Abstract
Alginate-based hydrogels are frequently employed in biomedical fields, where their size and uniformity are important for applications such as encapsulating pharmaceuticals or biological agents. In this paper, we study alginate droplet formation in a flow-focusing microfluidic device with a rectangular orifice of \(30\times 100\) microns in the presence of different types of surfactants in the continuous phase. This study looks at how surfactant type and concentration affect the generation of alginate microdroplets using droplet-based microfluidics, as well as how they affect the created alginate gel beads. To make alginate hydrogels, we used an external ionic cross-linking approach. Alginate hydrogels are formed by on-chip emulsifying a sodium alginate solution in an oil phase and collecting the emulsions in a bath containing calcium ions. Off-chip introduction of the cross-linking agents will reduce clogging issues that are common in droplet microfluidics. The diameter of microdroplets varies depending on the surfactant added to the continuous phase, either Span 80 or Tween 80, and the flow rate ratio between the dispersed and continuous phases. The conditions under which microdroplets form (the flow rate of the continuous phase and the surfactant supplied to it) determine the degree of gelation, shape, size, and monodipersity of hydrogels. Tween 80 emulsification results in the most homogeneous, totally gelled, tail-shaped hydrogels, whereas Span 80 emulsification results in smaller hydrogels with a more irregular size distribution. Using Span 80 resulted in partial gelation, necessitating a change in the collection bath (such as adding ethyl acetate to the oil phase).
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
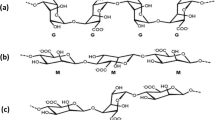
Droplet-based microfluidics has been shown to be capable of producing highly repeatable monodisperse droplets. This technology can be used to produce functional polymeric microparticles by solidifying droplets in a variety of ways. Following that, the microparticles of uniform size can be used to encapsulate pharmaceuticals or biological agents (Kim et al. 2014). Polysaccharides (alginate, chitosan, etc.) as natural polymers are suitable options for microgel generation owing to their biodegradability and biocompatibility (Ma et al. 2017). Alginate, an acidic polymer derived from algae, made up of the monomers \(\beta\)-D-mannuronic acid (M) and \(\alpha\)-L-guluronic acid (G), gels in the presence of divalent metal cations. When divalent cations bind to the carboxylates in the G-blocks to form the ’egg-box’ structures, alginate readily forms hydrogel (Voo et al. 2016). This polymer has demonstrated intriguing properties in a variety of fields and applications, ranging from medicinal to environmental protection (Vicini et al. 2017). The easiest method of fabricating alginate microgels is to drip alginate microdroplets into a solution containing the cross-linking agent. Using droplet-based microfluidics, the desired emulsion (mostly an aqueous solution of alginate-in-oil emulsion) is produced, and gelation follows. Ionic gelation can be accomplished in two ways: by adding the insoluble or poorly soluble cross-linking agent salt to the alginate solution and activating the gelation with acidified oils (internal method), or by adding the cross-linking agent to previously fabricated alginate microdroplets (external or diffusion method) (Chen et al. 2021). The cross-linking agent in the form of a calcium salt solution can be applied to the microdroplets either off-chip or on-chip in the external approach. The pre-fabricated droplets are dripped into the calcium solution in the off-chip one (Liu et al. 2018). The particle size and size distribution, which is commonly stated as a coefficient of variation, are key parameters in the synthesis of microparticles. Additionally, their morphology is crucial, especially for drug delivery applications. During the production of alginate microgels, these parameters can be manipulated by modifying the external or internal variables that may influence the quality of the alginate beads (Lin et al. 2013). Alginate type, its concentration (viscosity), and crosslinking type are internal variables, while dispersed and continuous phase flow rates, crosslinking method, and interfacial tension between the two phases are external variables in the droplet-microfluidic method. Using an external approach, the effects of internal variables on microfluidic-based alginate microbead formation have been studied previously (Lin et al. 2013; Hu et al. 2015), which demonstrated that increasing alginate viscosity and decreasing CaCl2 concentration both increased the size of the microbeads. Surfactants play a critical role in droplet formation by reducing the interfacial tension and preventing coalescence. In general, the surfactant is chosen according to the HLB (Hydrophilic-lipophilic Balance) indicator and the desired emulsion type, oil in water (o/w) or water in oil (w/o). Surfactants are one of the parameters that control the size of the droplets in microfluidics. According to He et al. (2020), droplet sizes can be reduced in a flow-focusing geometry by increasing a single surfactant Span 80, (Sorbitan monooleate) concentration in the oil phase at a constant flow rate for the two phases. Peng et al. (2011) adjusted only the concentration of Tween 20 in the water phase to generate different size of monodispersed water in oil droplets. The effect of surfactant addition on thinning and dynamics of droplet pinch off in a flow-focusing device is studied by Kiratzis et al. (2022). In bulk method of alginate microgels formation, such as emulsification or dripping methods, it was stated that the kind and concentration of the surfactants have an effect on the characteristics of the generated microgels, such as their size (Lupo et al. 2014), shape (Sadeghi et al. 2021; Davarcı et al. 2016), stability (Damiati 2020), and mechanical properties (Kaygusuz et al. 2016). Davarcı et al. (2016) added a surfactant (Tween 20) to the CaCl2 solution in the gelation bath and reported that increasing the surfactant concentration increases droplet penetration into the bath in dripping method. Using the bulk emulsification technique, Chaemsawang et al. (2018) produced alginate microparticles and reported that no microparticles formed in the absence of surfactant or when Span 80 was used as a surfactant in the oil phase. However, when Tween 80 was used as the surfactant in the oil phase, microparticles developed. Additionally, it was mentioned that when Tween 80 concentrations rise above a certain point, microparticle size decreases and sphericalness increases. Numerous surfactants have been reported to be used in the droplet microfluidics-based synthesis of alginate microbeads. The most commonly employed surfactant in the oil phase is Span 80, a w/o emulsifier with an HLB value of 4.3 and a hydrophobic character. In the on-chip introduction of the cross-linking agents either using internal (Damiati 2020; Wang et al. 2017) or external (Zhang et al. 2006; Kim et al. 2017; Yu et al. 2019; Zou et al. 2019; Hu et al. 2012) gelation procedures, Span 80 was added to the oil phase as the carrier fluid. This surfactant was also used in the collection bath of the procedures involving the off-chip introduction of the cross-linking agent (Yeh et al. 2009; Wang et al. 2012; Capretto et al. 2008). Tween 80, (Polysorbate 80) has also been used in the oil phase of alginate-in-oil emulsions, although it is known as a hydrophilic surfactant (HLB=15). It was also used as an emulsifier in the acidic oil in a container into which CaCO3-containing alginate microdroplets were dripped (Huang et al. 2007). Additionally, the continuous phase was prepared by mixing Tween 80 and mineral oil in an internal and on-chip manufacturing of alginate microbeads (Zhang et al. 2006).
