Abstract
Purpose
High-resolution ultrasound is increasingly used in the diagnosis of carpal tunnel syndrome; yet little is known about gender differences in clinical presentation and ultrasound findings.
Materials and methods
In this high-resolution ultrasound-based retrospective study in 170 cases, we assessed gender influence in CTS in terms of the severity of neural alterations by wrist-to-forearm ratio (WFR), epineural thickening, loss of fascicular anatomy, as well as classical signs and symptoms. The control group consisted of 42 wrists.
Results
Women present with a greater WFR at first admission are affected more often bilaterally, and report less subjective pain intensity, while men report fewer nightly pain episodes at higher WFR. Loss of fascicular anatomy is three times more frequent in women. An increase in epineural thickness, loss of fascicular anatomy, and involvement of more than 1.5 fingers correlate significantly with WFR regardless of sex.
Conclusion
Women differ significantly from men in terms of clinical presentation and ultrasound findings upon first diagnosis of CTS, which should be included in further diagnostic considerations.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Carpal tunnel syndrome (CTS) is the most common and impairing peripheral entrapment neuropathy, with a varying prevalence of 4–16 % [1] and a distribution favoring women [2]. There is a well-established and highly significant gender difference in incidence and prevalence of CTS. The reason for this gender imbalance is not well known: possible explanations are mainly assumed occupation based [2]. Still, even the same occupation does not change the imbalance between men and women [3]. In most cases, CTS is qualified as idiopathic [4]. Well-known etiologies for secondary CTS include tenosynovitis of the flexor tendons, joint ganglions, accessory muscle bellies, tendon sheath fibromas, amyloid deposits, and thrombosis of a persistent median artery [5].
However, the indication for surgery is still based on clinical presentation [6]. As the severity of symptoms does not always correlate with the extent of neural damage [7], further diagnostic measures comprise electrophysiological testing (EDx) [8] and high-resolution ultrasound imaging (HRUS). While EDx is well established, a high rate of false positives is reported in a healthy population [9].
Chronic compression of a nerve leads to demyelination, fibrosis, and finally nerve fiber degeneration [10], which may present as an increase in CSA or a loss of fascicular texture at or proximal to the segment of compression of the median nerve. Only recently, epineural thickening has been described as useful to differentiate mechanical from non-mechanical causes of ulnar neuropathy at the elbow [11].
As HRUS is an accessible and easy-to-use modality in this field, attempts have been made at HRUS-based objectification of neural alterations in CTS. Although existing studies show conflicting results in regard to the prognostic value of an increase in cross-sectional area [12–14], the calculation of the wrist-to-forearm ratio (WFR) was developed with promising diagnostic power [15–17] and is increasingly used in primary diagnosis of CTS. Still, patient age, duration of symptoms, and clinical score for CTS-related symptoms yield the strongest predictive information [18, 19], and clinical presentation to date overrules other contradictory technical findings [6]. Gender aspects have not received much attention in regard to diagnostic and prognostic value; only one recent study found gender and body mass index (BMI) to be prognostic factors [12]. To examine whether there are gender differences in clinical presentation and HRUS findings, we examined 170 cases with clinically verified CTS.
Materials and methods
Technical prerequisites
All scans described below were performed with a Philips iU22® (Philips, Bothell, Washington, USA) using a broadband linear array probe (12–5 or 17–5 MHz): all regions were scanned always in two perpendicular planes following the algorithm and landmarks defined below. All images were stored in the institution’s Agfa® PACS. Data were handled according to the World Medical Association Declaration of Helsinki (59th WMA Assembly, Seoul, 2008) in the prevailing version. Institutional review board approval was further granted by means of a general waiver for studies with retrospective data analysis (Ethikkommission, Medical University Innsbruck; 2009/02/20).
Retrospective patient assessment
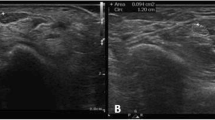
In this retrospective study, we evaluated the following features in 170 cases (129 patients) with clinical suspicion of CTS (44 with bilateral affection), 55 males (mean age 57.4 ± 16 years) and 115 females (mean age 55.1 ± 15.3 years), by a standardized questionnaire: maximum subjective pain via a visual analog scale (VAS [20]), the fingers affected by dysesthesia/paresthesia, and the presence of typical nocturnal episodes of pain (‘brachialgia paresthetica nocturna’). Furthermore, we assessed WFR, loss of neural texture (defined as lack of discernibility of individual fascicles) (see exemplary Fig. 1), epineural thickening (defined as epineural thickness exceeding 0.5 mm, 3 averages) taken from routinely performed studies for diagnostic purposes.
