Abstract
A method is proposed for rapid prototyping of glass microfluidic devices utilizing a commercial micromilling machine. In the proposed approach, micromilling is performed with the glass substrates immersed in cool water, which could efficiently remove debris and increase the life of milling tools. We also investigate the effects of spindle speed, feed rate, cutting depth, cooling mode, and tool type on finished channel geometries, bottom surface roughness, and burring along the channel sides. It was found that low cutting depths, high spindle speeds and low feed rate produce smoother channels. Several functional microfluidic devices were demonstrated with this rapid prototyping method. The results confirm that the proposed micromilling technique represents a viable solution for the rapid and economic fabrication of glass-based microfluidic chips. We believe that this method will greatly improve the accessibility of glass microfluidic devices to researchers.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
1 Introduction
The properties of the material, such as machinability, surface charge, molecular adsorption, electroosmotic flow mobility, optical properties, and biocompatibility, are critical for the successful application of microfluidic devices. Now many different materials, such as silicon, glass, polymer, and paper are available for developing microfluidic devices (Nge et al. 2013; Ren et al. 2014, 2013). Of all the materials commonly used in microfluidics, glass remains a primary option due to its many favorable properties and good compatibility for biology applications, such as great chemical and thermal stability, excellent optical transparency, high mechanical strength, good electric insulation, low biomolecules absorption, and low water permeability (Iliescu et al. 2012). Glass microchips are commonly fabricated by standard photolithography combined with wet chemical etching (Bu et al. 2004; Lin et al. 2001; Stjernström and Roeraade 1998) or deep reactive ion etching (DRIE) to transfer the channel patterns into the wafer surface (Akashi and Yoshimura 2006; Park et al. 2005). However, these operations require well-trained operators and complicated and expensive equipment in clean rooms that are not always readily accessible. Thus, alternative low-cost technologies have been developed in recent years to construct microchannels on glass substrates for prototyping applications. For example, Rodriguez et al. (2003) and Allen and Chiu (2008) developed a direct patterned etching method that transfers patterns of microchannels in poly (dimethylsiloxane) (PDMS) onto glass. They conformally sealed PDMS molds to a glass surface for confining the etching solution, which then defined the etched pattern. However, the replication processes utilized to fabricate the PDMS microchannels involved the construction of a master using photolithography. A similar issue is associated with thermal imprinting methods. This microfabrication technology presses a mold onto glass substrates at a temperature higher than the glass transition temperature (Tg) to transfer the pattern of mold onto glass surface (Huang et al. 2012; Ju et al. 2015; Tseng et al. 2014). It is also claimed to be cost-effective, but the construction of molds used for glass imprinting requires materials and sophisticated techniques that sometimes are not readily available. Recently, some researchers have demonstrated the feasibility of utilizing laser machining techniques to fabricate a network of microchannels on glass (Bulushev et al. 2016; Cheng et al. 2005; Hwang et al. 2004; Nieto et al. 2015, 2014). However, these methods almost require an ultrafast (e.g., femtosecond or nanosecond) laser system which is expensive and not readily available. In addition, it is difficult for laser machining techniques to fabricate channels with rectangular cross-sections.
For rapid prototyping of glass microfluidic devices, several simple and low-cost approaches have been presented to prepare wet etching masks for fabrication of glass microfluidic devices. For example, a toner-mediated lithographic technology for fabricating glass microchannels was proposed by Coltro et al. (2007). In this approach, the pattern used for wet chemical etching with HF was first printed on wax paper and then thermally transferred onto glass surfaces. Santana et al. reported a method for fabricating glass microchannels by wet chemical etching using masks made by xurography in vinyl adhesive films, in which the patterns of microchannels were transferred to the vinyl adhesives using a cutting plotter (de Santana et al. 2013). Despite their simplicity and economy, these methods involve corrosive chemicals, and are not suitable for fabricate channels with high aspect ratios.
Micromilling is an alternative method for rapid prototyping microdevices for microfluidic applications. This technique creates microstructures via cutting tools that remove bulk material. Compared to other rapid prototyping techniques, micromilling has some advantages due to its fast fabrication time, independence from cleanroom facilities, and low cost of equipment and materials. Recent works have shown micromilling to be effective for microfluidic devices (Carugo et al. 2016; Chen et al. 2014a, b; Guckenberger et al. 2015; Hupert et al. 2007; Wilson et al. 2011). These works have focused on two strategies: (1) machining the mold used in subsequent fabrication steps (e.g., embossing or injection molds) (Carugo et al. 2016; Hupert et al. 2007; Wilson et al. 2011) or (2) machining microchannels and features directly into the final part (Chen et al. 2014a, b). However, all works reported in the literature concern the fabrication of polymer-based microfluidic devices by micromilling, and no data were shown on using micromilling to create glass microfluidic devices.