The objective of this study is to figure out how external factors, which are chosen to be continuous phase flow rate and the type and concentration of the surfactant added to this phase affect how alginate beads form. We will alter the alginate microbeads by regulating the continuous phase and ensuring that the dispersed phase has a constant composition and flow rate. Due to channel clogging issues, we chose low-viscosity alginate, which is the best type for a microfluidic approach. To our knowledge, no research has been conducted on how the type and amount of surfactant in the continuous phase affects the production of alginate microgels using droplet-based microfluidics, and the studies in this field have focused on the effect of surfactant only on droplet formation. The size, throughput, and dispersity of alginate microdroplets, as well as the size and shape of synthesized microgels in the presence of different surfactants, are characterized. We used a conventional PDMS (Polydimethylsiloxane) flow-focusing microfluidic chip equipped with two inlets and one outlet. As the continuous phase, we used different amounts of Span 80 or Tween 80 in mineral oil. Alginate microdroplets are generated in different dynamic regimes, depending on the flow rate ratio of the two phases and the interfacial tension. To make alginate microgels, we introduced cross-linking agents off-chip and from the outside of the microgels. This strategy was derived from Hu et al. (2015) method with modifications. Our findings illustrate the effects of the flow rate ratio (between continuous and dispersed phases) and surfactant content on the characteristics of alginate microdroplets. We also looked at how the flow rate ratio, type of surfactant, and concentration affected the microgels that were made. Also, we came up with a way to change the shape of the microgels by adding ethyl acetate to the oil phase of the collecting bath.
2 Experimental
2.1 Materials
Light mineral oil (kinematic viscosity at \(25\,^\circ\)C=20.2\(-\)22.2 cSt), Sodium Alginate (Product number W201502, kinematic viscosity in water at \(25\,^\circ\)C=19.4 cSt), Calcium Chloride, and surfactants (Span 80 and Tween 80) were purchased from Sigma-Aldrich Chemicals, USA. A purification system (Milli-DI) in a laboratory produced deionized water (DI water). The alginate solutions (the dispersed phase) of 1 wt% were made by dissolving the required amount of alginate powder in DI water and stirring for 24 h at room temperature using a magnetic stirrer. The Calcium Chloride aqueous solution (5 M) was prepared by stirring the solution for 20 min to fully solubilize the powder. Polydimethylsiloxane (PDMS, Sylgard 184) and a curing agent were bought from Dow Corning in Michigan, USA, to fabricate the microfluidic device.
2.2 Interfacial tension measurement
The interfacial tension between an alginate solution and a mineral oil that contained surfactants (1, 3, and 5 wt% of Span 80) was measured to be (4.8, 4.3, and 4 mN/m), and (1, 1.2, and 1.5 wt% of Tween 80) to be (5, 4.9, and 4.8 mN/m). Jikan CAG-20, an automatic device for measuring static and dynamic contact angles, surface and interfacial tensions, was used to measure interfacial tension between the two phases at room temperature.
2.3 Device fabrication
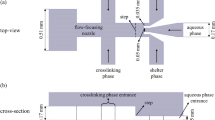
The silicon mold was created using the photolithography procedure following spin-coating the SU8-photoresist on a 2 inches silicon wafer. In a 10:1 ratio, PDMS and curing agent were mixed. Following degassing, the mixture was poured into the mold and degassed again. The PDMS was then cured for at least 30 min at \(75\,^\circ\)C. After peeling away the PDMS from the mold, inlet and outlet holes were punched with a 1 mm punching tool. To bond the PDMS and a glass microscope slide and seal the microchannels, they are exposed to oxygen plasma for 60 s. Figure 1a depicts the streamlined workflow outlining the key processes in creating our PDMS chip. The fabricated chip is stored one week before trials since the oxygen plasma will reverse the PDMS intrinsic hydrophobicity (Jiang et al. 2022).
2.4 Set up of the microfluidic device
The two phases were injected separately into the microfluidic chip using a syringe pump (Longer, TS-1B/W0109-1B) at the specified flow rate, the dispersed phase ( Qd) being an aqueous solution of alginate and the continuous phase (Qc) being a mixture of oil and oil-soluble surfactants. Figure 1b and Fig. 1c show microscopic images of micropillars placed at each inlet to act as filters. It should be noted that due to the small size of the orifice, the risk of unwanted particles clogging our droplet microfluidic chip is high. One method for preventing blockage is to implement mechanical filters at the device’s inlets. Using SEM microscopy, the average height of the columns that function as membrane filtration is 72.5 \(\upmu \hbox {m}\), which allows the debris-free liquids to flow smoothly through the 100 \(\upmu \hbox {m}\)-deep microchannel. At the junction of the microfluidic flow-focusing chip, the highly monodispersed alginate droplets are formed. The minimum feature size of the microfluidic droplet generator, the orifice, is 30 \(\upmu \hbox {m}\). The width of the outlet channel is 110 \(\upmu \hbox {m}\), while the width of the side channels and the main channel is both 100 \(\upmu \hbox {m}\). 100 \(\upmu \hbox {m}\) is a constant depth in all channels. The flow rate of the dispersed phase is set at 0.9 \(\upmu \hbox {L}/\hbox {min}\), lowest flow rate that our syringe pump can handle, and it is varied from 0.9 \(\upmu \hbox {L}/\hbox {min}\) to 7.2 \(\upmu \hbox {L}/\hbox {min}\) for the continuous phase. The flow rate of the alginate solution has been reduced because it is typically used to encapsulate rare or costly substances. The process is monitored using an inverted microscope (Nikon, Eclipse T100) equipped with a high-speed video camera (Basler A1300).