The inclusion criteria were as follows:
-
Typical clinical constellation for CTS such as typical dysesthesia/paresthesia, nocturnal pain episodes, and abnormal clinical neurophysiologic findings.
-
Available HRUS imaging of the median nerve [standardized series of HRUS images including the measurements of maximum cross section area (CSA) of the median nerve proximal to the entry into the carpal tunnel or within the carpal tunnel and at the level of the proximal third of the pronator quadratus muscle for WFR calculation, modified from [15] (as described in [16]), measurement of the maximum thickness of the hyperechoic epineurium, and depiction of the inner texture at the carpus at the point of greatest nerve CSA].
-
The exclusion criteria were as follows:
-
Secondary causes for CTS.
-
Diabetes mellitus and autoimmune or connective tissue diseases such as vasculitis or collagenosis.
Control group
A control group consisting of 21 healthy volunteers (11 females, 10 males) was included; both wrists were examined in each case. Exclusion criteria were current CTS, known history of CTS, nightly pain episodes, paresthesia/dysesthesia of the hands, prior wrist surgery, pregnancy, diabetes mellitus or autoimmune disease. All participants provided informed consent prior to inclusion.
Statistical analysis
Clinical and HRUS data were segregated by gender and accordingly grouped in a dichotomous fashion: loss of neural texture (=normal texture/texture masked), epineural thickening (=thin epineurium/thick epineurium, cut-off 0.5 mm), extent of finger involvement (cut-off 1.5 fingers), VAS maximum pain (VAS cut-off 5), and episodes of nightly hand pain (=no nightly hand pain/nightly hand pain). The following data analyses and descriptive statistics were performed using PASW Statistics 17.0 (SPSS Inc., Chicago, Illinois, USA). Quantitative data were described with mean values and standard deviation. Two-tailed student T Test, Chi-square test, and two-tailed Fisher’s exact test were used to determine significance values between the groups. A p value <0.05 was assumed as statistically significant. For presentation, box plots and profile plots were generated (Figs. 3, 4, 5).
Results
General results
Both women and men showed an M-shaped distribution of incidence with peaks at the ages of 40–55 and 65–70 years, although this was more pronounced in women (Fig. 2).
Overall bilateral affection was 34.9 %. The mean CSA of the median nerve at the level of the pronator quadratus was 0.08 ± 0.02 cm2 (range 0.036–0.19) and 0.19 ± 0.06 cm2 (range 0.08–0.47) at the carpus. The according standard deviation of 25 and 31 % of the base value illustrates a rather high inter-individual variability of median nerve CSA. Mean WFR was 2.54 ± 0.9 (male 2.28 ± 0.74; female 2.66 ± 0.95); according to the state-of-the-art cut-off value for WFR of 1.4, the overall diagnostic sensitivity of this HRUS feature was 95.9 %. Symptomatic patients had significantly higher values for WFR (2.54 ± 0.9 vs. 1.1 ± 0.26; p < 0.0001), rate of epineural thickening (62.9 vs. 2.4 %; p < 0.0001), and loss of fascicular texture (12.4 vs. 0 %; p = 0.017).
In symptomatic patients, the extent of finger involvement (p = 0.043), hypoechoic loss of inner neural texture of the median nerve at the restricted segment (p = 0.027), and epineural thickening (p = 0.006) correlated well with WFR (see Fig. 3); the combination of those markers led to a significance of p = 0.02. Nightly arm pain (p > 0.05) and maximum subjective pain intensity measured by VAS (p > 0.05) did not yield any significant correlation (see Fig. 3).
