Abstract
Purpose
Ascites can cause compression of the inferior vena cava (IVC), leading to increased renal venous pressure and renal congestion. Previously, the left renal vein diameter in liver cirrhosis patients with ascites was measured using computed tomography, showing that enlargement of the left renal vein diameter affects the prognosis. Herein, the diameter and flow velocity of the renal veins were measured using ultrasonography.
Methods
Abdominal ultrasonography was performed on 186 patients. The patients were divided into four groups: normal liver (n = 102), liver cirrhosis (LC) without ascites (n = 37), LC with ascites (n = 30), and congestive liver (n = 17). Ultrasonographic measurements for diameter and flow velocity of the IVC, left renal vein main trunk, and segmental renal vein were performed.
Results
The left renal vein diameter increased in the following order: normal liver, LC, LC with ascites, and congestive liver groups (P < 0.001). IVC flow velocity was lower and left renal vein diameter was larger in the congestive liver and LC with ascites groups. These results suggest that the two groups have different pathological conditions, but the mechanism of renal congestion is similar. In patients with LC, IVC compression due to ascites might cause blood stagnation and renal congestion.
Conclusion
The left renal vein and IVC can be measured using ultrasonography. It might help in furthering our understanding of the pathophysiology of renal congestion in these patients.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
In congestive heart failure (CHF), increased central venous pressure is associated with a decline in renal function due to renal venous hypertension, renal congestion, and renal failure. This condition also has a poor prognosis and significant morbidity, and may lead to death [1, 2]. Fluid retention associated with CHF leads to an increase in renal interstitial pressure, which compresses renal blood vessels and tubules, and impairs glomerular filtration and renal medullary blood flow [3]. Renal congestion alone is associated with increased mortality and morbidity in patients with heart failure. Renal congestion is also linked to cirrhotic ascites, which frequently causes compression of the inferior vena cava (IVC), an increase in renal venous pressure, and dilation of the renal veins. In a previous study using computed tomography (CT) to measure renal vein diameter, it was shown that patients who had refractory cirrhotic ascites and left renal vein dilation had high mortality rates [4]. To date, a few studies have measured the renal vein diameter or renal flow velocity using the harmless and painless color Doppler ultrasonography [5, 6].
The aim of this study was to assess the clinical significance of ultrasonography in measuring renal vein diameter and flow velocity in patients with and without liver disease. These measurements might be used as prognostic markers and may ultimately assist in treating cases of liver disease, renal disease, and CHF.
Materials and methods
Patient selection and eligibility
This cross-sectional, observational study involved 186 consecutive patients examined between April 2015 and March 2017 at our hospital. The patients were divided into four groups: normal liver (n = 102), liver cirrhosis (LC) without ascites (n = 37), LC with ascites (n = 30), and congestive liver (n = 17). The inclusion criteria were: age ≥ 20 years; patients in the normal liver group had no history of chronic liver disease or heart disease and were negative for hepatitis B surface antigens and hepatitis C virus antibodies, while patients with LC were divided into two groups, i.e., those with no or minimal ascites, and those with more-than-minimal ascites. Patients on artificial dialysis, patients with only one kidney, and patients with a gastrorenal or splenorenal shunt were excluded. In the normal liver and LC groups, patients with heart disease were excluded. In the congestive liver group, patients with other chronic liver diseases were excluded. LC was diagnosed based on the history of chronic liver disease, physical examination, blood examination, and findings of LC and/or portal hypertension (nodular liver, splenomegaly, and collaterals including gastroesophageal varices) on CT, ultrasonography, or gastrointestinal endoscopy. The diagnosis of congestive liver was based on the elevation of aspartate aminotransferase, alanine aminotransferase, alkaline phosphatase, or γ-glutamyl transferase levels, dilation of the hepatic veins, and decreased respiratory movement of the IVC. In all cases, clinical data were obtained from hospital records. The Nihon University Institutional Review Board approved this study. Informed consent was obtained by an opt-out method, because this study was retrospective in design and targeted cases from routine clinical practice.
Clinical assessment and blood tests, and various imaging studies
In this study, clinical assessments and general blood tests (total bilirubin, blood urea nitrogen, creatinine, sodium levels, and serum albumin) were performed for each patient on the same day as that of the ultrasound examination.
The presence or absence of esophageal varices was confirmed in patients with liver cirrhosis who underwent upper gastrointestinal endoscopy within 3 months before and after ultrasonography.