2.5 Characterization of alginate microdroplets
The image processing program, ImageJ, is used to on-chip measuring of the droplet diameters and a Python script calculates the frequency of droplet generation. All the experiments were repeated 5 times, and the average value was taken as the result. We measured the spherical and non-spherical droplets using Feret diameter. It quantifies the minimum distance between parallel tangents of droplets at an arbitrary angle. We repeated the experiment three times at each flow rate, calculating five different feret diameters at random angles for each of 50 droplets. The average of these 150 values was used to determine the droplet diameter for each droplet generated under different surfactant concentration and flow rate ratio conditions.
2.6 Characterization of alginate microgels
A tube was used to continually deliver alginate droplets to a 30 mL beaker. The collecting bath contained 2.5 mL of 5 M CaCl2 aqueous solution and 15 mL of mineral oil. The concentration of CaCl2 was chosen based on Lin et al. (2013) who studied the effect of CaCl2 on the formation of alginate beads. Due to slow gelation, they indicate that more diluted solutions will form beads in the shape of a tail. The tip of the droplet’s feeding tube was immersed in mineral oil in a fixed position, allowing the droplet to travel smoothly from the tube to the gelation bath. Microdroplets transformed into microgels after passing across the interface between mineral oil and the cross-linking layer. The shape and size of the gels are determined by the flow rate ratio of the dispersed and continuous phases, as well as the type and concentration of surfactants in the carrier fluid (oil phase). The inverted microscope was used to track the evolution of droplets into microgels. Figure 2 depicts the entire setup.
2.7 Microgels collection
The microgels that have been collected are washed repeatedly. A CaCl2 aqueous solution containing 1 wt% Tween 80 was used to wash the microgels to eliminate any remaining mineral oil solutions. To remove the oil, the microparticles were rinsed many times with isopropyl alcohol. The beads were then freeze-dried for 24 h (at \(-20\,^\circ\)C) to eliminate any remaining water. The beads were ready for SEM microscopy after being coated with gold using a sputter coater machine.
3 Results and discussion
3.1 Effect of flow rate ratio and surfactant addition on the emulsion microdroplets
a The influence of surfactant addition and flow rate ratio on the microdroplet diameter and the regime of alginate microdroplets formation in surfactant-laden mineral oil. The flow patterns observed in the microfluidic device are shown using different symbols. b-e Dynamic regimes of microdroplet formation b Dripping \(Q_c\)=7.2 \(\upmu \hbox {L}/\hbox {min}\), c Squeezing \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\), d Jetting \(Q_c\)=3 \(\upmu \hbox {L}/\hbox {min}\), e Threading \(Q_c\)=0.9 \(\upmu \hbox {L}/\hbox {min}\)
In the first step of our alginate microgel fabrication method, highly monodisperse w/o Na-alginate droplets were formed in a microfluidic flow-focusing device. The interfacial tension between the dispersed and continuous phases, as well as the flow rate of these two immiscible phases can be used to regulate size-controllable droplet formation in these devices. The effect of the flow rate ratio and surfactant addition on the alginate microdroplet formation in surfactant-laden mineral oil is shown in Fig. 3a.
Nonionic surfactants as interfacial tension manipulators were introduced to the continuous phase to change the interfacial tension between mineral oil and alginate aqueous solution. Interfacial tension was measured to be (4.8, 4.3, and 4) mN/m between an alginate solution and mineral oil with surfactants (1, 3, and 5 wt% of Span 80), and (5, 4.9, 4.8) for (1, 1.2, and 1.5 wt%) of Tween 80, respectively. The use of two types of surfactants in mineral oil at the same concentration and flow rate results in distinct droplet production behavior (See Supplemental Video 1.avi and Video 2.avi). The produced droplets get smaller and closer together in the outlet microchannel as the surfactant concentration increases. To put it another way, the frequency of droplet formation increases with the surfactant addition. Due to the decreased interfacial tension in surfactant-laden fluids, a decreasing trend in droplet size with increasing surfactant concentration, either Span 80 or Tween 80, was seen. According to Sinzato et al. (2017) Tween 80 requires a lower concentration than Span 80 to achieve the same reduction in interfacial tension, which is most likely due to the presence of hydrophilic chains, which may result in a higher free energy of adsorption. Although the higher efficiency of Tween 80 was approved by our interfacial tension test between surfactant-laden oils and Na-alginate solution, Fig. 3a also approve that slight increase in Tween 80 concentration (0.5wt%) leads to 32.1% average reduction in the fabricated droplet diameter. On the other hand, 10.41% average reduction in droplet diameter necessitate 4wt% increase in Span 80 concentration. We used Span 80 at various concentrations of 1, 3, and 5 wt% and Tween 80 at various percentages of 1, 1.2 and 1.5 wt%. All concentrations are higher than CMC (Critical Micelle Concentration) of each surfactant to be sure that kinetics are controlled by equilibrium interfacial tension (Paiboon et al. 2022). According to Ratledge et al. (1984), the required HLB for water in mineral oil is around 4, which is consistent with the HLB value for Span 80. However, using the microfluidic droplet generator, we were able to observe the formation of aqueous alginate solution droplets in mineral oil using Tween 80, whose HLB value was 15. It should be noted that Tween 80 has a limited concentration range and was insoluble in mineral oil at concentrations greater than 1.5 wt% due to its water-soluble nature.
