Abstract
The aim of this cross-sectional study was to develop a Frailty at Risk Scale (FARS) incorporating ten well-known determinants of frailty: age, sex, marital status, ethnicity, education, income, lifestyle, multimorbidity, life events, and home living environment. In addition, a second aim was to develop an online calculator that can easily support healthcare professionals in determining the risk of frailty among community-dwelling older people. The FARS was developed using data of 373 people aged ≥ 75 years. The Tilburg Frailty Indicator (TFI) was used for assessing frailty. Multivariate logistic regression analysis showed that the determinants multimorbidity, unhealthy lifestyle, and ethnicity (ethnic minority) were the most important predictors. The area under the curve (AUC) of the model was 0.811 (optimism 0.019, 95% bootstrap CI = −0.029; 0.064). The FARS is offered on a Web site, so that it can be easily used by healthcare professionals, allowing quick intervention in promoting quality of life among community-dwelling older people.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
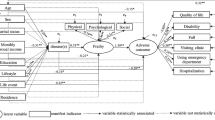
Frailty may be defined as a dynamic state affecting an individual who experiences losses in one or more domains of human functioning (physical, psychological, and social), which is caused by the influence of a range of variables and increases the risk of adverse outcomes (Gobbens et al., 2010a). The influencing variables and adverse outcomes are presented in an integral conceptual model of frailty (Gobbens et al., 2010b). As the model shows, possible outcomes of frailty are disability (Liu et al., 2019), increased healthcare utilization (Fried et al., 2001; Rockwood et al., 2005), and mortality (Shamliyan et al., 2013; Vermeiren et al., 2016). Influencing factors (i.e., potential determinants of frailty) included in the model are: age, sex, marital status ethnicity, education, income, lifestyle, multimorbidity, life events, and home living environment (Gobbens et al., 2010b).
Many scales have been developed to assess multidimensional frailty, including physical, psychological, and social domains, among community-dwelling older people, such as the Frailty Index (FI) (Mitnitski et al., 2002), the Electronic Frailty Index (eFI) (Clegg et al., 2016), the Groningen Frailty Indicator (GFI) (Schuurmans et al., 2004), the SUNFRAIL tool (Gobbens et al., 2020), and the Tilburg Frailty Indicator (TFI) (Gobbens et al., 2010d). The last scale, the TFI, especially fits perfectly with the integral conceptual model of frailty. To prevent or postpone frailty, it is important to identify determinants of frailty. Numerous studies have been carried out on the associations between individual and combinations of determinants and frailty (Collard et al., 2012; Freer & Wallington, 2019; Gobbens et al., 2010c; Niederstrasser et al., 2019; Ocampo-Chaparro et al., 2019; van Assen et al., 2016; Yadav et al., 2019). For instance, a systematic review showed that the prevalence of frailty increased with age and was higher among women than men (Collard et al., 2012), and in a sample of 47,768 individuals aged ≥ 65 years, similar findings were reached (van Assen et al., 2016). In addition, a study among community-dwelling older people aged 75 years or older observed that medium income, an unhealthy lifestyle, and multimorbidity were associated with frailty, after controlling for other determinants (age, sex, education, and life events) (Gobbens et al., 2010c). Another study stressed the importance of environmental factors in predicting frailty (Freer & Wallington, 2019).
It is important that healthcare professionals have at their disposal a scale with which determinants of frailty easily can be identified, since the findings of such an assessment tool can provide healthcare professionals with points of leverage for interventions to prevent or postpone frailty. As far as we know, such a scale does not yet exist. Therefore, the main aim of the present study was to develop a Frailty at Risk Scale (FARS) incorporating all determinants included in both the integral conceptual model of frailty and part A of the TFI. In addition, we aimed to develop an online calculator that can easily support primary care professionals in determining the risk of frailty among community-dwelling older people.
Methods
Study population and data collection
For the present cross-sectional study, we used a sample of 479 community-dwelling people aged ≥ 75 years, referring to a 42% response rate, which has also been used in previous studies (Gobbens et al., 2010c, d). The participants were resident in (blinded for review) the Netherlands. In 2008, they received a self-report questionnaire by mail containing scales for determining frailty, quality of life, and disability.