Gender-specific results
Within our control group, we found no significant differences in regard to WFR (women 1.06 ± 0.23 vs. men 1.13 ± 0.29; p = 0.41), epineural thickness (women 0.27 ± 0.06 mm, men 0.31 ± 0.09 mm; p = 0.13), or loss of neural texture (none in either group). In one male volunteer, we found an epineural thickness of 0.5 mm (see Table 1). Gender-specific analysis between patients and controls showed that symptomatic men also had a significantly higher mean WFR (2.28 ± 0.74 vs. 1.13 ± 0.29; p < 0.0001) and higher rate of epineural thickening (56.4 vs. 5.0 %; p < 0.0001), but not of loss of fascicular texture (5.5 vs. 0 %; p = 0.56). In symptomatic women, WFR (2.66 ± 0.95 vs. 1.06 ± 0.23; p < 0.0001) and rate of epineural thickening (66.1 vs. 0 %; p < 0.0001) were significantly higher than in controls; the rate of loss of fascicular texture showed no significant difference (15.7 vs. 0 %; p = 0.08) (see also Table 1).
In symptomatic cases, we found a significant difference in WFR between women (2.66 ± 0.95) and men (2.28 ± 0.74) (p = 0.009), with the diagnostic sensitivity in men being lower than in women (89.1 vs. 99.1 %, WFR cut-off 1.4). There was only a non-significant trend of higher bilateral affection in women: women were affected bilaterally in 36.9 % of cases, whereas men only in 31 % (p > 0.05).
Epineural thickness, inner texture changes, and amount of fingers involved were all correlated to an increase in WFR in both sexes (see Fig. 4). While epineural thickening was encountered in 56.4 % of men and 66.1 % of women (p = 0.24), loss of fascicular anatomy was three times more likely in women (5.5 vs. 15.6 %, respectively), although it was not statistically significant (p = 0.08).
Gender-specific box plots of the features described and depicted in Fig. 3
Additional profile plots were built to assess possible base effects of gender on HRUS features and clinical features vs. WFR (see Fig. 5): Male patients showed a stronger WFR correlation to epineural thickening and female patients for loss of inner neural texture; the amount of finger involvement correlated well with WFR regardless of patient sex (see Figs. 4, 5).
Sex did not affect the correlation between WFR and loss of neural texture, epineural thickening, and amount of finger involvement, while subjective pain measured by VAS showed no correlation to WFR in men and a paradoxical negative correlation in women. Furthermore, there was an inverse correlation between WFR and the presence of nightly arm pain in men
Nightly arm pain correlated positively with an increase in WFR in women and inversely in men. WFR did not correspond with VAS-assessed pain intensity in men, while higher WFR paradoxically led to lower VAS scores in women.
Discussion
General aspects
In light of recent advances at quantifying neural changes in CTS via HRUS, a possible gender influence on clinical presentation and HRUS findings has, to our knowledge, not been examined. The decision whether to treat a patient conservatively or surgically still relies on reviewing clinical history and EDx and HRUS findings as no single test can reliably predict outcome [12].
Basically, we could reproduce findings of previous studies [2, 4, 8] as we also found the highest incidence of CTS at the age of 40–50 years, and HRUS had a rather high sensitivity for (preliminarily known) CTS by using the WFR.
Furthermore, we could demonstrate a good correlation between WFR and several putative indicators of neural compression-induced damage such as epineural thickening and masking of inner neural texture, regardless of patient sex: epineural thickening is considered a sign of compression-induced reduction in neural perfusion and consequent edema and fibroblast invasion of the epineurium [4]. A loss of neural texture, on the other hand, reflects edema and swelling of neural tissue, indicative of at least restricted perfusion and concurrent and consequent alterations on cellular and subcellular levels of the neuron upon sustained or repeated increase in pressure [10]. A standardized inclusion of epineural thickening and loss of texture into HRUS evaluation might provide further information about the quality and quantity of neuronal damage. Edema possibly reflects an acute component of neural damage, while epineural thickening is probably linked to a longer history of compression and thus may hint at a greater disease progression and less favorable prognosis.
VAS-based assessment of maximum subjective pain was not associated with an increase in WFR, which is in line with findings from a previous study that demonstrated a lack of correlation between subjective pain and neural alterations [7]; the same held true for nightly pain episodes.
Gender-specific aspects
In line with the literature [21], we found no significant gender differences in regard to WFR, epineural thickness, or loss of fascicular texture in a healthy control group. All measurement parameters also differed significantly between symptomatic patients and controls. Gender-specific analysis revealed this to be true for WFR and rate of epineural thickening, but not for loss of fascicular texture. The latter may be explained by the low occurrence rate in men and the smaller sample size in controls.