Another regulating parameter that influences droplet size and frequency of formation is the volume flow ratio (the ratio of dispersed and continuous flow rates). With a constant dispersed flow rate (\(Q_d\)= 0.9 \(\upmu \hbox {L}/\hbox {min}\)), droplet diameter decreased with increasing the continuous phase flow rate as expected because of an increase in shear stress acting on the droplet surface. As shown in Fig. 3a, the droplet formation regime was controlled by the input flow rates and the type of surfactant applied to the oil phase. The fluids behaviour in the flow-focusing generators can occur in a variety of modes (Paiboon et al. 2022), dripping (Fig. 3b), squeezing (Fig. 3c), jetting (Fig. 3d), and threading without droplet formation (Fig. 3e) were observed in our experiments.
In the dripping regime, only a portion of the width of the outlet channel is obstructed by the dispersed thread while the thread fully contacts the main channel during the squeezing regime. In the jetting regime, the instability breaks the thread into droplets, whereas in the threading regime, two parallel phase streams flow along the outlet channel. In all regimes of droplet formation, droplet diameter is reduced with an increase in the flow rate of the continuous phase. It is shown that dripping and squeezing occurred exclusively when Tween 80 was employed as the surfactant in the low volume flow ratio, whereas employing Span 80 led to jetting and threading at all the input continuous phase flow rates. The transition from jetting to dripping with an increase in \(Q_c\) and maintaining \(Q_d\) at a constant rate in flow-focusing devices, which could be observed in the Tween-laden continuous phase, was consistent with the findings of previous studies on droplet regimes in cross-junctions (Paiboon et al. 2022).
In Fig. 4, the influence of Capillary number linked to the continuous phase on dimensionless diameter of the generated droplets is shown. Capillary number is defined as:
where \(\mu\) is the dynamic viscosity of the liquid, \(\sigma\) is the interfacial tension between the two fluid phases, \(U_c\) is characteristic velocity defined as:
where \(A_i\) is inlet channel area. The increase in Capillary number caused by the simultaneous change in interfacial tension and the flow rate of the continuous phase reduces droplet size and increase the frequency of formation. The Capillary number of the continuous phase is increased to transition the droplet generation regime from jetting to squeezing (and then to dripping). The Capillary number for the jetting-to-squeezing droplet generation regime is shown for 1 and 1.2 wt% Tween 80. Squeezing is expected to be observed when \(Ca_c\) is less than 0.01 but in our experiments it was observed in higher range. The type and concentration of the surfactant, variations in the channel and orifice dimensions, flow rates, and viscosity ratios can all contribute to differences in the threshold values of capillary number.
Numerous renowned scaling laws for the droplet size variation with the flow rate ratio of both phases and the capillary number of the phases have been presented by researchers. Cubaud and Mason (2008) presented droplet diameter calculations in each droplet generation regime in square microchannels. According to them, in the jetting regime, the dimensionless diameter in square microchannels is only dependent on the flow rate ratio as:
where w is the width of the microchannel. For low viscosity contrast between the phases, as in our experiment, Fu et al. (2012) modified the relation as:
The diameter of the droplets produced during the jetting regime when 5 wt% Span 80 was used, exhibited good agreement with this relation with just a 8.9% variation. The droplets generated in the presence of various Span 80 and Tween 80 concentrations exhibited greater deviation.
In the dripping mode, which was only seen when Tween 80 was employed as the surfactant, the relationship established by Fu et al. (2012) as:
estimates the droplet diameter rather well with a slight variation, 6.4%, when 1.2 wt% Tween 80 was utilized.
Figure 5 depicts the alginate droplet formation frequencies in surfactant-laden mineral oil. The smallest droplet, measuring \(50.8\pm 0.5\) \(\upmu \hbox {m}\) in diameter, and the highest generation frequency (126 Hz), resulted from the lowest interfacial tension, the highest Span 80 concentration (5 wt%), and the highest volume flow ratio. The biggest microdroplets, \(216.3\pm 0.5\) \(\upmu \hbox {m}\) in diameter, were formed at the lowest frequency (7 Hz) in presence of the lowest concentration of Tween 80 (1 wt%).
Dependence of droplet formation frequency on flow rate ratios and surfactant types and concentrations. The surfactants in the continuous phase are either Span 80 (with varying concentration of 1, 3, and 5 wt%) or Tween 80 (with varying concentration of 1, 1.2, and 1.5 wt%). The higher or lower concentration of Tween 80 inhibited droplet formation, resulting in droplet coalescence or mixing of the two phases
Figure 6 shows droplets in the microfluidic chip’s outlet when the maximum and minimum concentrations of Span 80 and Tween 80, 1 and 5 wt% for the first and 1 and 1.5 wt% for the second, are applied to mineral oil at its maximum and minimum flow rates (0.9 and 7.2 \(\upmu \hbox {L}/\hbox {min}\)). Droplets in the outlet reservoir were not restricted by the lateral walls. When the greatest concentration of Tween 80 (1.5 wt%) was employed as an emulsifier in mineral oil, droplets adhered to each other at a reduced volume flow rate of the continuous phase (lower than 4 \(\upmu \hbox {L}/\hbox {min}\)). This phenomenon is thought to be caused by the fact that droplets remain in the outlet reservoir rather than conveying to the collection tube due to low oil phase flow. Tween 80’s hydrophilic nature in the confined reservoir destabilises the emulsion.
