonary transit times between those studies and our data support the use of the delayed input function model to estimate regional pulmonary vascular transit times.
We chose to use the AIC to compare the two models of 18F-FDG kinetics. This statistical criterion is based on the concept of minimizing both the average error between model prediction and data as well as the number of model parameters.1,19 Thus, the delayed input function model received a penalty for its additional parameter, t delay. In spite of this, we found lower AIC values in 53 out of 54 ROIs, suggesting that including the delay as a model parameter results in an overall improvement of model quality that justifies the additional parameter. Also, the positive relationship between the difference in AIC between models and the magnitude of the local lung delay suggests that errors in the prediction of early 18F-FDG kinetics by the right heart input function model were dependent on the size of the delay itself. This finding is consistent with theoretical expectations, as the early kinetics of a region with a large delay would be poorly described by the plasma function of the right heart, since the tracer would arrive much earlier in the right heart than in the microcirculation of the lung. In such conditions, allowing a shift of the right heart plasma function in time should significantly improve the model description of local kinetics, a presumption directly supported by our data.
We found important differences between 18F-FDG kinetics parameters estimated with the delayed input function and right heart input function models. The right heart input function model yielded significantly lower estimates of F B and higher estimates of k 1 and k 2. These parameters describe the blood pool size and the transfer of tracer between plasma and tissue, and are associated with the description of the early stages of 18F-FDG kinetics. We did not find major differences between the models for K i , F e, and k 3, consistent with previous evidence that K i measurements in the brain are relatively insensitive to the input function.12 While we were not able to compare parameter estimates of each model to a gold-standard method, the improved accuracy of the delayed input function model estimates of k 1, k 2, and F B is supported by two key findings: (1) improved description of 18F-FDG kinetics with this model (as indicated by lower AIC values); and (2) accuracy of perfusion estimates, which were computed from the F B and t delay parameters of the model. Because the right heart input function model does not provide estimates of t delay (i.e., it is assumed to be 0), it is not possible to derive perfusion estimates with this model for comparison, as the denominator of Eq. (5) would equal zero.
These observed differences in kinetic parameter estimates may have implications for the interpretation of 18F-FDG-PET studies. For example, the parameter blood volume (F B), for which we found a small but systematic underestimation when the input function delay was ignored, was recently proposed by Pouzot et al. as a useful surrogate for regional perfusion.26 However, because their method only estimates relative and not absolute perfusion, and the F B values between the models were highly correlated (r = 0.99), the incorporation of the input function delay in their approach may not afford any improvement in precision. Importantly, we found no major differences between models in the parameters K i , F e, and k 3, which have been used in several studies to assess regional inflammatory cell activity.5–7,15,18,34,44 Given the recent evidence of the relevance of K i , F e, and k 3 to investigate the early stages of lung injury,7,8,44 this finding indicates that use of a three-compartment model without the input function delay is acceptable if these are the primary parameters of interest. However, future studies interested in measurements of k 1, k 2, or F B should consider using the delayed input function model to improve parameter accuracy.
Our measurements of relative perfusion derived with the delayed input function model showed strong linear associations with measurements of perfusion based on the 13NN-saline technique, an established method validated against gas exchange measurements.20,22,34,38,39 The robust agreement between these techniques was supported by several findings, including (1) best-fit lines comparing the two techniques not different from the line of identity; (2) no relationship between measurement differences and perfusion magnitude; and (3) reasonable limits of agreement (see Fig. S1, Online Resource 2). We also found that the agreement between the two techniques decreased when ROI size was reduced (see Fig. S2, Online Resource 2). Presumably, this finding was related to decreased signal-to-noise ratios in smaller ROIs, such that parameter accuracy was reduced in smaller ROIs, a trend previously described for PET-based measurements.42
The relation between blood volume and perfusion measurements revealed a strong linear association with positive intercepts and slopes less than 1 in all conditions. The finding of a positive intercept is consistent with the concept of mean circulatory filling pressure,13 in which a positive pressure and blood volume are observed in the circulation following interruption of the cardiac output. Fuld et al. also observed the presence of a positive intercept of blood volume for zero flow using CT-based measurements of pulmonary blood flow and blood volume.10 In contrast, Pouzot et al. found a negligible intercept when comparing 18F-FDG derived blood volume with 15O-H2O perfusion,26 which is inconsistent with that physiological principle of mean circulatory filling pressure. This discrepancy could be related to the underestimation of blood volume by Pouzot et al. in consequence of their non-consideration of the delay, as shown in the current work, and supports the relevance of considering a delay for improved physiological description. Contrary to the suggestion of Pouzot et al. that pulmonary transit times are homogeneous throughout the lungs,26 regional pulmonary vascular delays in our study were highly heterogeneous, in agreement with numerous previous studies on the distributions of pulmonary capillary transit times.3,27,40,41 Overall, these findings suggest that while blood volume may be correlated with perfusion in most lung conditions, more accurate quantification of perfusion can be achieved by additionally accounting for regional transit times, according to Eq. (4).