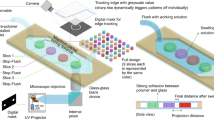
Herein we demonstrate that micromilling is also suitable for fabricating glass microchannels by immersing the substrate in water and employing the appropriate cutting parameters. Figure 1 schematically illustrates the steps of micromilling glass microstructures. The method consists of the following steps: layout design, computer numerically controlled (CNC) milling, and debris removal, which is much faster than the lithography process. In addition, micromilling can also be used to fabricate multi-level microfluidic structures during the same milling procedure simply by typing the parameters into the control system, which allows combining more functions and applications into the microfluidic device for integrating microfluidic system. Without a clean room facility or the highly corrosive acid, HF, the overall development time is within hours, which makes it easy for researchers to prototype glass microfluidic devices in academic laboratories.
2 Experimental section
2.1 Materials and instruments
In this work, commercially available soda–lime microscopic glass slides (75 mm × 25 mm × 1 mm, Feizhou Corporation, Yancheng, China) were used as substrates for micromilling the channels. For enclosed microfluidic devices, sealing glass slides were also composed of the same substrates. For open microfluidic devices, a surface superhydrophobization was performed before micromilling by coating the glass substrate with commercially available superhydrophobic agent (NC319, Nanocoating Co., Ltd, China). A micromilling machine (JY4030, Jieke Automation, Inc., China) was used to engrave user-defined features into the glass substrates. Two-fluted flat nose or ball nose micro-end mills with TiN coatings were used to create the desired channel geometries.
2.2 Fabrication procedure
First, a customized microfluidic device design was created using a CAD software. This data was then imported into the CNC engraving software for generating tool paths and outputting g-code which was used to control milling tools. The glass substrate was fixed to the worktable using toe clamps, and then a certain amount of water was added into bath until covering the glass substrates, which was used to cool the workpiece and remove chips during the machining process. After finishing the milling process, the glass substrate was washed by ultrasonication in deionized water and blown dry with forced air. After cleaning, both channel geometry and surface character of the device were assessed via microscopy and scanning electron microscopy (SEM).
2.3 Device bonding
For enclosed devices, a calcium-assisted glass-to-glass bonding technique was used to assemble the whole-glass microfluidic devices as previously reported (Allen and Chiu 2008). The cleaned slide and coverslip were immersed in a solution mixed with a 0.5% Alconox (Alconox, Inc. White Plains, NY) and 0.5% (w/v) calcium (II) acetate hydrate, and then rinsed with a stream of DI water for about 30 s. After rinsing, the two pieces of glass were brought into full contact and dried at 60 °C for 1 h. If no defects were found, the glass assembly was clamped by clips and placed in a 115 °C oven for 2 h to create an irreversible seal.
3 Results and discussion
3.1 Glass patterning via micromilling
Glass is an excellent material for use as a microfluidic device substrate because it has great chemical and thermal stability, excellent optical transparency, and high electrical resistivity. It would be ideal to fabricate glass microstructures through mechanical micromachining for some rapid prototyping applications of glass-based microfluidic devices, but the brittle nature of glass makes machining difficult. The machined surface is usually fractured and requires additional finishing processes that are costly and time consuming. Fortunately, it is found that the glass can be machined in a ductile regime to form smooth, chip free structures (Arif et al. 2011; Bifano et al. 1991). Ductile machining occurs if the strain applied allows for the material to behave plastically. To achieve this, the applied strain rate must be controlled by applying the correct machining parameters: tool choice, translation speed, rotational speed, and depth of cut (Neo et al. 2012). Typically, reducing the translation/rotational speed and depth of cut subsequently decreases the strain rate and thus can initiate ductile mode machining. Hence, to achieve fracture-free and high-quality glass microfluidic devices with an acceptable productivity, we explored a large machine parameter space by varying spindle rotational speeds from 2000 to 8000 rpm, translational speeds from 0.25 to 5 mm/min and cut depths from 20 to 300 µm; these cutting parameters are being chosen according to machine capability, realistic material removal rates and moderation of forces exerted on the tool. In addition, water was used as coolant to stabilize the temperature of milling tools, whilst providing lubrication at the cutting interface and removes cutting debris. We note that dry-mode machining results in catastrophic sample failure so coolant is necessary (Fig. 2a).