Effect of \(Q_c\) and surfactant concentration on the size distribution of alginate droplets. \(Q_d\) is set at 0.9 \(\upmu \hbox {L}/\hbox {min}\) during the whole experiment. a-b Span 80 and Tween 80 concentration was 1 wt% and \(Q_c\) is 0.9 \(\upmu \hbox {L}/\hbox {min}\),respectively. c Span 80 concentration was 5 wt% and \(Q_c\) is 7.2 \(\upmu \hbox {L}/\hbox {min}\), d Tween 80 concentration was 1.5 wt% and \(Q_c\) is 7.2 \(\upmu \hbox {L}/\hbox {min}\). (Scale bar: 70 \(\upmu \hbox {m}\))
3.2 Effect of the flow rate ratio and surfactant addition on the alginate hydrogels
The external gelation method was used to complete the gelation procedure of the produced droplets. Droplets were transported to a collection bath, flowed smoothly to the top layer of mineral oil, fell through the mineral oil, and reached the interface between mineral oil and the aqueous solution of cross-linking agent below. According to Hu et al. (2015), the external gelation method introduces additional experimental parameters that allow for greater control over the properties of the alginate microgel generated and it will reduce clogging issues that are common in droplet microfluidic-based microgel fabrication. As previously discussed, on-chip control of the size and frequency of the formation of Na-Alginate droplets is possible via flow rate ratio adjustment and surfactant addition.
Phase inversion occurs when the w/o emulsion passes through the interface and reaches the calcium chloride solution and the droplets begin to gel (Sinzato et al. 2017). The type of surfactant used in the continuous phase influences the gelation process. When Tween 80, as the hydrophilic surfactant, is used as an emulsifier in the droplet generator microfluidic chip’s continuous phase, phase inversion occurs quickly, calcium ions diffuse into the microdroplets, and are completely partitioned to the aqueous phase. In contrast, when lipophilic surfactant, Span 80 is used, the w/o emulsion is fully stabilized, the droplets pass through the oil phase in the collection bath and float in the oil-crosslinking solution interface. Excessive stabilization of the water-in-oil emulsion may prevent it from destabilising and inverting, which is required to recover the gel beads. The microgels formed from microdroplets in mineral oil with Span 80 at different flow rates are illustrated in Fig. 7. As expected, the continuous phase’s higher flow rate has resulted in smaller hydrogels.
In each hydrogel quantification, a total of 100 microgels were taken into consideration after 24 h of their transferring to the collection bath. Inadequate gelation resulted in non-spherical hydrogels for which equivalent diameter based on the projected area is calculated. When comparing droplets with corresponding hydrogels, it is clear that the droplet size decreases following gelation, even when the procedure was not totally completed. Researchers have previously reported on the decrease in droplet size owing to gelation (Liu et al. 2018). The majority of the reduction in microdroplet diameter after transfer to the gelation bath and introducing the crosslinking agent is attributable to the release of water from the droplets into the crosslinking solution, with a minor contribution from the difference in osmotic pressure (Marquis et al. 2014).
Effect of \(Q_c\) on the size of alginate hydrogels when Span 80 concentration was 1 wt%. \(Q_d\) is set at 0.9 \(\upmu \hbox {L}/\hbox {min}\). a \(Q_c\)= 0.9 \(\upmu \hbox {L}/\hbox {min}\) b \(Q_c\)= 4 \(\upmu \hbox {L}/\hbox {min}\) c \(Q_c\)= 6 \(\upmu \hbox {L}/\hbox {min}\), d \(Q_c\)= 7.2 \(\upmu \hbox {L}/\hbox {min}\). (Scale bar: 70 \(\upmu \hbox {m}\))
Table 1 and Table 2 are used to summarize all of the characteristics of the droplets and related hydrogels following ionic gelation. The mean diameter of the droplets and the equivalent diameter of the hydrogels are reported in the tables for each surfactant concentration and flow rate of the continuous phase. Furthermore, CV (coefficient of variation) was calculated to discuss the homogeneity of the generated droplets and hydrogels.
According to Table 1, the smallest hydrogels (equivalent diameter= \(40.2\pm 0.5\) \(\upmu \hbox {m}\)) were generated in the presence of 5 wt% Span 80 in the continuous phase when \(Q_c\) was adjusted to its maximum value of 7.2 \(\upmu \hbox {L}/\hbox {min}\). A \(20.9\%\) reduction in the size of the smallest droplet was observed after its gelation was completed. After gelation, the largest droplet generated in the Span-laden continuous phase was reduced \(17.4\%\) in size. By increasing the continuous flow rate from 0.9 to 4 \(\upmu \hbox {L}/\hbox {min}\), 6 \(\upmu \hbox {L}/\hbox {min}\) and, 7.2 \(\upmu \hbox {L}/\hbox {min}\) and using 1 wt% Span 80 as surfactant in the continuous phase, the average hydrogel size decreased by \(47\%\), \(60\%\), and \(62\%\) respectively. When 5 wt% Span 80 is used, the decline in the average hydrogel size was \(46\%\), \(61\%\), and \(66\%\) as \(Q_c\) increased. Assuming \(Q_c\) is set at 0.9 \(\upmu \hbox {L}/\hbox {min}\), increasing Span 80 concentration from 1 wt% to 5 wt% resulted in a \(10\%\) decrease in average hydrogel size. Setting \(Q_c\) to 7.2 \(\upmu \hbox {L}/\hbox {min}\) resulted in a \(22\%\) reduction in size due to surfactant addition (from 1 wt% to 5 wt%).
As reported in Table 2, the largest hydrogel formed in the presence of 1 wt% Tween 80 had an equivalent diameter of \(100.8\pm 0.5\) \(\upmu \hbox {m}\) when \(Q_c\) was set to 4 \(\upmu \hbox {L}/\hbox {min}\) and the smallest one, equivalent diameter= \(42.4\pm 0.5\) \(\upmu \hbox {m}\) ) was obtained from 1.5 wt% Tween 80 when \(Q_c\) was 7.2 \(\upmu \hbox {L}/\hbox {min}\). As a result, the corresponding droplet size reductions after gelation were \(41\%\) and \(17\%\) for the smallest and largest droplets, respectively. By increasing the continuous flow rate from 4 \(\upmu \hbox {L}/\hbox {min}\) to 6 \(\upmu \hbox {L}/\hbox {min}\), then to 7.2 \(\upmu \hbox {L}/\hbox {min}\) and using 1 wt% Tween 80 as a surfactant in the continuous phase, the average hydrogel size was reduced by \(6\%\), and \(13\%\). When 1.5 wt% Tween 80 was used, the average hydrogel size decreased by \(4\%\), and \(35\%\) as the \(Q_c\) value increased, respectively. Increasing Tween 80 concentration from 1 wt% to 1.5 wt% reduced average hydrogel size by \(53\%\) when \(Q_c\) was set to 4 \(\upmu \hbox {L}/\hbox {min}\). When \(Q_c\) is set to 7.2 \(\upmu \hbox {L}/\hbox {min}\), a \(51\%\) reduction in hydrogel size was achieved due to the application of surfactant (from 1 wt% to 1.5 wt%). As a consequence, utilizing higher concentration of Tween 80 reduced the hydrogel size greatly when compared to an equivalent droplet.