In patients with congestive liver, N-terminal prohormone of brain natriuretic peptide (NT-proBNP) in the blood, cardiothoracic ratio (CTR) on chest X-ray, and ejection fraction (EF) and transtricuspid pressure gradient (TRPG) using transthoracic echocardiography were confirmed within 3 months before and after ultrasonography.
In patients with liver cirrhosis and congestive liver, CT was performed within 3 months before and after ultrasonography, and the left renal vein diameter was measured on CT images. The left renal vein diameter was measured on CT at the main trunk of the renal vein [4]. Measurements were performed using non-contrast CT.
Ultrasonographic measurement of the IVC and renal veins
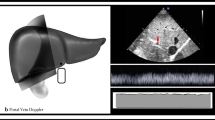
Ultrasonographic measurements of the diameter and the flow velocity of the IVC, left renal vein main trunk, and segmental renal vein in the supine position were taken. The ultrasonographic equipment used in this study included LOGIQ S8 (GE Healthcare Japan, Tokyo, Japan), Xario, Aplio 300 (Canon Medical Systems, Tokyo, Japan), and Aloka α10 (Hitachi Aloka Medical Ltd., Tokyo, Japan). The IVC parameters were measured with a sagittal scan. The largest diameter during exhalation was measured. The venous flow velocity was measured three times when the heart rate was stable, and the velocity recorded was the average of the three measurements. The IVC was measured at the caudal end of the liver. The left renal vein diameter and flow velocity were measured at the main trunk of the renal vein, which is downstream from the confluence of branched veins. The left renal vein was measured, because the right renal vein sometimes branches into multiple renal veins connecting to the IVC (occurs in approximately 15% of patients). The left renal vein, which runs ventral to the aorta, was measured on a transverse scan. The segmental renal vein was measured on the left intercostal scan (Fig. 1).
All tests were performed by two specific physicians who were board-certified Fellows of the Japan Society of Ultrasonics in Medicine (FJSUM) (M.K., N.M.; 5 and 15 years of imaging experience, respectively).
Statistical analyses
The data are shown as mean ± standard deviation. Patient characteristics were compared using the Mann–Whitney U test and the χ2 test. Normal distribution was assessed with the Kolmogorov–Smirnov test. P values < 0.05 were considered statistically significant for all analyses. All statistical analyses were performed using EZR (Saitama Medical Center, Jichi Medical University, Saitama, Japan), a graphical user interface for R (R Foundation for Statistical Computing, Vienna, Austria). EZR is a modified version of R Commander, enabling statistical functions frequently used in biostatistics.
Results
Blood test results by patient group
One hundred eighty-six patients satisfied the study inclusion criteria. Table 1 summarizes patient background, clinical characteristics, and blood test data. In the LC and LC with ascites groups, total serum bilirubin levels were higher than that in the normal liver group (P < 0.001). Serum albumin levels decreased in the following order: normal liver group, LC group, congestive liver group, and LC with ascites group (P < 0.001). Serum sodium levels were lower in the LC and LC with ascites groups compared to the normal liver group (P < 0.001).
Success rate of ultrasonographic measurement of left renal vein diameter and flow velocity
The success rate of measuring left renal vein diameter was 96% in the normal liver group, 97% in the LC group, 63% in the LC with ascites group, and 100% in the congestive liver group. The success rate of measuring left renal vein flow velocity was 91%, 76%, 37%, and 88%, respectively. The success rate of measuring segmental renal vein diameter was 94%, 95%, 100%, and 94%, respectively. The success rate of measuring segmental renal vein flow velocity was 91%, 81%, 87%, and 77%, respectively. In the normal liver, LC without ascites, and congestive liver groups, the success rate of measuring left renal vein diameter was more than 70% (Fig. 2).
Success rates for ultrasonographic measurements of left renal vein diameter and flow velocity. In the normal liver, LC without ascites, and congestive liver groups, the success rate for measuring left renal vein diameter was more than 70%. In the LC with ascites group, the success rate for measuring left renal vein diameter and velocity was low (P = 0.013, P = 0.016)
Normally distributed left renal vein diameters in normal liver group
The diameter and flow velocity of the left renal vein were normally distributed in the normal liver group (P = 0.012 and < 0.001, respectively) (Fig. 3). In this group, the diameter of the left renal vein did not correlate with height, weight, or diameter of the IVC (P = 0.085, 0.922, and 0.468, respectively).