SEM images of machined microchannels of glass under different cutting parameters. a axial depth of cut: 100 µm, feed rate: 0.5 mm/min, spindle rate: 8000 rpm, lubrication: dry; b axial depth of cut: 100 µm, feed rate: 5 mm/min, spindle rate: 8000 rpm, lubrication: water; c axial depth of cut: 100 µm, feed rate: 0.5 mm/min, spindle rate: 8000 rpm, lubrication: water; d axial depth of cut: 300 µm, feed rate: 0.5 mm/min, spindle rate: 8000 rpm, lubrication: water
It can be found that fracture-free channels could be cut in soda–lime glass by micro-end milling, if the feed rate was maintained below 0.5 mm/min at a spindle speed of 8000 rpm and the depth of cut was below 100 µm (Fig. 2b). Under these optimal milling conditions, the milled glass surfaces had low surface roughness and thus exhibited low scattering losses and excellent optical transparency (as shown in Fig. S1 in Supporting Information), which was beneficial for optical detection and imaging applications. Additionally, it was also observed that the average surface roughness increased with the feed rate (Fig. 2c), and notable brittle cracks remained on the edges of the finished channel when increasing the depth of cut (Fig. 2d). This is perhaps because the shear stress applied at the cutting face exceeds the material’s plastic limit thus creating cracks that propagate and chip away material, producing an unsmooth, pitted and cracked surface at higher feed rate and greater depth of cut. In addition to surface quality, we also assessed the precision and accuracy of the micromilled devices by comparing the obtained channel widths with the designed channel widths. As shown in Fig. S2, the microchannels from 300 to 1000 µm wide exhibited less than 6% deviation from the expected widths, i.e., in these devices the measured channel widths well-matched the designed channel widths. Furthermore, for all the measured channel widths, the relative standard deviations (RSDs) are very small (see Table S1 in Supporting Information), indicating that the channel to channel reproducibility is quite acceptable.
Micromilling provides the widest range of feature capabilities with the least added process complexity. Particularly, this method is capable of making complex three-dimensional (3D) features that may be impractical or unfeasible with other methods. This is demonstrated by the microchannels such as stepped depth and graded depth channels showed in Fig. 3a, b. Channels of varying depth can be made all in the same process, something that requires multiple process steps in a lithography-based PDMS fabrication approach. The graded channel, which has a continuously varying depth, would be extremely difficult to produce in a lithography-based process. Besides, the method also can fabricate microfluidic channels with complex cross-sectional geometries, not just rectangular channels as those achieved by traditional photolithographic methods (Fig. 3c, d). The present results confirm that crack-free glass microchannels with complex profiles can be successfully fabricated using a widely available milling machine.
The main instrument in this technique is a desktop CNC milling machine, which costs $1500. This makes this technique easily accessible to most labs. Furthermore, this technique possesses the ability to fabricate a part directly from a three-dimensional (3D) CAD model, making it easier and faster to convert design concepts to working prototypes. Compared to other prototyping techniques (Table 1), this method provides a rapid, low-cost, and highly flexible solution for glass microfluidic device prototyping.
3.2 Device examples
To demonstrate the feasibility of micromilling for the fabrication of glass microfluidic devices, several typical microfluidic devices were fabricated and tested, respectively. The first example demonstrated is a Y-shape diffusive mixer with serpentine channel of a rectangular cross-section (300 µm × 50 µm) (Fig. 4a). To demonstrate operation of this device, colored dye solutions were injected through the inlets, with well-defined laminar flow observed at the confluence of the injected streams and formation of a smooth color gradient by diffusion within the downstream straight segment (Fig. 4a). However, the two dye solutions achieved nearly complete diffusive mixing over the first half-serpentine section. The observed performance is similar to that reported for mixers made using conventional lithographic-based fabrication techniques. The second example is a T-junction droplet generator with stepped channels (Fig. 4b). This device consists of two small inlet branches that combine to connect with a wider and deeper main channel downstream. The inlet branches are 300 µm wide, 50 µm deep and 10 mm long, while the main channel is 800 µm wide, 250 µm deep and 43 mm long. With paraffin oil containing 0.2% (w/w) Span-80 as the continuous phase and colored water as the dispersed phase, stable water-in-oil droplet generation was observed (see the inset image in Fig. 4b and Video S1 in ESI), as is the case with most well-fabricated microchannels. Image analysis performed to characterize droplet generation displays a narrow droplet distribution (Fig. S3). In addition to enclosed microfluidic devices, open microfluidic devices were also demonstrated. In contrast to the conventional enclosed fluidic systems, open-air microfluidic devices offer several advantages such as direct environmental accessibility, no cavitation/interfacial obstruction, clear optical path, and compatibility with biological experiments. To create an open microfluidic device on the glass substrate, a simple two-step procedure was performed. First, a superhydrophobic coating was applied to the glass slide by spraying a commercially available superhydrophobic coating agent (NC319, Nanocoating Co., Ltd, China). Then, the hydrophilic patterns were directly written on the prepared superhydrophobic glass slide with micromilling. The first open microfluidic device example is a simple straight microchannel of depth 50 µm and width 300 µm. On such an open-air microfluidic device, the hydrophilic patterns define the wetting boundary to direct flow, and surface tension-driven passive pumping is used to drive the flow (Lam et al. 2002). As shown in Fig. 4c, the pressure difference in the two droplets drove a flow from the smaller droplet to the larger one until the smaller droplet completely collapsed. The other open microfluidic device is a micromilling-patterned hydrophilic spots array (Fig. 4d). Using the wettability contrast of the superhydrophobic patterned glass slide, we dispensed droplets of dye solution and kept them restricted and fixed in the wettable spot due to the difference in surface tension compared with the superhydrophobic surrounding regions. Such an open microfluidic device could provide a much more accessible and cost-effective array-based platform for bioassays.