Raising the flow rate ratio decreased the droplet and hydrogels homogeneity. Generally hydrogels had a smaller diameter and a higher CV value than precursor Na-alginate droplets. Continuous phases containing the highest concentration of Tween 80 produced the most monodisperse microdroplets with the narrowest size distribution, when \(Q_c\) was set to 4 \(\upmu \hbox {L}/\hbox {min}\), droplets with an average diameter of \(87.4\pm 0.5\) \(\upmu \hbox {m}\) and a CV of \(2.4\%\) formed. In our experiments, corresponding hydrogels with an average projected area of \(7993.4\pm 0.5\) \(\upmu \hbox {m}^{2}\) and an average CV of \(7.5\%\) demonstrated the narrowest hydrogel size distribution. When 1 wt% Span 80 in mineral oil was employed as the continuous phase, the lowest homogeneity (CV=19.4%) was observed in the generated droplets when \(Q_c\) was set to 7.2 \(\upmu \hbox {L}/\hbox {min}\). It’s worth noting that increasing Span 80 resulted in a decrease in CV. When Tween 80 is added to the continuous phase, a similar effect is observed, and increasing the Tween 80 concentration in mineral oil decreases the CV value and results in an even narrower size range.
As shown in Fig. 8(a-e), when Tween 80 is applied to the continuous phase during droplet formation, adequate gelation occurred in the collecting bath regardless of the \(Q_c\) used. In these images, the hydrogels formed in presence of 1, 1.2, and 1.5 wt% Tween 80 in the mineral oil with different \(Q_c\) is illustrated. When \(Q_c\) was maintained at 0.9 \(\upmu \hbox {L}/\hbox {min}\), hydrogel formation failed at all Tween 80 concentrations. This is most likely due to the coalescence of droplets during conveyance to the gelation bath. Because of their smaller size, the higher magnification is used to photograph hydrogels in the highest concentration of Tween 80.
Using the controllability of the procedure provided by the external gelation procedure, the insufficient gelation due to Span 80 presence in the continuous phase may be enhanced. For instance, we employed a 50/50 (v/v) mixture of ethyl acetate and mineral oil as the collecting bath’s top layer. \(Q_c\) of the continuous phase (containing 3 wt% Span 80) was 0.9 \(\upmu \hbox {L}/\hbox {min}\) and as a result, adequate gelation occurred. Ethyl acetate is marginally soluble in water (National Center for Biotechnology Information 2022) and facilitated phase inversion and the gelation by increasing the cross-linking agent’s delivery to the droplets. Figure 8f shows the SEM image of one of these hydrogels after washing and freeze-drying. Figure 9g shows the SEM image of a dried tail-shaped hydrogel synthesised in the presence of 1.5 wt% Tween 80 when \(Q_c\) was set at 4 \(\upmu \hbox {L}/\hbox {min}\).
Effect of \(Q_c\) and concentration of Tween 80 on the size of tail-shaped hydrogels. The continuous phase composed of a 1 wt% Tween 80 in mineral oil at \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\). b1.2 wt% Tween 80 in mineral oil at \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\). (c) 1.2 wt% Tween 80 in mineral oil at \(Q_c\)=7.2 \(\upmu \hbox {L}/\hbox {min}\). d 1.5 wt% Tween 80 in mineral oil at \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\), observed with different magnification. e 1.5 wt% Tween 80 in mineral oil at \(Q_c\)=7.2 \(\upmu \hbox {L}/\hbox {min}\), observed with different magnification. (Scale bar is 3 \(\upmu \hbox {m}\).) f An SEM image of a dried gel prepared in presence of 3 wt% Span 80 in the continuous phase and a 50/50 (v/v) mixture of ethyl acetate and mineral oil as the collecting bath’s top layer (Scale bar: 50 \(\upmu \hbox {m}\)) when \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\) g An SEM image of a dried gel prepared in presence of 1.5 wt% Tween 80 in the mineral oil as continuous phase when \(Q_c\)=4 \(\upmu \hbox {L}/\hbox {min}\). (Scale bar: 50 \(\upmu \hbox {m}\))
3.3 Effect of the flow rate ratio and surfactant addition on monodispersity
Gelation not only caused shrinkage in the created hydrogels when compared to the corresponding hydrogel, but it also reduced the homogeneity of the hydrogels. Utilizing 1 wt% span-laden mineral oil at \(Q_c\)=7.2 \(\upmu \hbox {L}/\hbox {min}\) produced microdroplets with an average diameter of \(62.9\pm 0.5\) \(\upmu \hbox {m}\) and a CV of 16.3%. After gelation, our less uniform hydrogels with a mean equivalent diameter of \(51.6\pm 0.5\) \(\upmu \hbox {m}\) and the lowest CV of all created hydrogels (CV=19.4%) are developed (Fig. 9(a-b)). Tween 80 at its maximum concentration (1.5%) and flow rate (7.2 \(\upmu \hbox {L}/\hbox {min}\)) was utilized as the continuous phase with droplets of average diameter of \(72.6\pm 0.5\) \(\upmu \hbox {m}\) resulting in hydrogels with equivalent diameter of \(42.6\pm 0.5\) \(\upmu \hbox {m}\), which equals 41.6% shrinkage, the highest recorded shrinkage in this study (Fig. 9(c-d)).