Measures
Determinants
Part A of the TFI was used in order to collect data on the determinants of frailty: age, sex, marital status ethnicity, education, income, lifestyle, multimorbidity, life events, and home living environment (Gobbens et al., 2010d). Table 1 shows the questions and response categories referring to these ten determinants.
Frailty
We used part B of the TFI to assess frailty. This part contains 15 items referring to the physical (eight items), psychological (four items), and social (three items) domains of frailty (Gobbens et al., 2010d). The score ranges from 0 to 15, with higher scores indicating greater frailty. The cut-off point for distinguishing non-frail and frail older people is 5 (Gobbens et al., 2010d). Previous studies have demonstrated that the TFI is a valid and reliable questionnaire for assessing frailty among community-dwelling older people (Dong et al., 2017; Gobbens et al., 2010d, 2012; Santiago et al., 2013; Uchmanowicz et al., 2016).
Statistical analysis
We used counts and percentages to describe the baseline characteristics of the participants. All variables were dichotomized for the modeling. The Chi-square test was used as a univariate technique to compare the dichotomous variables with respect to the dichotomized outcome variable. A p-value < 0.05 was considered as significant. Cramer’s V, a statistic derived from the Chi-square value, was used as an association measure. Values toward 0 indicate weak association and values toward 1 indicate strong association. For the multivariate analysis, we used logistic regression with all ten dichotomized predictor variables and the dichotomized outcome (frail vs non-frail). The predictive performance of the model was measured using the area under the curve (AUC). An AUC > 0.700 was considered as an indication for good predictive performance (Steyerberg, 2009). Internal validation was done using bootstrapping (1000 repetitions) to gain insight in the optimism of the logistic regression model. External validation was performed using a dataset consisting of comparable participants in 2013. A nomogram was constructed based on the transformed coefficients (coefficients divided by the maximum of the coefficients and multiplied by 100) of the logistic regression model. Based on the nomogram, we designed a calculator that is accessible on the Web (blinded for review). For the analyses, we used R version 3.4.4. (R Core Team, 2018).
Results
Table 1 shows the participant characteristics. We dichotomized the variables that had more than two categories. Age was recoded into the categories < 85 and ≥ 85 years, and frailty score was recoded into the categories < 5 (non-frail) and ≥ 5 (frail), and we deleted the participants with missing values for one or more variables, leaving 373 participants. The percentage missing values for the variables varied from 0.0% to 8.6%. Table 1 also shows the p-values of the Chi-square test for each characteristic. Six out of ten characteristics had p-values < 0.05.
Figure 1 shows the univariate association of the variables measured by Cramer’s V. The outcome variable (frail or non-frail) had the strongest association with diseases and/or chronic disorders, followed by the variable lifestyle.
Table 2 shows the points for each variable based on multivariate analysis with the logistic regression technique. Table 2 also shows that variable diseases and/or chronic disorders had the highest influence on the outcome variable (frail vs non-frail), whereas variable education had the lowest influence.
Figure 2 is the nomogram derived from the logistic regression analysis with all ten predictors and frail/non-frail as outcome. An example of the use of this nomogram: Suppose the participant is a woman with an income ≤ 1800 euro. The total number of points for that participant is then 27 + 39 = 66, and the corresponding estimated probability for being frail is then approximately 0.15. Our online calculator (blinded for review), which automatically calculates the points and the probability for being frail for a participant, presents the same results. This calculator is prefilled for the most favorable situation, leading to a probability for being frail of 6%. By clicking on the radio buttons, the characteristics of a participant can be changed.
The AUC of model was 0.811 (optimism 0.019, 95% bootstrap CI = − 0.029–0.064).