3.3 Cutting limitations
Although this method has many attractive features, it is not without limitation. The primary limitation of this method is the resolution of the fabricated glass microchannels, which is currently limited to ∼ 100 µm. The resolution of the milled glass features is mainly determined by the dimensions of the milling tools. It is worth noting that there are some advancements in the manufacture of ultra-small micro-end mills. For example, several manufacturers (e.g., Harvey Tool and Performance Micro Tool) offer end mills with diameters of 0.001 in (25 µm) and smaller. Together with high-end, advanced milling systems, the cutting resolution that can be achieved with such tools will likely reach new limits. In addition, unpredictable cutting tool life and premature tool failure are also major problems for glass micromilling. However, it should be possible, with proper tool condition monitoring systems installed, micromilling can also be successfully performed with acceptable cutting tool life and productivity to create miniaturized feature on glass materials.
4 Conclusion
In the present work, we have explored the application of micromilling method to rapid prototyping of glass microfluidic devices. Several typical glass microfluidic devices were demonstrated with this micromilling-based rapid prototyping method. The complete fabrication process from device design concept to working device can be completed in a matter of minutes and hours without photolithographic processes or chemicals, thus enabling researchers without cleanroom facilities to quickly prototype and test microfluidic designs in glass. In addition, glass microfluidic devices with complex features can be readily achieved with micromilling, which might be difficult or impossible to achieve with lithography. Thus, despite its limitation in resolution, this low-cost and straight forward rapid prototyping method for fabricating glass microfluidic devices should be attractive for the microfluidics/LoC community.
References
Akashi T, Yoshimura Y (2006) Deep reactive ion etching of borosilicate glass using an anodically bonded silicon wafer as an etching mask. J Micromech Microeng 16:1051
Allen PB, Chiu DT (2008) Calcium-assisted glass-to-glass bonding for fabrication of glass microfluidic devices. Anal Chem 80:7153–7157
Arif M, Rahman M, San WY (2011) Ultraprecision ductile mode machining of glass by micromilling process. J Manuf Process 13:50–59
Bifano TG, Dow T, Scattergood R (1991) Ductile-regime grinding: a new technology for machining brittle materials. J Eng Ind 113:184–189
Bu M, Melvin T, Ensell GJ, Wilkinson JS, Evans AG (2004) A new masking technology for deep glass etching and its microfluidic application. Sens Actuators A Phys 115:476–482
Bulushev E, Bessmeltsev V, Dostovalov A, Goloshevsky N, Wolf A (2016) High-speed and crack-free direct-writing of microchannels on glass by an IR femtosecond laser. Opt Laser Eng 79:39–47
Carugo D, Lee JY, Pora A, Browning RJ, Capretto L, Nastruzzi C, Stride E (2016) Facile and cost-effective production of microscale PDMS architectures using a combined micromilling-replica moulding (µMi-REM) technique. Biomed Microdevices 18:4
Chen P-C, Pan C-W, Lee W-C, Li K-M (2014a) An experimental study of micromilling parameters to manufacture microchannels on a PMMA substrate. Int J Adv Manuf Tech 71:1623–1630
Chen P-C, Pan C-W, Lee W-C, Li K-M (2014b) Optimization of micromilling microchannels on a polycarbonate substrate. Int J Precis Eng Man 15:149–154
Cheng J-Y, Yen M-H, Wei C-W, Chuang Y-C, Young T-H (2005) Crack-free direct-writing on glass using a low-power UV laser in the manufacture of a microfluidic chip. J Micromech Microeng 15:1147–1156
Coltro WKT, Piccin E, da Silva JAF, do Lago CL, Carrilho E (2007) A toner-mediated lithographic technology for rapid prototyping of glass microchannels. Lab Chip 7:931–934