Raising the flow rate ratio reduces homogeneity, whereas increasing the concentration of surfactants in the continuous phase increases homogeneity in both droplets and, as a result, the associated hydrogels. In Fig. 10, the relation between the CV value of droplets and the hydrogels is illustrated. The droplets are more homogeneous than the corresponding hydrogels. Tween 80 produced more homogeneous hydrogels, and it can be deduced that reducing the flow rate of the continuous phase of Tween 80-laden mineral oil will promote homogeneity far more pronouncedly than Span-80-laden continuous phase.
Size distribution histograms of alginate droplets a, c and corresponding hydrogels b, d. Flow rate of the continuous phase is set to 7.2 \(\upmu \hbox {L}/\hbox {min}\) and \(Q_d\)= 0.9 \(\upmu \hbox {L}/\hbox {min}\). a-b1 wt% Span 80 and c-d1.5 wt% Tween 80 is used as the surfactant in the continuous phase
4 Conclusions
This study looked at the formation of alginate microdroplets in the presence of varying concentrations of two different types of surfactants in a microfluidic flow-focusing device. The dispersed phase flowed at a constant rate and the continuous phase was mineral oil containing varying percentages of either Span 80 or Tween 80, the surfactant. As anticipated according to Peng et al. (2011); Kiratzis et al. (2022); Lupo et al. (2014), it was discovered experimentally that decreasing the interfacial tension between the continuous and dispersed phases (by adding surfactants) reduces the diameter of the generated droplet. Increasing the flow rate ratio has the same effect on droplet size as expected. The droplet detachment process (droplet formation regime) was also found to be impacted by both interfacial tension and flow rate ratio. To investigate the interfacial tension reduction, Span 80 with concentration varying from 1 to 3 wt% and Tween80 with varying concentration of 1 to 1.5 wt% was added to the continuous phase and the flow rate of this phase was increased gradually. The smallest droplets were obtained at the highest concentration of Span 80 and the maximum flow rate ratio. Therefore, with the combined effect of surfactant addition and varying the outer phase flow rate, an accurate prediction of droplet size is attainable. When the cross-linking agent is introduced, this programmable microfluidic generation of alginate droplets will result in a variable hydrogel. We also investigated the external and off-chip gelation of alginate droplets, as well as the effect of flow rate ratio and surfactant on the properties of the hydrogels formed. The hydrogel’s size, monodispersity, and degree of gelation were all determined by the \(Q_c\) and surfactant in the continuous phase. Tween 80 emulsification produces the most homogeneous, completely gelled, tail-shaped hydrogels, whereas Span 80 emulsification produces smaller hydrogels with a more uneven size distribution. Using Span 80 caused partial gelation, necessitating a collection bath change (such as adding ethyl acetate to the oil phase). Hydrogels are distinct from one another in terms of their size, shape, and monodispersity; taken together, these characteristics determine how they might be exploited for the delivery of drugs or cells.
Data Availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Capretto L, Mazzitelli S, Balestra C, Tosi A, Nastruzzi C (2008) Effect of the gelation process on the production of alginate microbeads by microfluidic chip technology. Lab Chip 8(4):617–621
Chaemsawang W, Prasongchean W, Papadopoulos KI, Sukrong S, Kao WJ, Wattanaarsakit P (2018) Emulsion cross-linking technique for human fibroblast encapsulation. International Journal of Biomaterials 2018:
Chen M, Bolognesi G, Vladisavljević GT (2021) Crosslinking strategies for the microfluidic production of microgels. Molecules 26(12):3752
Cubaud T, Mason TG (2008) Capillary threads and viscous droplets in square microchannels. Phys Fluids 20(5):053302
Damiati S (2020) In situ microfluidic preparation and solidification of alginate microgels. Macromol Res 28(11):1046–1053
Davarcı F, Turan D, Ozcelik B, Poncelet D (2016) The influence of solution viscosities and surface tension on calcium-alginate microbead formation using dripping technique. Food Hydrocolloids 62:
Fu T, Wu Y, Ma Y, Li HZ (2012) Droplet formation and breakup dynamics in microfluidic flow-focusing devices: From dripping to jetting. Chem Eng Sci 84:207–217
He T, Wang W, Chen B, Wang J, Liang Q, Chen B (2020) 5-fluorouracil monodispersed chitosan microspheres: Microfluidic chip fabrication with crosslinking, characterization, drug release and anticancer activity. Carbohydrate polymers 236:116094
Hu Y, Azadi G, Ardekani AM (2015) Microfluidic fabrication of shape-tunable alginate microgels: Effect of size and impact velocity. Carbohydrate Polym 120:38–45
Hu Y, Azadi G, Ardekani AM (2015) Microfluidic fabrication of shape-tunable alginate microgels: Effect of size and impact velocity. Carbohydrate polymers 120:38–45
Hu Y, Wang Q, Wang J, Zhu J, Yang Y (2012) Shape controllable microgel particles prepared by microfluidic combining external ionic crosslinking. Biomicro Fluid 6:26502–265029
Huang K-S, Lai T-H, Lin Y-C (2007) Using a microfluidic chip and internal gelation reaction for monodisperse calcium alginate microparticles generation. Front Biosci-Landmark 12(8):3061–3067
Jiang B, Guo H, Chen D, Zhou M (2022) Microscale investigation on the wettability and bonding mechanism of oxygen plasma-treated pdms microfluidic chip. Appl Surface Sci 574:151704
Kaygusuz H, Evingür GA, Pekcan Ö, von Klitzing R, Erim FB (2016) Surfactant and metal ion effects on the mechanical properties of alginate hydrogels. Int J Biol Macromol 92:220–224