Discussion
Frail community-dwelling older people have a high risk of disability (Liu et al., 2019), increased healthcare utilization (e.g., hospitalization and institutionalization) (Fried et al., 2001; Rockwood et al., 2005), and premature death (Shamliyan et al., 2013; Vermeiren et al., 2016). Moreover, frailty is associated with lower quality of life (Kojima, Iliffe, Jivraj, et al., 2016). It is therefore important to have insight into the determinants of frailty so that healthcare professionals can identify older people who are at increased risk of frailty, which allows the opportunity to carry out interventions to prevent or delay frailty. Currently, many studies have been conducted that provide evidence on individual determinants of frailty. In the present study, we expanded this viewpoint. We aimed to create a Frailty at Risk Scale (FARS) in which we included ten well-known determinants listed in the integral conceptual model of frailty and the TFI: age, gender, marital status, education, income, ethnicity, lifestyle, multimorbidity, life events, and home living environment (Gobbens et al., 2010b, 2010d).
In the model with all ten determinants, the AUC for predicting frailty (frail, non-frail) was 0.811, which can be considered an indication for a good predictive performance (Steyerberg, 2009). A nomogram derived from logistic regression analysis showed the points that must be given to the ten determinants used to predict frailty. These points range from 20 (education) up to and including 100 (diseases and/or chronic disorders). Not surprisingly, diseases and/or chronic disorders, referring to as multimorbidity, are the most important predictor of frailty. A systematic review and meta-analysis conducted by the Joint Action ADVANTAGE WP4 Group observed that multimorbidity was associated with frailty in pooled analyses (odds ratio = 2.27; 95% CI = 1.97−2.62, 12 = 47.7%) (Vetrano et al., 2019). Our logistic regression analysis also demonstrated that an unhealthy lifestyle and ethnicity were important predictors for frailty; in the FARS, 81 and 73 points were attributed to these two determinants, respectively. Previous studies also supported these findings. An unhealthy lifestyle is characterized by poor dietary habits, poor oral health, sedentary behavior, smoking, and excessive use of alcohol. For each of these factors, there is evidence that they are associated with frailty (Amiri & Behnezhad, 2019; Hakeem et al., 2019; Kojima, Iliffe, Liljas et al., 2017; Schoufour et al., 2019). However, it should be mentioned that a physical definition, the phenotype of frailty (Fried et al., 2001), was often used. With regard to the predictor ethnicity, ethnic minority migrants living in economically developed countries showed higher prevalence figures of frailty than white, indigenous older people (Majid et al., 2020). Moreover, in a sample of 47,768 Dutch people aged ≥ 65 years, very large frailty differences, assessed with the TFI, existed between autochthonous people and people belonging to ethic groups (i.e., Surinamese, Turks, Moroccans) (van Assen et al., 2016).
Based on multivariate logistic regression analyses, nine determinants of frailty had the expected association with the dichotomized frailty score (frail, non-frail). The exception was education. Several studies provided evidence that a low educational level was associated with frailty (Etman et al., 2012; Hoogendijk et al., 2014). For instance, in a sample of 1,205 Dutch people aged 65 years or older, it was observed that those with a low educational level had higher odds of being physically frail than those with a high educational level (relative index of inequality odds ratio, 2.94; 95% CI = 1.84−4.71) (Hoogendijk et al., 2014); however, this effect was reduced by 76% after adjustment for other predictors (e.g., income and number of chronic diseases) (Hoogendijk et al., 2014). In the present study, the coefficient of higher education in the univariate analysis with logistic regression was −0.166, indicating that higher education decreases the probability for being frail. In the multivariate analysis, the coefficient of higher education was 0.313, indicating that higher education increases the probability for being frail. Both coefficients, however, were not significant (p-values 0.568 and 0.422, respectively), so these effects can be considered as very small and an explanation for the low number of points allocated to education (only 20). Because previous studies used a physical definition of frailty, we recommend future studies to examine the association between education and multidimensional frailty, including physical, psychological, and social components.
Some limitations of our study should be mentioned. First, some variables have low frequencies in the categories. In particular, this applies to ethnicity, life events, and satisfaction with living environment. This may have influenced our findings. Secondly, our prediction model is based on one sample of community-dwelling older people. The generalizability of our findings can therefore be called into question. Because it is important to establish whether our model could also predict frailty in the future, we carried out an additional analysis using the same sample with a follow-up of five years (n = 140); the mean age was 83.8 (standard deviation = 3.1); the percentage of women was 49.3%. The AUC turned out to be 0.750, which can be regarded as good (Steyerberg, 2009). However, because our prediction model is only based on data of community-dwelling older people, validating in other populations (e.g., residents of assisted living facilities, hospitalized older people) is recommended. In addition, we recommend further validation of the FARS using other multidimensional frailty scales, e.g., the FI (Mitnitski et al., 2002), and more unidimensional frailty scales such as the Phenotype of Frailty (Fried et al., 2001), and the Clinical Frailty Scale (CFS) (Rockwood et al., 2005). Finally, the response rate was 42%. The consequence of non-response may be an underestimation of frailty among community-dwelling older people. This also reduces the generalizability of our findings.