Correlation between the IVC and left renal vein diameters, height, and age
In the normal liver group, the diameter of the IVC correlated with height (r = 0.347, P = 0.001) and age (r = − 0.436, P < 0.001), while the diameter of the left renal vein did not correlate with height (r = 0.186, P = 0.091) or age (r = − 0.024, P = 0.816).
Group-by-group comparison of the IVC, left renal vein, and segmental renal vein diameter
In the congestive liver group, the diameter of the IVC was larger compared to the normal liver, LC, and LC with ascites groups (P < 0.001). In the normal liver group, the diameter of the IVC was larger compared to the LC with ascites group (P = 0.012). In the normal liver group, the diameter of the left renal vein was smaller compared to the LC with ascites and congestive liver groups (P = 0.05 and < 0.001, respectively). The diameter of the left renal vein gradually increased in the following order: normal liver, LC, LC with ascites, and congestive liver groups (P < 0.001). There was no significant difference in segmental renal vein diameters between groups (Fig. 4).
Group-by-group comparison of IVC, left renal vein, and segmental renal vein diameter. a The diameter of the IVC in the congestive liver group was larger than that in the normal, LC, and LC with ascites groups (P < 0.001, P < 0.001, P < 0.001, respectively). The diameter of the IVC in the normal group was larger than that in the LC with ascites group (P = 0.012). b The diameter of the left renal vein was gradually increased in the following order: normal, LC, LC with ascites, and congestive liver groups (P < 0.001). The diameter of the left renal vein in the normal group was smaller than that in the LC with ascites and congestive liver groups (P = 0.05 and P < 0.001, respectively). c No significant differences were found in the segmental renal vein diameters between groups
Group-by-group comparison of IVC, left renal vein, and segmental renal vein flow velocities
In the normal liver group, the IVC flow velocity was greater compared to the LC with ascites group (P = 0.037). There was no significant difference in the flow velocity of the left renal vein and segmental renal vein between groups (Fig. 5).
Group-by-group comparison of IVC, left renal vein, and segmental renal vein flow velocities. a The flow velocity of the IVC in the normal group was faster than that in the LC with ascites group (P = 0.037). b The flow velocity of the left renal vein showed no significant differences between groups. c The flow velocity of the segmental renal vein showed no significant differences between groups
Group-by-group comparison of the ratio of IVC/left renal vein diameter
The median of the ratio of IVC/left renal vein diameter was 2.56 in the normal liver group, 1.87 in the LC group, 1.85 in the LC with ascites group, and 2.99 in the congestive liver group. In the LC group, the ratio of IVC/left renal vein diameter was lower compared to the normal liver group (P = 0.002). In the congestive liver group, the ratio of IVC/left renal vein diameter was higher compared to the LC group (with or without ascites; P = 0.019 and P < 0,001, respectively) (Fig. 6).
Group-by-group comparison of the ratio of IVC/left renal vein diameter. In the LC group, the ratio of IVC/left renal vein diameter was lower compared to the normal liver group (P = 0.002). In the congestive liver group, the ratio of IVC/left renal vein diameter was higher compared to the LC group (with or without ascites; P = 0.019 and P < 0,001, respectively)
Correlation between left renal vein diameter measured by ultrasonography and CT
Of the patients with congestive liver and liver cirrhosis (with or without ascites), the left renal vein could be measured in 54 patients using CT. The left renal vein measured by ultrasonography and CT showed correlation (r = 0.309, P = 0.047) (Fig. 7).
Correlation between left renal vein diameter and esophageal varices
There was no correlation between left renal vein diameter and esophageal varices (P = 0.725).
Correlation between NT-proBNP, CTR, EF, and TRPG and IVC and left renal vein diameter
Left renal vein diameter and CTR showed correlation (r = 0.575, P = 0.040). IVC and CTR tended to correlate (r = 0.574, P = 0.051). NT-proBNP, EF, and TRPG did not correlate with IVC (P = 0.501, P = 0.908, and P = 0.585, respectively) and left renal vein diameter (P = 0.256, P = 0.460, and P = 0.244, respectively).
Discussion
In the present study, ultrasonographic measurements of renal vein diameter and flow velocity were taken. The success rate of measuring left renal vein diameter and flow velocity in the LC with ascites group was lower compared to other groups, since ascites may impair visualization of the vessels, causing difficulties in measuring the diameter and flow velocity.