The sensitivity for CTS in our study was higher for women than for men, with false negatives occurring in about 10 % of men and only 1 % in women. The uneven gender distribution and differing WFR at presentation might factor in here, though.
Female patients had a higher mean WFR than men and were more often affected bilaterally: this finding apparently conflicts with at least one previous study, where a significantly higher severity was described in men using EDx testing [22]. Although this may be due to differences in study populations, electrophysiological alterations very likely do not directly translate into morphological changes. EDx and HRUS possibly offer complementary rather than confirmatory information in CTS. Another explanation might be a later disease stage with fascicular atrophy in men upon first diagnosis. WFR may even be a marker of acute damage rather than severity. Edema might signify an earlier stage of CTS, with a later reduction in WFR and epineural thickening being a sign of chronic changes to the nerve [10]. As we did not follow up patients, we cannot provide functional data on outcome. Men have been shown to have a worse post-surgical outcome than women, just like elderly patients and smokers [12]. The influence of patient sex is still being debated according to a review of the literature [23], though, which may also stem from the low number of studies with a focus on gender differences in CTS. Still, differences in the pathophysiological background, susceptibility, or even nociception may be causative, such as gender-specific neural and connective tissue properties, as discussed in recent publications [24, 25]. Interestingly, WFR was completely independent from VAS-based maximum subjective pain in men. A higher WFR was associated with less subjective pain in women, prompting us to question whether patient symptoms should act as quantifier for CTS, or rather as a qualifier.
While men and women showed a comparable rate of epineural thickening, women had a three-fold higher rate of loss of fascicular anatomy. Furthermore, men had less nightly pain episodes at a higher WFR, whereas women exhibited lower maximum pain intensity at a higher WFR. Equally paradox findings exist for EDx, where either strong or absent changes in EDx were linked with poor post-surgical outcome [19].
The drawbacks of our study were its retrospective nature, the small sample size, and its immanent pre-selection bias: our patients had already been diagnosed clinically as highly likely for CTS; thus, there was no control group. We furthermore did not quantify EDx findings, as we expected no significant gain of information in the face of high clinical suspicion for CTS. As patients were included consecutively, more women were included in our study due to the known gender imbalance in regard to incidence.
To date, there have been no studies with a focus on gender differences in HRUS-based diagnostics in CTS. Gender-specific differences in neural susceptibility or nociception may be attributed to a different disease progression in men and women following differences in pain thresholds and pain perception [24, 25]. Women might feel more pain at an earlier disease stage, and thus be more likely to consult a physician. This might explain why women exhibit a greater WFR at first admission and why men, possibly being diagnosed later, suffer from a worse clinical outcome.
A different explanation for our findings in men might be a systematic underdiagnosis, as CTS may still be considered a classical ‘female’ disease. Men might delay consultation of a physician or might later be referred for further diagnostic workup.
Thus, general practitioners, neurologists, orthopedists, and radiologists should be aware that men may present later, with fewer or paradox symptoms and a lower WFR.
Conclusion
Overall, we were able to demonstrate a significant divergence of clinical symptoms from HRUS findings with a strong gender influence, to some extent calling into question the role of undifferentiated use of HRUS in quantification of primary CTS. The inclusion of epineural thickening and loss of fascicular anatomy could increase the prognostic value of a sonographic assessment of a patient with suspected CTS.
Further awareness is needed in regard to a patient’s sex, as women and men appear to present with a different set of symptoms and at different disease stages. Prospective studies should be carried out to elucidate this.
References
Ibrahim I, Khan W, Goddard N, et al. Carpal tunnel syndrome: a review of the recent literature. Open Orthop J. 2012;6:69–76.
McDiarmid M, Oliver M, Ruser J, et al. Male and female rate differences in carpal tunnel syndrome injuries: personal attributes or job tasks? Environ Res. 2000;83:23–32.
Jenkins P, Srikantharajah D, Duckworth A, et al. Carpal tunnel syndrome: the association with occupation at a population level. J Hand Surg Eur. 2013;38:67–72.
Uchiyama S, Itsubo T, Nakamura K, et al. Current concepts of carpal tunnel syndrome: pathophysiology, treatment and evaluation. J Orthop Sci. 2010;15:1–13.