The product in our study is a calculator that is accessible on the Web. This calculator allows healthcare professionals to easily enter data, on the basis of which risk of frailty is automatically calculated. If the risk of frailty is high, healthcare professionals may be able to deploy interventions to prevent frailty, e.g., lifestyle interventions, dealing with chronic diseases and life events, and contributing to greater satisfaction with one’s living environment/home. Another option is to use part B of the TFI to determine the extent to which frailty already exists. The outcome of this assessment will support healthcare professionals in carrying out interventions focused on improving physical, psychological, and social frailty.
In conclusion, in the present study we developed a FARS, characterized by an excellent AUC for predicting frailty, containing ten questions referring to determinants of frailty that can be used to determine the risk level for an individual older person being frail. The determinants to which most weight has been assigned are: diseases and/or chronic disorders (present), lifestyle (unhealthy), and ethnicity (ethnic minority migrants). We offer the FARS on a Web site, so that the instrument can be used by healthcare professionals without a threshold, allowing timely intervention in promoting quality of life among community-dwelling older people.
Data availability and material
All data were pseudonymized and stored in a central and secure server at (blinded for review). Furthermore, we have complied with the law with regard to personal data privacy information (Dutch Data Protection Authority.
References
Amiri S, Behnezhad S (2019) Systematic review and meta-analysis of the association between smoking and the incidence of frailty. Neuropsychiatry 33(4):198–206. https://doi.org/10.1007/s40211-019-0315-4
Central Committee on Research Involving Human Subjects (2010). Does your study have to be reviewed? Available from: http://www.ccmo-online.nl/ Home >for investigators >Review step plan RC >WMO). Assessed June 15 2010).
Clegg A, Bates C, Young J, Ryan R, Nichols L, Teale EA, Mohammed MA, Parry J, Marshall T (2016) Development and validation of an electronic frailty index using routine primary care electronic health record data. Age Age 45(3):353–360. https://doi.org/10.1093/ageing/afw039
Collard RM, Boter H, Schoevers RA, Oude Voshaar RC (2012) Prevalence of frailty in community-dwelling older persons: a systematic review. J Am Geriatr Soc 60(8):1487–1492. https://doi.org/10.1111/j.1532-5415.2012.04054.x
Dong L, Liu N, Tian X, Qiao X, Gobbens RJJ, Kane RL, Wang C (2017) Reliability and validity of the Tilburg Frailty Indicator (TFI) among Chinese community-dwelling older people. Arch Gerontol Geriatr 73:21–28. https://doi.org/10.1016/j.archger.2017.07.001
Etman A, Burdorf A, Van der Cammen TJ, Mackenbach JP, Van Lenthe FJ (2012) Socio-demographic determinants of worsening in frailty among community-dwelling older people in 11 European countries. J Epidemiol Commun Health 66(12):1116–1121. https://doi.org/10.1136/jech-2011-200027
Freer K, Wallington SL (2019) Social frailty: the importance of social and environmental factors in predicting frailty in older adults. Br J Commun Nurs 24(10):486–492. https://doi.org/10.12968/bjcn.2019.24.10.486
Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, Seeman T, Tracy R, Kop WJ, Burke G, McBurnie MA, Cardiovascular Health Study Collaborative Research Group (2001) Frailty in older adults: evidence for a phenotype. J Gerontol Series A, Biol Sci Med Sci 56(3):146–156
Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010a) Toward a conceptual definition of frail community dwelling older people. Nurs Outlook 58(2):76–86. https://doi.org/10.1016/j.outlook.2009.09.005
Gobbens RJ, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010b) Towards an integral conceptual model of frailty. J Nutr, Health Ag 14(3):175–181. https://doi.org/10.1007/s12603-010-0045-6
Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010c) Determinants of frailty. J Am Med Dir Assoc 11(5):356–364. https://doi.org/10.1016/j.jamda.2009.11.008
Gobbens RJ, van Assen MA, Luijkx KG, Wijnen-Sponselee MT, Schols JM (2010d) The Tilburg frailty indicator: psychometric properties. J Am Med Dir Assoc 11(5):344–355. https://doi.org/10.1016/j.jamda.2009.11.003
Gobbens RJ, van Assen MA, Luijkx KG, Schols JM (2012) The predictive validity of the Tilburg Frailty Indicator: disability, health care utilization, and quality of life in a population at risk. Gerontologist 52(5):619–631. https://doi.org/10.1093/geront/gnr135
Gobbens RJJ, Maggion M, Longobucco Y, Barbolini M (2020) The validity of the Sunfrail tool: a cross-sectional study among Dutch community-dwelling older people. J Frailty Ag 9(4):219–225. https://doi.org/10.14283/jfa.2020.4
Hakeem FF, Bernabé E, Sabbah W (2019) Association between oral health and frailty: a systematic review of longitudinal studies. Gerodontology 36(3):205–215. https://doi.org/10.1111/ger.12406
Hoogendijk EO, van Hout HP, Heymans MW, van der Horst HE, Frijters DH, Broese van Groenou MI, Deeg DJH, Huisman M (2014) Explaining the association between educational level and frailty in older adults: results from a 13-year longitudinal study in the Netherlands. Ann Epidemiol 24(7):538-544.e532. https://doi.org/10.1016/j.annepidem.2014.05.002
Kojima G, Iliffe S, Jivraj S, Walters K (2016) Association between frailty and quality of life among community-dwelling older people: a systematic review and meta-analysis. J Epidemiol Commun Health 70(7):716–721. https://doi.org/10.1136/jech-2015-206717
Kojima G, Iliffe S, Liljas A, Walters K (2017) Non-linear association between alcohol and incident frailty among community-dwelling older people: a dose-response meta-analysis. Biosci Trends 11(5):600–602. https://doi.org/10.5582/bst.2017.01237
Liu HX, Ding G, Yu WJ, Liu TF, Yan AY, Chen HY, Zhang AH (2019) Association between frailty and incident risk of disability in community-dwelling elder people: evidence from a meta-analysis. Public Health 175:90–100. https://doi.org/10.1016/j.puhe.2019.06.010
Majid Z, Welch C, Davies J, Jackson T (2020) Global frailty: the role of ethnicity, migration and socioeconomic factors. Maturitas 139:33–41. https://doi.org/10.1016/j.maturitas.2020.05.010
Mitnitski AB, Graham JE, Mogilner AJ, Rockwood K (2002) Frailty, fitness and late-life mortality in relation to chronological and biological age. Biomed Cent Geriatr 2:1. https://doi.org/10.1186/1471-2318-2-1
Niederstrasser NG, Rogers NT, Bandelow S (2019) Determinants of frailty development and progression using a multidimensional frailty index: evidence from the English Longitudinal Study of Ageing. Public Lib Sci one 14(10):e0223799. https://doi.org/10.1371/journal.pone.0223799
Ocampo-Chaparro JM, Reyes-Ortiz CA, Castro-Flórez X, Gómez F (2019) Frailty in older adults and their association with social determinants of Health. SABE Colombia Study Colombian Medica (Cali) 50(2):89–101. https://doi.org/10.25100/cm.v50i2.4121
R Core Team. R: A language and environment for statistical computing. 2018. https://www.R-project.org/.