Parameters provided by the delayed input function model may be useful for new clinical applications of pulmonary 18F-FDG-PET. The ability to simultaneously obtain measurements of regional lung perfusion and metabolism with a single PET scan may facilitate future investigations of acute lung injury, wherein both perfusion and metabolism may show important and related changes.6 Additionally, the technique may be useful for applications in oncology. For example, Miles et al. showed that the relationships between tumor blood flow and metabolism in non-small cell lung cancer depend on tumor size and stage.21 Also, Bernstine et al. recently showed that time-to-peak measurements derived from early 18F-FDG kinetics are better than standardized uptake value for discriminating hepatocellular carcinomas from background liver tissue.2 Thus, simultaneous measurement of regional lung perfusion, delay times, and metabolism could be used for the grading of lung cancers based on tumor functional properties.
Some limitations of the delayed input function model are worth noting. First, the model requires an estimate of the cardiac output, which may not be available in all clinical or research applications. Nonetheless, cardiac output may be estimated from body surface area with reasonable accuracy,9 and errors in cardiac output do not have a substantial effect on delay estimates (see Online Resource 3). Another limitation is that inclusion of the additional parameter t delay adds uncertainty to parameter estimates. Below ROI sizes of about 50 mL, corresponding to 12 ROIs in each animal, we were not able to reliably achieve model convergence in all ROIs, with some returning non-physiologic parameter values. Thus, the resolution of regions that can be studied with the model is limited by the noise present in 18F-FDG kinetics. It is possible that improved signal-to-noise ratio with newer PET scanners, motion correction, or higher 18F-FDG doses would allow for smaller ROIs to be studied. The uncertainty associated with an additional parameter may also preclude the application of more complex compartmental models, such as the four-compartment model,33 since increasing numbers of parameters can have an undesirable effect on model convergence and parameter accuracy. Extension of the input function delay to other compartmental models will require further study and validation.
Our experimental approach could be improved by optimizing the 18F-FDG infusion and imaging protocol to better capture early 18F-FDG kinetics. Because our early PET frames were 10 s each, we relied on linear interpolation between the frames to estimate the delay with sub-second resolution. The accuracy of delay estimates could potentially be improved by using PET frames on the order of 2.5 s during the first minutes of imaging when the 18F-FDG arrives in the right heart and lungs, limiting the effect of errors due to interpolation. Nonetheless, the favorable comparison of lung-specific delays with previously published pulmonary capillary transit times,41 together with the accuracy of perfusion measurements based on estimated delays, suggest that interpolation errors were likely negligible in this study. Additionally, use of a bolus infusion may provide a better signal from which to estimate the difference in tracer arrival between the right heart and the lungs. We used a moderate rate of tracer infusion (approximately 1.3 mL/s), as this was the fastest pump setting available.
In conclusion, we have developed a new model of pulmonary 18F-FDG kinetics that accounts for delays in tracer arrival in the lungs relative to the site of plasma activity measurement. The model provides estimates of vascular transport delays between the right heart and distinct lung regions, and allows for significant improvements in prediction of 18F-FDG kinetics and in parameter estimation, justifying the use of the model over simpler models. Additionally, measurements of perfusion derived from the model parameters are highly accurate based on comparisons with an established 13NN-saline PET technique. The proposed method can be applied during routine clinical imaging by acquiring dynamic PET images and fitting the model to the imaged 18F-FDG kinetics in order to obtain simultaneous measurements of pulmonary transit times, perfusion, and inflammation with a single protocol.
References
Akaike, H. A new look at the statistical model identification. IEEE Trans. Autom. Control 19:716–723, 1974.