Kopf H, Loizides A, Mostbeck G, et al. Diagnostic sonography of peripheral nerves: indications, Examination techniques and pathological findings. Ultraschall in Med. 2011;32:242–63.
American Academy of Neurology. Practice parameter for carpal tunnel syndrome (summary statement). Report of the quality standards subcommittee of the American Academy of Neurology. Neurology. 1993;43:2406–9.
Nunez F, Vranceanu A, Ring D. Determinants of pain in patients with carpal tunnel syndrome. Clin Orthop Relat Res. 2010;468:3328–32.
Patijn J, Vallejo R, Janssen M, et al. Carpal tunnel syndrome. Pain Pract. 2011;11:297–301.
Atroshi I, Gummesson C, Johnsson R, et al. Prevalence of carpal tunnel syndrome in a general population. JAMA. 1999;282:153–8.
Rempel D, Diao E. Entrapment neuropathies: pathophysiology and pathogenesis. J Electromyogr Kinesiol. 2004;14:71–5.
Plaikner M, Loizides A, Loescher W, et al. Thickened hyperechoic outer epineurium, a sonographic sign suggesting snapping ulnar nerve syndrome? Ultraschall Med. 2013;34:58–63.
Bland J, Rudolfer S. Ultrasound imaging of the median nerve as a prognostic factor for carpal tunnel decompression. Muscle Nerve. 2014;49:741–4.
Soyupek F, Yesildag A, Kutluhan S, et al. Determining the effectiveness of various treatment modalities in carpal tunnel syndrome by ultrasonography and comparing ultrasonographic findings with other outcomes. Rheumatol Int. 2012;32:3229–34.
Mondelli M, Filippou G, Aretini A, et al. Ultrasonography before and after surgery in carpal tunnel syndrome and relationship with clinical and electrophysiological findings. A new outcome predictor? Scand J Rheumatol. 2008;37:219.
Hobson-Webb L, Massey J, Juel V, et al. The ultrasonographic wrist-to-forearm median nerve area ratio in carpal tunnel syndrome. Clin Neurophysiol. 2008;119:1353–7.
Klauser A, Halpern E, De Zordo T, et al. Carpal tunnel syndrome assessment with US: value of additional cross-sectional area measurements of the median nerve in patients versus healthy volunteers. Radiology. 2009;250:171–7.
Cartwright M, Hobson-Webb L, Boon A, et al. Evidence-based guideline: neuromuscular ultrasound for the diagnosis of carpal tunnel syndrome. Muscle Nerve. 2012;46:287–93.
Bland J. Carpal tunnel syndrome. BMJ. 2007;335:343–6.
Bland J. Do nerve conduction studies predict the outcome of carpal tunnel decompression? Muscle Nerve. 2001;24:935–40.
Price D, McGrath P, Rafii A, et al. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17:45–56.
Boehm J, Scheidl E, Bereczki, et al. High-resolution ultrasonography of peripheral nerves: measurements on 14 nerve segments in 56 healthy subjects and reliability assessments. Ultraschall Med. 2014;35:459–67.
Becker J, Nora D, Gomes I, et al. An evaluation of gender, obesity, age and diabetes mellitus as risk factors for carpal tunnel syndrome. Clin Neurophysiol. 2002;113:1429–34.
Turner A, Kimble F, Gulyás K, et al. Can the outcome of open carpal tunnel release be predicted? A review of the literature. ANZ J Surg. 2010;80:50–4.
Wiesenfeld-Hallin Z. Sex differences in pain perception. Gend Med. 2005;2:137–45.
Mogil J. Sex differences in pain and pain inhibition: multiple explanations of a controversial phenomenon. Nat Rev Neurosci. 2012;13:859–66.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Ethical statement
All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, as revised in 2008 (59th WMA Assembly, Seoul, 2008).
Conflict of interest
The authors Leonhard Gruber, Hannes Gruber, Peter Schullian, Tanja Djurdjevic, and Alexander Loizides declare that there is no conflict of interest.
About this article
Cite this article
Gruber, L., Gruber, H., Djurdjevic, T. et al. Gender influence on clinical presentation and high-resolution ultrasound findings in primary carpal tunnel syndrome: do women only differ in incidence?. J Med Ultrasonics 43, 413–420 (2016). https://doi.org/10.1007/s10396-016-0707-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10396-016-0707-z