Correlations between IVC diameter, height, and age have been reported in earlier studies; height has a positive correlation and age has a negative correlation with IVC diameter [7, 8]. The results in this study are consistent with those reports. The IVC diameter decreases, because the right atrium pressure decreases during aging [8]. On the other hand, the left renal vein is not affected by age, because it is a peripheral vein and far from the heart. This may explain why the diameter of the left renal vein was not correlated with height and age. Furthermore, this study found no correlation between the IVC and left renal vein diameter. Thus, the diameter and flow velocity of the renal veins are independent of body height or weight.
In addition, left renal vein diameter could be used as an independent factor for the evaluation of renal congestion, because it does not correlate with serum urea nitrogen and creatinine levels, which proves that these are not influenced by renal function [4]. Therefore, left renal vein diameter may be used as an independent parameter. In a previous report, it was shown that the median overall survival for LC patients with a left renal vein diameter ≥ 11 mm, as measured by CT, was less than that for patients with a left renal vein diameter < 11 mm [4]. This proves that a larger left renal vein diameter is associated with a poor prognosis in patients with LC.
The diameter of the left renal vein can be measured with ultrasonography. Compared to CT, ultrasonography has several merits, such as no radiation exposure, easy use at bedside, and repeatable examination.
It is known that heart failure causes renal venous hypertension leading to renal failure. The same mechanism could be underlying cirrhotic ascites. Compression of the IVC due to ascites probably causes congestion, which leads to the dilation of the left renal vein and renal congestion. Experiments on pigs showed that intra-abdominal pressure correlated with renal venous pressure [9, 10]. Studies also showed that an increase in ascetic fluid level increased the renal venous pressure in patients with LC [9]. Moreover, in the supine position, IVC compression by the liver also leads to increased IVC pressure [11]. Thus, in cases of decompensated LC, IVC compression by the liver and the ascetic fluid may cause renal venous hypertension.
Renal congestion has been studied in experimental models [12,13,14,15]. These studies showed that histological damage began with interstitial edema in the tubular epithelium, which progressed to necrosis [12]. Furthermore, studies also showed obstruction of the proximal tubule lumen by swollen cells, ischemia associated with the accumulation of cell debris [13], impaired renal cortical flow [14], tubulointerstitial injury, glomerular injury, and hypoxia in the medullary thick ascending limbs [15]. Recently, the significance of color Doppler ultrasonography for the evaluation of renal congestion has been reported [6, 16, 17]. A monophasic waveform in the intrarenal veins (abnormal, as opposed to biphasic or continuous) revealed by color Doppler ultrasonography indicates the presence of renal congestion, which is possibly associated with increased mortality [6, 17] or hospitalization due to heart failure [16]. Treatments for renal congestion have been developed recently [2, 18]. Tolvaptan [18, 19] and cell-free and concentrated ascites reinfusion therapy (CART) may improve renal congestion. Renal congestion also occurs during the anhepatic phase of liver transplantation [20] and in other conditions such as abdominal compartment syndrome [21].
The present study has several limitations. First, the sample size of the LC and congestive liver groups was relatively small and included patients of specifically Japanese ethnicity. Second, various types of ultrasonography equipment were used in this study. The methods of measuring IVC and renal veins were unified to minimize the possibility of error. Third, the accuracy of the IVC and renal vein measurements was not sufficiently reliable in patients with ascites or obesity, because deep attenuation influences the sensitivity of color Doppler ultrasonography.
Conclusion
In conclusion, this study showed that ultrasonography can be used for measuring the renal vein diameter and flow velocity. In patients with liver cirrhosis, the diameter of the left renal vein tended to increase and that of the IVC tended to decrease. Compression of the IVC by cirrhotic ascites causes congestion similar to that caused by CHF. Future research on renal congestion will benefit from this new method of measurement.
References
Ross EA. Congestive renal failure: the pathophysiology and treatment of renal venous hypertension. J Card Fail. 2012;18:930–8.
Mullens W, Abrahams Z, Francis GS, et al. Importance of venous congestion for worsening of renal function in advanced decompensated heart failure. J Am Coll Cardiol. 2009;53:589–96.
Mori T, Ohsaki Y, Oba-Yabana I, et al. Diuretic usage for protection against end-organ damage in liver cirrhosis and heart failure. Hepatol Res. 2017;47:11–22.
Matsumoto N, Ogawa M, Kumagawa M, et al. Renal vein dilation predicts poor outcome in patients with refractory cirrhotic ascites. Hepatol Res. 2018;48:E117–25.