Bernstine, H., M. Braun, N. Yefremov, Y. Lamash, R. Carmi, D. Stern, A. Steinmetz, J. Sosna, and D. Groshar. FDG PET/CT early dynamic blood flow and late standardized uptake value determination in hepatocellular carcinoma. Radiology 260:503–510, 2011.
Capen, R. L., W. L. Hanson, L. P. Latham, C. A. Dawson, and W. W. Wagner, Jr. Distribution of pulmonary capillary transit times in recruited networks. J. Appl. Physiol. 69(473–478):1990, 1985.
Chen, D. L., M. A. Mintun, and D. P. Schuster. Comparison of methods to quantitate 18F-FDG uptake with PET during experimental acute lung injury. J. Nucl. Med. 45:1583–1590, 2004.
Chen, D. L., and D. P. Schuster. Positron emission tomography with [18F]fluorodeoxyglucose to evaluate neutrophil kinetics during acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 286:L834–L840, 2004.
Costa, E. L., G. Musch, T. Winkler, T. Schroeder, R. S. Harris, H. A. Jones, J. G. Venegas, and M. F. Vidal Melo. Mild endotoxemia during mechanical ventilation produces spatially heterogeneous pulmonary neutrophilic inflammation in sheep. Anesthesiology 112:658–669, 2010.
de Prost, N., E. L. Costa, T. Wellman, G. Musch, M. R. Tucci, T. Winkler, R. S. Harris, J. Venegas, B. Kavanagh, and M. F. Vidal Melo. Effects of ventilation strategy on distribution of lung inflammatory cell activity. Crit. Care 17:R175, 2013.
de Prost, N., E. L. Costa, T. Wellman, G. Musch, T. Winkler, M. R. Tucci, R. S. Harris, J. G. Venegas, and M. F. Vidal Melo. Effects of surfactant depletion on regional pulmonary metabolic activity during mechanical ventilation. J. Appl. Physiol. 111:1249–1258, 2011.
de Simone, G., R. B. Devereux, S. R. Daniels, G. Mureddu, M. J. Roman, T. R. Kimball, R. Greco, S. Witt, and F. Contaldo. Stroke volume and cardiac output in normotensive children and adults. Assessment of relations with body size and impact of overweight. Circulation 95:1837–1843, 1997.
Fuld, M. K., A. F. Halaweish, S. E. Haynes, A. A. Divekar, J. Guo, and E. A. Hoffman. Pulmonary perfused blood volume with dual-energy CT as surrogate for pulmonary perfusion assessed with dynamic multidetector CT. Radiology 267:747–756, 2013.
Galletti, G. G., and J. G. Venegas. Tracer kinetic model of regional pulmonary function using positron emission tomography. J. Appl. Physiol. 93:1104–1114, 2002.
Guo, H., R. A. Renaut, and K. Chen. An input function estimation method for FDG-PET human brain studies. Nucl. Med. Biol. 34:483–492, 2007.
Guyton, A. C., D. Polizo, and G. G. Armstrong. Mean circulatory filling pressure measured immediately after cessation of heart pumping. Am. J. Physiol. 179:261–267, 1954.
Harris, R. S., D. B. Willey-Courand, C. A. Head, G. G. Galletti, D. M. Call, and J. G. Venegas. Regional VA, Q, and VA/Q during PLV: effects of nitroprusside and inhaled nitric oxide. J. Appl. Physiol. 92:297–312, 2002.
Hartwig, W., E. A. Carter, R. E. Jimenez, R. Jones, A. J. Fischman, C. Fernandez-Del Castillo, and A. L. Warshaw. Neutrophil metabolic activity but not neutrophil sequestration reflects the development of pancreatitis-associated lung injury. Crit. Care Med. 30:2075–2082, 2002.
Herrero, P., J. J. Hartman, M. J. Senneff, and S. R. Bergmann. Effects of time discrepancies between input and myocardial time-activity curves on estimates of regional myocardial perfusion with PET. J. Nucl. Med. 35:558–566, 1994.
Huyer, W., and A. Neumaier. Global optimization by multilevel coordinate search. J. Glob. Optim. 14:331–355, 1999.
Jones, H. A., R. J. Clark, C. G. Rhodes, J. B. Schofield, T. Krausz, and C. Haslett. In vivo measurement of neutrophil activity in experimental lung inflammation. Am. J. Respir. Crit. Care Med. 149:1635–1639, 1994.