Kim JH, Jeon TY, Choi TM, Shim TS, Kim S-H, Yang S-M (2014) Droplet microfluidics for producing functional microparticles. Langmuir 30(6):1473–1488
Kim C, Park K-S, Kim J, Jeong S-G, Lee C-S (2017) Microfluidic synthesis of monodisperse pectin hydrogel microspheres based on in situ gelation and settling collection. J Chem Technol Biotechnol 92(1):201–209
Kiratzis I, Kovalchuk NM, Simmons MJ, Vigolo D (2022) Effect of surfactant addition and viscosity of the continuous phase on flow fields and kinetics of drop formation in a flow-focusing microfluidic device. Chem Eng Sci 248:117183
Lin Y-S, Yang C-H, Hsu Y-Y, Hsieh C-L (2013) Microfluidic synthesis of tail-shaped alginate microparticles using slow sedimentation. Electrophoresis 34(3):425–431
Liu Y, Tottori N, Nisisako T (2018) Microfluidic synthesis of highly spherical calcium alginate hydrogels based on external gelation using an emulsion reactant. Sensors and Actuators B: Chemical 283:
Lupo B, Maestro A, Porras M, Gutiérrez JM, González C (2014) Preparation of alginate microspheres by emulsification/internal gelation to encapsulate cocoa polyphenols. Food HydrocolL 38:56–65
Ma T, Gao X, Dong H, He H, Cao X (2017) High-throughput generation of hyaluronic acid microgels via microfluidics-assisted enzymatic crosslinking and/or diels-alder click chemistry for cell encapsulation and delivery. Appl Mater Today 9:49–59
Marquis M, Davy J, Fang A, Renard D (2014) Microfluidics-assisted diffusion self-assembly: Toward the control of the shape and size of pectin hydrogel microparticles. Biomacromole 15(5):1568–1578
National Center for Biotechnology Information (2022). PubChem Compound Summary for CID 8857, Ethyl acetate. https://pubchem.ncbi.nlm.nih.gov/compound/Ethyl-acetate/ (accessed March.14, 2022)
Paiboon N, Surassmo S, Ruktanonchai UR, Soottitantawat A (2022) Hydrodynamic control of droplet formation in narrowing jet and tip streaming regime using microfluidic flow-focusing. Int J Multiphase Flow 150:104013
Peng L, Yang M, Guo S-S, Liu W, X-z Zhao (2011) The effect of interfacial tension on droplet formation in flow-focusing microfluidic device. Biomed Microdev 13(3):559–564
Ratledge C, Dawson PSS, Rattray J (1984) Biotechnol Oils Fats Indus, vol 11. The American Oil Chemists Society, USA
Sadeghi D, Solouk A, Samadikuchaksaraei A, Seifalian AM (2021) Preparation of internally-crosslinked alginate microspheres: Optimization of process parameters and study of ph-responsive behaviors. Carbohydrate Polymers 255:117336
Sinzato YZ, Dias NJS, Cunha FR (2017) An experimental investigation of the interfacial tension between liquid-liquid mixtures in the presence of surfactants. Exp Thermal Fluid Sci 85:370–378
Vicini S, Mauri M, Wichert J, Castellano M (2017) Alginate gelling process: Use of bivalent ions rich microspheres. Polymer Eng Sci 57(6):531–536
Voo W-P, Ooi C-W, Islam A, Tey B-T, Chan E-S (2016) Calcium alginate hydrogel beads with high stiffness and extended dissolution behaviour. Europe Polymer J 75:343–353
Wang Q, Liu S, Yang F, Gan L, Yang X, Yang Y (2017) Magnetic alginate microspheres detected by mri fabricated using microfluidic technique and release behavior of encapsulated dual drugs. Int J Nanomed 12:4335
Wang Q, Zhang D, Xu H, Yang X, Shen A, Yang Y (2012) Microfluidic one-step fabrication of radiopaque alginate microgels with in situ synthesized barium sulfate nanoparticles. Lab Chip 12:4781–6
Yeh C-H, Zhao Q, Lee S-J, Lin Y-C (2009) Using a t-junction microfluidic chip for monodisperse calcium alginate microparticles and encapsulation of nanoparticles. Sens Actuat Phys 151(2):231–236
Yu D, Dong Z, Lim H, Chen Y, Ding Z, Sultana N, Wu J, Qin B, Cheng J, Li W (2019) Microfluidic preparation, shrinkage, and surface modification of monodispersed alginate microbeads for 3d cell culture. RSC Adv 9(20):11101–11110
Zhang H, Tumarkin E, Peerani R, Nie Z, Sullan RMA, Walker GC, Kumacheva E (2006) Microfluidic production of biopolymer microcapsules with controlled morphology. Journal of the american chemical societyJ Am Chem Soc 128(37):12205–12210
Zou Q, Hou F, Wang H, Liao Y, Wang Q, Yang Y (2019) Microfluidic one-step preparation of alginate microspheres encapsulated with in situ-formed bismuth sulfide nanoparticles and their photothermal effect. Europe Polym J 115:282–289
Acknowledgements
This work was supported by the UK Research and Innovation, Research England, International investment initiative (I3) [3DMED] and by INSF [98001236]. We would like to thank Prof. Matteo Santin, and Prof. Alidad Amirfazli for helpful discussions.
Funding
Partial financial support was received from UKRI (UK Research and Innovation).
Author information
Authors and Affiliations
Contributions
The authors confirm contribution to the paper as follows. Conceptualization : M. Oveysi, G. Peregrino , Dr. V. Bazargan, Prof. M. Marengo. Investigation : M. Oveysi:, A, Zaker, G. Peregrino. Software: A. Zaker, G. Peregrino, Dr. V. Bazargan. Visualization: M. Oveysi, A. Zaker. Writing - original draft: M. Oveysi. Writing - review & editing: A. Zaker, G. Peregrino , Dr. V. Bazargan, Prof. M. Marengo. Funding acquisition: Prof. M. Marengo.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file 1 (avi 1679 KB)
Supplementary file 2 (avi 4116 KB)
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Oveysi, M., Zaker, M.A., Peregrino, G. et al. Droplet-based fabrication of alginate hydrogel microparticles in presence of surfactants. Microfluid Nanofluid 27, 45 (2023). https://doi.org/10.1007/s10404-023-02655-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-023-02655-2