Kudo Y, Mikami T, Nishida M, et al. Altered oscillation of Doppler-derived renal and renal interlobar venous flow velocities in hypertensive and diabetic patients. J Med Ultrason. 2017;44:305–14.
Iida N, Seo Y, Sai S, et al. Clinical implications of intrarenal hemodynamic evaluation by Doppler ultrasonography in heart failure. JACC Heart Fail. 2016;4:674–82.
Patil S, Jadhav S, Shetty N, et al. Assessment of inferior vena cava diameter by echocardiography in normal Indian population: a prospective observational study. Indian Heart J. 2016;68:26–30.
Masugata H, Senda S, Okuyama H, et al. Age-related decrease in inferior vena cava diameter measured with echocardiography. Tohoku J Exp Med. 2010;222:141–7.
Henrilsen JH, Ring-Larsen H. Raised renal venous pressure: direct cause of renal sodium retention in cirrhosis. Lancet. 1988;2:112.
Bloomfield GL, Blocher CR, Fakhry IF, et al. Elevated intra-abdominal pressure increases plasma renin activity and aldosterone levels. J Trauma. 1997;42:997–1004.
Nakao S, Come PC, McKay RG, et al. Effects of positional changes on inferior vena caval size and dynamics and correlations with right-sided cardiac pressure. Am J Cardiol. 1987;59:125–32.
Manenti A, Botticelli A, Baraldi A, et al. Experimental acute renal congestion in rat: histological observations. Pathologica. 1989;81:523–6.
Hemmi S, Matsumoto N, Jike T, et al. Proximal tubule morphology in rats with renal congestion: a study involving the in vivo cryotechnique. Med Mol Morphol. 2015;48:92–103.
Komuro K, Seo Y, Yamamoto M, et al. Assessment of renal perfusion impairment in a rat model of acute renal congestion using contrast-enhanced ultrasonography. Heart Vessels. 2018;33:434–40.
Shimada S, Hirose T, Takahashi C, et al. Pathophysiological and molecular mechanisms involved in renal congestion in a novel rat model. Sci Rep. 2018;8:16808.
Puzzovivo A, Monitillo F, Guida P, et al. Renal venous pattern: a new parameter for predicting prognosis in heart failure outpatients. J Cardiovasc Dev Dis. 2018;5:52.
Husain-Syed F, Birk HW, Ronco C, et al. Doppler-derived renal venous stasis index in the prognosis of right heart failure. J Am Heart Assoc. 2019;8:e013584.
Mori T, Hirose T, Kinugasa S. Treatment of renal congestion by tolvaptan. Hypertens Res. 2019;42:745–8.
Chiba H, Seo Y, Sai S, et al. Renoprotective effects of tolvaptan in hypertensive heart failure rats depend on renal decongestion. Hypertens Res. 2019;42:319–28.
Li ZX, Wang MC, Zhang YC, et al. Hemodynamics and vasoactive substance levels during renal congestion that occurs in the anhepatic phase of liver transplantation. World J Gastroenterol. 2015;21:5482–7.
Kamimura H, Watanabe T, Sugano T, et al. A case of hepatorenal syndrome and abdominal compartment syndrome with high renal congestion. Am J Case Rep. 2017;18:1000–4.
Acknowledgements
The authors thank Editage (https://www.editage.jp) for the English language review. The authors also thank Masanori Abe (Professor, Division of Nephrology, Hypertension, and Endocrinology, Department of Internal Medicine) and Keisuke Matsusaki (Kanamecho Hospital) for research support.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Masahiro Kaneko, MD; Naoki Matsumoto, MD, PhD; Mariko Kumagawa, MD; Yukinobu Watanabe, MD; Midori Hirayama, MD; Hiroshi Nakagawara, MD, PhD; Toshiki Yamamoto, MD, PhD; Masahiro Ogawa, MD, PhD; and Mitsuhiko Moriyama, MD, PhD declare that they have no conflicts of interest.
Ethical statements
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1964 and later versions.
Informed consent
Informed consent was obtained from all patients for being included in the study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
About this article
Cite this article
Kaneko, M., Matsumoto, N., Kumagawa, M. et al. Renal vein measurement using ultrasonography in patients with cirrhotic ascites and congestive heart failure. J Med Ultrasonics 48, 225–234 (2021). https://doi.org/10.1007/s10396-021-01088-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-021-01088-0