Landaw, E. M., and J. J. DiStefano, III. Multiexponential, multicompartmental, and noncompartmental modeling. II. Data analysis and statistical considerations. Am. J. Physiol. 246:R665–R677, 1984.
Melo, M. F., R. S. Harris, J. D. Layfield, and J. G. Venegas. Topographic basis of bimodal ventilation-perfusion distributions during bronchoconstriction in sheep. Am. J. Respir. Crit. Care Med. 171:714–721, 2005.
Miles, K. A., M. R. Griffiths, and C. J. Keith. Blood flow-metabolic relationships are dependent on tumour size in non-small cell lung cancer: a study using quantitative contrast-enhanced computer tomography and positron emission tomography. Eur. J. Nucl. Med. Mol. Imaging 33:22–28, 2006.
Musch, G., J. D. Layfield, R. S. Harris, M. F. Melo, T. Winkler, R. J. Callahan, A. J. Fischman, and J. G. Venegas. Topographical distribution of pulmonary perfusion and ventilation, assessed by PET in supine and prone humans. J. Appl. Physiol. 93:1841–1851, 2002.
Musch, G., J. G. Venegas, G. Bellani, T. Winkler, T. Schroeder, B. Petersen, R. S. Harris, and M. F. Melo. Regional gas exchange and cellular metabolic activity in ventilator-induced lung injury. Anesthesiology 106:723–735, 2007.
Pajevic, S., S. L. Bacharach, R. E. Carson, and G. H. Weiss. Effects of time delay in cardiac blood flow measurements by bolus H2(15)O. IEEE Trans. Med. Imaging 16:294–300, 1997.
Patlak, C. S., R. G. Blasberg, and J. D. Fenstermacher. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data. J. Cereb. Blood Flow Metab. 3:1–7, 1983.
Pouzot, C., J. C. Richard, A. Gros, N. Costes, F. Lavenne, D. Le Bars, and C. Guerin. Noninvasive quantitative assessment of pulmonary blood flow with 18F-FDG PET. J. Nucl. Med. 54:1653–1660, 2013.
Presson, Jr., R. G., T. M. Todoran, B. J. De Witt, I. F. McMurtry, and W. W. Wagner, Jr. Capillary recruitment and transit time in the rat lung. J. Appl. Physiol. 83(543–549):1997, 1985.
Rhodes, C. G., P. Wollmer, F. Fazio, and T. Jones. Quantitative measurement of regional extravascular lung density using positron emission and transmission tomography. J. Comput. Assist. Tomogr. 5:783–791, 1981.
Richard, J. C., M. Janier, F. Decailliot, D. Le Bars, F. Lavenne, V. Berthier, M. Lionnet, L. Cinotti, G. Annat, and C. Guerin. Comparison of PET with radioactive microspheres to assess pulmonary blood flow. J. Nucl. Med. 43:1063–1071, 2002.
Sackner, M. A., N. Atkins, J. Goldberg, N. Segel, S. Zarzecki, and A. Wanner. Pulmonary arterial blood volume and tissue volume in man and dog. Circ. Res. 34:761–769, 1974.
Saha, D., K. Takahashi, N. de Prost, T. Winkler, M. Pinilla-Vera, R. M. Baron, and M. F. Vidal Melo. Micro-autoradiographic assessment of cell types contributing to 2-deoxy-2-[(18)F]fluoro-D-glucose uptake during ventilator-induced and endotoxemic lung injury. Mol. Imaging Biol. 15:19–27, 2012.
Schroeder, T., M. F. Vidal Melo, G. Musch, R. S. Harris, J. G. Venegas, and T. Winkler. Image-derived input function for assessment of 18F-FDG uptake by the inflamed lung. J. Nucl. Med. 48:1889–1896, 2007.
Schroeder, T., M. F. Vidal Melo, G. Musch, R. S. Harris, J. G. Venegas, and T. Winkler. Modeling pulmonary kinetics of 2-deoxy-2-[(18)F]fluoro-d-glucose during acute lung injury. Acad. Radiol. 15:763–775, 2008.
Schroeder, T., M. F. Vidal Melo, G. Musch, R. S. Harris, T. Winkler, and J. G. Venegas. PET imaging of regional 18F-FDG uptake and lung function after cigarette smoke inhalation. J. Nucl. Med. 48:413–419, 2007.