Rockwood K, Song X, MacKnight C, Bergman H, Hogan DB, McDowell I, Mitnitski A (2005) A global clinical measure of fitness and frailty in elderly people. Can Med Assoc J 173(5):489–495. https://doi.org/10.1503/cmaj.050051
Santiago LM, Luz LL, Mattos IE, Gobbens RJ, van Assen MA (2013) Psychometric properties of the Brazilian version of the Tilburg frailty indicator (TFI). Arch Gerontol Geriatr 57(1):39–45. https://doi.org/10.1016/j.archger.2013.03.001
Schoufour JD, Overdevest E, Weijs PJM, Tieland M (2019) Dietary protein, exercise, and frailty domains. Nutrients. https://doi.org/10.3390/nu11102399
Schuurmans H, Steverink N, Lindenberg S, Frieswijk N, Slaets JP (2004) Old or frail: what tells us more? J Gerontol Series A, Biol Sci Med Sci 59(9):M962-965. https://doi.org/10.1093/gerona/59.9.m962
Shamliyan T, Talley KM, Ramakrishnan R, Kane RL (2013) Association of frailty with survival: a systematic literature review. Ageing Res Rev 12(2):719–736. https://doi.org/10.1016/j.arr.2012.03.001
Steyerberg E (2009) Clinical prediction models. a practical approach to development, validation, and updating, 1st edn. Springer-Verlag, New York
Uchmanowicz I, Jankowska-Polanska B, Uchmanowicz B, Kowalczuk K, Gobbens RJ (2016) Validity and reliability of the polish version of the Tilburg Frailty Indicator (TFI). J Frailty Ag 5(1):27–32. https://doi.org/10.14283/jfa.2015.66
van Assen MA, Pallast E, Fakiri FE, Gobbens RJ (2016) Measuring frailty in Dutch community-dwelling older people: Reference values of the Tilburg Frailty Indicator (TFI). Arch Gerontol Geriatr 67:120–129. https://doi.org/10.1016/j.archger.2016.07.005
Vermeiren S, Vella-Azzopardi R, Beckwee D, Habbig AK, Scafoglieri A, Jansen B, Bautmans I (2016) Frailty and the prediction of negative health outcomes: a meta-analysis. J Am Med Dir Assoc 17(12):1163.e1161-1163.e1117. https://doi.org/10.1016/j.jamda.2016.09.010
Vetrano DL, Palmer K, Marengoni A, Marzetti E, Lattanzio F, Roller-Wirnsberger R, Samaniego LL, Rodriguez-Manas L, Bernabei R, Onder G, Joint Action ADVANTAGE WP4 Group (2019) Frailty and multimorbidity: a systematic review and meta-analysis. J Gerontol Series A, Biol Sci Med Sci 74(5):659–666. https://doi.org/10.1093/gerona/gly110
Yadav UN, Tamang MK, Thapa TB, Hosseinzadeh H, Harris MF, Yadav KK (2019) Prevalence and determinants of frailty in the absence of disability among older population: a cross sectional study from rural communities in Nepal. Biomed Cent Geriatr 19(1):283. https://doi.org/10.1186/s12877-019-1290-0
Acknowledgements
The authors would like to thank the study participants for their contributions to this study.
Funding
No funds, grants, or other support was received.
Author information
Authors and Affiliations
Contributions
RG was involved in study concept and design, acquisition of subjects and data, and preparation of manuscript (drafting, final approval). TP was involved in study concept and design, analysis and interpretation of data, and preparation of manuscript (drafting, final approval). Both authors agree to be accountable for all aspects of the work.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest for this work.
Ethical approval
For this study, medical ethics approval was not necessary as particular treatments or interventions were not offered or withheld from respondents. The integrity of the respondents was not encroached upon as a consequence of participating in this study, which is the main criterion in medical–ethical procedures in the Netherlands (Central Committee on Research Involving Human Subjects, 2010). This research was conducted according to the guidelines for good clinical practice. The researchers did not make the questionnaire long so the burden on participants would be limited; the average time for completing the questionnaire was 20 min. In addition, the questionnaire contained measures that have already been used in many previous studies among older people.
Consent to participate
Informed consent related to detailing the study (e.g., information about the purpose of the study) and maintaining confidentiality was observed.
Additional information
Responsible Editor: Morten Wahrendorf
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Gobbens, R.J.J., van der Ploeg, T. Frailty at Risk Scale (FARS): development and application. Eur J Ageing 19, 301–308 (2022). https://doi.org/10.1007/s10433-021-00628-4
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10433-021-00628-4