de Santana PP et al (2013) Fabrication of glass microchannels by xurography for electrophoresis applications. Analyst 138:1660–1664
Guckenberger DJ, de Groot TE, Wan AM, Beebe DJ, Young EW (2015) Micromilling: a method for ultra-rapid prototyping of plastic microfluidic devices. Lab Chip 15:2364–2378
Huang C-Y, Kuo C-H, Hsiao W-T, Huang K-C, Tseng S-F, Chou C-P (2012) Glass biochip fabrication by laser micromachining and glass-molding process. J Mater Process Tech 212:633–639
Hupert ML, Guy WJ, Llopis SD, Shadpour H, Rani S, Nikitopoulos DE, Soper SA (2007) Evaluation of micromilled metal mold masters for the replication of microchip electrophoresis devices. Microfluid Nanofluid 3:1–11
Hwang D, Choi T, Grigoropoulos C (2004) Liquid-assisted femtosecond laser drilling of straight and three-dimensional microchannels in glass. Appl Phys A Mater Sci Process 79:605–612
Iliescu C, Taylor H, Avram M, Miao J, Franssila S (2012) A practical guide for the fabrication of microfluidic devices using glass and silicon. Biomicrofluidics 6:016505
Ju J, Lim S, Seok J, Kim S-m (2015) A method to fabricate low-cost and large area vitreous carbon mold for glass molded microstructures. Int J Precis Eng Man 16:287–291
Lam P, Wynne KJ, Wnek GE (2002) Surface-tension-confined microfluidics. Langmuir 18:948–951
Lin C-H, Lee G-B, Lin Y-H, Chang G-L (2001) A fast prototyping process for fabrication of microfluidic systems on soda-lime glass. J Micromech Microeng 11:726
Neo WK, Kumar AS, Rahman M (2012) A review on the current research trends in ductile regime machining. Int J Adv Manuf Technol 63:465–480
Nge PN, Rogers CI, Woolley AT (2013) Advances in microfluidic materials, functions, integration and applications. Chem Rev 113:2550–2583
Nieto D, Delgado T, Flores-Arias MT (2014) Fabrication of microchannels on soda-lime glass substrates with a Nd: YVO 4 laser. Opt Laser Eng 63:11–18
Nieto D, Couceiro R, Aymerich M, Lopez-Lopez R, Abal M, Flores-Arias MT (2015) A laser-based technology for fabricating a soda-lime glass based microfluidic device for circulating tumour cell capture. Colloids Surf B 134:363–369
Park J, Lee N-E, Lee J, Park J, Park H (2005) Deep dry etching of borosilicate glass using SF 6 and SF 6/Ar inductively coupled plasmas. Microelectron Eng 82:119–128
Ren K, Zhou J, Wu H (2013) Materials for microfluidic chip fabrication. Accounts Chem Res 46:2396–2406
Ren K, Chen Y, Wu H (2014) New materials for microfluidics in biology. Curr Opin Biotech 25:78–85
Rodriguez I, Spicar-Mihalic P, Kuyper CL, Fiorini GS, Chiu DT (2003) Rapid prototyping of glass microchannels. Anal Chim Acta 496:205–215
Stjernström M, Roeraade J (1998) Method for fabrication of microfluidic systems in glass. J Micromech Microeng 8:33–38
Tseng S-F, Chen M-F, Hsiao W-T, Huang C-Y, Yang C-H, Chen Y-S (2014) Laser micromilling of convex microfluidic channels onto glassy carbon for glass molding dies. Opt Laser Eng 57:58–63
Wilson ME, Kota N, Kim Y, Wang Y, Stolz DB, LeDuc PR, Ozdoganlar OB (2011) Fabrication of circular microfluidic channels by combining mechanical micromilling and soft lithography. Lab Chip 11:1550–1555
Acknowledgements
This work was supported by grants from the National Natural Science Foundation of China (nos. 61771078 and 61271161), the Chongqing Research Program of Basic Research and Frontier Technology (no. cstc2017jcyjB0182) and the Fundamental Research Funds for the Central Universities (no. 106112016CDJXZ238826).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary material 2 (WMV 649 KB)
Rights and permissions
About this article
Cite this article
Ku, X., Zhang, Z., Liu, X. et al. Low-cost rapid prototyping of glass microfluidic devices using a micromilling technique. Microfluid Nanofluid 22, 82 (2018). https://doi.org/10.1007/s10404-018-2104-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s10404-018-2104-y