Sergiacomi, G., F. Bolacchi, M. Cadioli, M. L. Angeli, F. Fucci, S. Crusco, P. Rogliani, G. Pezzuto, F. Romeo, E. Mariano, and G. Simonetti. Combined pulmonary fibrosis and emphysema: 3D time-resolved MR angiographic evaluation of pulmonary arterial mean transit time and time to peak enhancement. Radiology 254:601–608, 2010.
Sokoloff, L., M. Reivich, C. Kennedy, M. H. Des Rosiers, C. S. Patlak, K. D. Pettigrew, O. Sakurada, and M. Shinohara. The [14C]deoxyglucose method for the measurement of local cerebral glucose utilization: theory, procedure, and normal values in the conscious and anesthetized albino rat. J. Neurochem. 28:897–916, 1977.
van den Hoff, J., W. Burchert, W. Muller-Schauenburg, G. J. Meyer, and H. Hundeshagen. Accurate local blood flow measurements with dynamic PET: fast determination of input function delay and dispersion by multilinear minimization. J. Nucl. Med. 34:1770–1777, 1993.
Vidal Melo, M. F., D. Layfield, R. S. Harris, K. O’Neill, G. Musch, T. Richter, T. Winkler, A. J. Fischman, and J. G. Venegas. Quantification of regional ventilation-perfusion ratios with PET. J. Nucl. Med. 44:1982–1991, 2003.
Vidal Melo, M. F., T. Winkler, R. S. Harris, G. Musch, R. E. Greene, and J. G. Venegas. Spatial heterogeneity of lung perfusion assessed with (13)N PET as a vascular biomarker in chronic obstructive pulmonary disease. J. Nucl. Med. 51:57–65, 2010.
Wagner, Jr., W. W., L. P. Latham, M. N. Gillespie, J. P. Guenther, and R. L. Capen. Direct measurement of pulmonary capillary transit times. Science 218:379–381, 1982.
Wagner, Jr., W. W., L. P. Latham, W. L. Hanson, S. E. Hofmeister, and R. L. Capen. Vertical gradient of pulmonary capillary transit times. J. Appl. Physiol. 61(1270–1274):1986, 1985.
Wellman, T. J., T. Winkler, E. L. Costa, G. Musch, R. S. Harris, J. G. Venegas, and M. F. Melo. Measurement of regional specific lung volume change using respiratory-gated PET of inhaled 13N-nitrogen. J. Nucl. Med. 51:646–653, 2010.
Wellman, T. J., T. Winkler, E. L. Costa, G. Musch, R. S. Harris, J. G. Venegas, and M. F. Vidal Melo. Effect of regional lung inflation on ventilation heterogeneity at different length scales during mechanical ventilation of normal sheep lungs. J. Appl. Physiol. 113:947–957, 2012.
Wellman, T. J., T. Winkler, E. L. Costa, G. Musch, R. S. Harris, H. Zheng, J. G. Venegas, and M. F. Vidal Melo. Effect of local tidal lung strain on inflammation in normal and lipopolysaccharide-exposed sheep*. Crit. Care Med. 42:e491–e500, 2014.
Zanotti-Fregonara, P., M. Fadaili el, R. Maroy, C. Comtat, A. Souloumiac, S. Jan, M. J. Ribeiro, V. Gaura, A. Bar-Hen, and R. Trebossen. Comparison of eight methods for the estimation of the image-derived input function in dynamic [(18)F]-FDG PET human brain studies. J. Cereb. Blood Flow Metab. 29:1825–1835, 2009.
Acknowledgments
This work was supported by grants R01-HL121228 and R01-HL086827 from the National Heart, Lung, and Blood Institute. The authors declare that they have no conflicts of interest. Two authors (T. Winkler and M. F. Vidal Melo) have a patent related to the computation of the image-derived input function used in the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
Associate Editor Merryn Tawhai oversaw the review of this article.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Wellman, T.J., Winkler, T. & Vidal Melo, M.F. Modeling of Tracer Transport Delays for Improved Quantification of Regional Pulmonary 18F-FDG Kinetics, Vascular Transit Times, and Perfusion. Ann Biomed Eng 43, 2722–2734 (2015). https://doi.org/10.1007/s10439-015-1327-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10439-015-1327-2